Abstract
Focal intranasal drug delivery to the olfactory cleft is a promising avenue for pharmaceuticals targeting the brain. However, traditional nasal sprays often fail to deliver enough medication to this specific area. We present a laminar fluid ejection (LFE) method for precise delivery of medications to the olfactory cleft. Using a 3D-printed model of the nasal passages, we determined the precise velocity and angle of insertion needed to deposit fluid at the olfactory cleft. Then, we conducted three proof-of-concept in-vivo imaging studies to confirm olfactory delivery in humans. First, we used Technetium-99 (a radiolabeled tracer) and methylene blue (a laboratory-made dye) to visualize olfactory deposition. Both tracers showed successful deposition. In a separate study, we used functional MRI (fMRI), to compare our LFE method with a conventional nasal spray while delivering insulin. From the fMRI results, we qualitatively observed focal decreases in brain activity in prefrontal cortex following insulin delivery. Overall, these preliminary results suggest that LFE offers a targeted approach to olfactory drug delivery, opening opportunities for access to the brain.Clinical and Translational Impact Statement - Focal deposition at the olfactory cleft is a promising target for delivering medication to the brain. We present in-human tests of a laminar fluid ejection method for intranasal drug delivery and demonstrate improvements over conventional nasal spray.
Keywords: Drug delivery, medical devices, neuroimaging, olfactory
I. Introduction
There is immense therapeutic potential for intranasal administration of drugs targeting the brain. Advantages of the intranasal route include that it is needleless and less painful than intravenous or intramuscular administration. Moreover, it offers faster delivery by largely bypassing the first-pass metabolism drugs are subjected to when administered via oral, intravenous, and intramuscular routes [1], [2], [3]. However, intranasal devices are severely limited by the volume of medication they can deliver, and medications targeting the brain often suffer from inconsistent pharmacokinetics when administered intranasally [1], [2]. A review of intranasal administration in emergency medicine and out-of-hospital settings found that intranasal administration of several drugs was not practical for adults due to the small volume limits of the devices [2]. An example of this limitation is highlighted in a clinical trial of intranasal ketamine for treatment-resistant depression, wherein the trial was halted early because participants could not tolerate the number of repeated nasal sprays that were necessary to deliver the required dose [4]. Of the many devices currently available to deliver drugs intranasally, most produce a turbulent spray that deposits small droplets of fluid diffusely throughout the lower nasal passages. Here, we present a new laminar fluid ejection (LFE), method to deliver medication focally to the olfactory cleft – an upper region of the nasal passages that may allow for transport to the brain. LFE dispenses medication with low force, offering potential benefits for biologics and lipid nanoparticles that may be altered or destroyed by the high shear forces of a typical nasal spray. The method dispenses formulations from a vantage point superior to the nasal valve, minimizing wastage and enabling consistent, accurate ejections.
A. Laminar Vs. Turbulent Flow
Laminar flow describes a smooth, regular movement of fluids where adjacent layers of particles experience relatively little mixing. Conversely, turbulent flow is characterized by chaotic, irregular motions with extensive mixing between adjacent layers. Turbulent behavior is better modeled by statistical, rather than analytical methods [5].
The turbulent spray of medications is associated with random deposition patterns within the nasal cavities, resulting in anatomical features being coated in proportion to their relative surface area rather than their desirability as a target site. The problem of spray deposition to the olfactory cleft is further complicated by the cleft's entrance profile which stymies the ingress of droplets. Specific deposition to the olfactory cleft is desirable for drugs targeting the brain [6].
We have developed and tested a laminar flow ejection (LFE) method, during which the medication is discharged as a continuous stream (see Fig. 1) and forms an intact bolus upon reaching the target site. We propose that the LFE method can facilitate precise deposition at the olfactory cleft in the upper nasal passages.
FIGURE 1.
(Left) the plume of
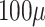 L fluid emitted from a conventional nasal spray device. (Right) Stream of
L fluid emitted from a conventional nasal spray device. (Right) Stream of
 L fluid emitted using the laminar fluid ejection (LFE) method.
L fluid emitted using the laminar fluid ejection (LFE) method.
B. Human Nasal Anatomy
The human nasal passages have a convoluted, labyrinthine anatomy divided into three main regions: (i) the nasal vestibule, (ii) the nasal turbinates, and (iii) the olfactory cleft (see Fig. 2).
FIGURE 2.
Schematic illustrating the convoluted human nasal passages. Neurons originating in the olfactory bulb protrude through small holes in the cribriform plate of the skull and into the olfactory cleft (highlighted in blue). Delivering a drug directly to the olfactory cleft may facilitate uptake in the central nervous system [4].
The nasal vestibule is the most ventral portion of the nasal passages and extends dorsally from the nostrils. The narrowest portion of the nasal vestibule (and of the nasal passages in general) is called the nasal valve. The nasal valve is the primary factor limiting the rate of airflow during respiration and deflects the majority of inhaled air into the respiratory nasal cavity and away from the olfactory cleft [6].
Bordering the nasal vestibule are three thin passages called the inferior, middle, and superior turbinates. As air flows past the turbinates, eddies form, significantly disrupting the flow of air [7]. Tissue in the turbinate regions is composed of ciliated and non-ciliated epithelial cells that secrete mucous. The ciliated mucosal cells are responsible for mucociliary clearance, which defends the respiratory system against harmful inhaled substances [8]. As air circulates through the turbinate eddies it is warmed, humidified, and deflected onto the mucus, clearing away inhaled substances. Cells in the turbinates are covered in microvilli which increases the surface area of these regions. Medication deposited in the turbinates will be absorbed systemically, failing to reach the brain [6].
The uppermost region of the nasal passages is the olfactory cleft, the region responsible for the sense of smell. The olfactory cleft comprises a relatively small portion of the internal nasal cavity. While anatomy can differ from person to person it is estimated that the olfactory cleft comprises 2-3% of the total volume, and roughly 5% of the total surface area of the internal nasal cavities [9], [10], [11]. The superior wall of the olfactory cleft is formed by a portion of bone at the base of the skull called the cribriform plate. Olfactory nerves project from the olfactory bulb at the base of the forebrain, through small holes in the cribriform plate, and directly into the olfactory cleft [12]. The olfactory nerve is one of only two cranial nerves that projects directly from the cerebrum [13]. (The other ten cranial nerves originate from the brainstem or midbrain.) Because it is innervated by the olfactory nerve, the olfactory cleft may allow medications direct access to frontal regions of the brain, making it an intriguing site for drugs targeting the central nervous system.
C. Drug Delivery to the Olfactory Cleft
The primary target for nose-to-brain drug delivery is the olfactory cleft [14], [15], [16]. A variety of mechanisms have been proposed as pathways from the olfactory cleft into the brain including axonal transport, perivascular pumping, bulk flow, lymphatic drainage, and endothelial transport through the olfactory nerve (for review, see [17]).
To even reach the olfactory cleft, drugs must surpass a range of defenses evolved to prevent harmful substances from entering the respiratory tract. Most of the air that enters the nasal valve is deflected back into the turbinates. As the force of inhalation is increased, the nasal valve is constricted, further deflecting air back into the turbinates and respiratory system. High-velocity airflow through the narrow nasal valve and turbulent eddies along the turbinates subject the drug to intense cleaving forces. If deposited in the nasal vestibule or turbinates, a drug targeting the brain might instead be inhaled, swallowed, or removed through enzymatic degradation or mucociliary clearance [18].
For decades, nasal sprays have been the established standard for noninvasive delivery of drugs into the nasal cavity [7]. These devices work well for the administration of topical agents, for decongestion, and for drugs targeting the respiratory system. When targeting the central nervous system however, recent research has established the inefficiency of conventional sprays compared to methods designed to deliver drugs more focally into the olfactory cleft [6], [19], [20]. Focal delivery promises to deposit a medication bolus directly to the desired area.
Conventional nasal sprays face several challenges for delivering pharmaceutical agents to the brain. The tip of most spray devices is typically short and does not extend past the nasal valve. The plume from a nasal spray deposits drug throughout the nasal cavity, significantly diluting any dose that may eventually reach the olfactory cleft. Moreover, spray devices typically atomize or vaporize the drug compound, creating small droplets that are degraded by high velocity airflow through the nasal valve. Shear forces can destroy some drug molecules and biologics as they are atomized into droplets [21]. With few exceptions, existing devices typically deliver only 5-8% of a dose to the olfactory cleft (see Table 1).
TABLE 1. Deposition in Olfactory Cleft (Existing Devices).
| Device | Description | Deposition (Ratio of Dose Delivered to Olfactory Cleft) | Ref. |
|---|---|---|---|
| Miaoling | Spray bottle | 4.59% | [22] |
| Apotex | Spray bottle | 0.71% | |
| Astelin | Spray bottle | 1.78% | |
| Nasonex | Spray bottle | 0.76% | |
| Drive Voyager Pro | Mesh nebulizer | Negligible | |
| Respironics | Ultrasonic nebulizer | Negligible | |
| Philips Respironics 1100312 InnoSpire Essence | Jet nebulizer | 6.7+/- 1.2% | |
| Pari Sinus | Jet nebulizer | 9+/- 1.7% | |
| Zetonna pMDI Spray | Pressurized metered dose inhaler | 12%

|
[23] |
| Custom high-force pMDI spray | Pressurized metered dose inhaler | 42%
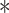
|
|
| N/A – CFD model of nasal spray | Computer simulation | 6.23% | [24] |
Deposition percentages in the olfactory cleft for several existing devices. These values are not directly comparable to each other, but overall demonstrate that deposition rates are generally in the range of 5-8%. *Value visually estimated from plot in reference manuscript.
A variety of approaches have been employed to surmount the many obstacles of intranasal drug delivery. These include manipulations to the dose volume, spray pattern, plume geometry, droplet size, velocity, viscosity, thixotropicity, and surface tension of drug vehicles/formulations (for review, see [20], [22]). Considering the myriad attempts to alter compounds so that they are “sprayable,” a key question arises: Why are we spraying in the first place? Is there a more efficient way to deliver medication directly to the olfactory cleft?
Here we present a novel method for intranasal drug delivery which we term laminar fluid ejection (LFE), to focally target the olfactory cleft. With this set of studies, our primary goals were to engineer a laminar fluid ejection device, test its feasibility for use in humans, and visualize olfactory deposition. Fig. 3 presents a graphical abstract of the various studies we conducted in pursuit of these goals. First, we conducted in-vitro tests to determine the conditions necessary (e.g. device angle, velocity) to deliver the laminar ejection as a continuous flow through the nasal passages. Next, we conducted pilot tests in humans to assess feasibility and visualize olfactory deposition, first with Technetium-99 (a radiolabeled-tracer) and then using methylene blue (a laboratory-made dye). Finally, we conducted a pilot test using functional magnetic resonance imaging to measure brain activity in response to LFE administration of insulin. Altogether, the results suggest that the LFE method efficiently deposits compound at the olfactory cleft, offering a promising opportunity for noninvasive delivery of pharmaceuticals directly to the central nervous system.
FIGURE 3.

Outline of the experimental goals and primary outcome measures for each of the studies presented in this manuscript.
II. Development of A Laminar Fluid Ejection Method
A. Device Description
We set out to develop a method of ejecting fluid precisely through a cannula for deposition at the olfactory cleft (see Fig. 4). Multiple prototype devices were created to test the LFE method. In general, all devices consisted of a 1mL polycarbonate syringe filled to 0.20mL of formulation. The syringe was capped with a cannula (inner diameter =1.35mm) and mounted on a carbon-fiber reinforced mechanism. A coil spring housed within the mechanism provided the force to compress the syringe. The spring preload was adjustable with the use of an external threaded collar and shims. The spring surrounded an orifice-based damper that could be filled with different weight silicone oils and orifice plates with different sized holes and quantity of holes. The mechanism used a simple trigger that released a syringe plunger guided by aluminum rails. This bar pushed the plunger shaft of the syringe. The syringe was held accurately and firmly in place in front of the mechanism.
FIGURE 4.

(A) Schematic of the early prototype of the laminar fluid ejection device that was used for in-vivo testing of delivery to the olfactory cleft in humans. (B & C) Updates to the device focus on optimization for self-administration, including a comfortable introducer that facilitates proper positioning in the nasal cavity, and an interlock to ensure users cannot depress the actuation button until the device has reached the correct position.
B. Aperture Testing
Aperture testing was conducted to evaluate the coherence of fluid post-ejection and to visually assess the laminar qualities of the ejected fluid. We tested fluids at two different viscosities (1cP and 50cP), each at a range of ejection velocities (2m/s – 27.7m/s). Device settings for a selection of velocities within this range were determined experimentally prior to conducting aperture testing. Velocity was determined using high-speed camera-footage of ejected fluid traveling a fixed distance from the tip of the canula. A small aperture (5.64mm in diameter) was placed 25mm away from the tip of the canula. The canula was supported by magnetic supports to reduce wobbling. A catching tray was placed on the opposite side of the aperture (see Fig. 5a)
FIGURE 5.
Experimental setups for laminar flow ejection method development. (A) Aperture testing was conducted to determine whether the method could efficiently deliver fluid through a small opening like the nasal passages. (B) Deposition testing was conducted to determine the optimal velocity for depositing fluids of various viscosities at the olfactory cleft. (C) A 3D-printed model of the human nasal passages was used to visualize deposition at the olfactory cleft.
The percent mass transferred from the syringe to the catching tray was calculated for each test-run (Mass in catching tray / Mass Ejected from syringe
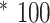 ). Tests of each combination of viscosity and speed were repeated three times. The mean percent mass transferred for each condition is presented in Table 2. (Note that the ejected mass is smaller than the total fill mass, as it does not include the residual left in the device. The residual left in the device did not meaningfully differ across conditions: 13.6% for 1cP, 10.4% for 50cP).
). Tests of each combination of viscosity and speed were repeated three times. The mean percent mass transferred for each condition is presented in Table 2. (Note that the ejected mass is smaller than the total fill mass, as it does not include the residual left in the device. The residual left in the device did not meaningfully differ across conditions: 13.6% for 1cP, 10.4% for 50cP).
TABLE 2. Aperture Testing.
| Viscosity (cP) | Velocity (m/s) | % Mass Transfer |
|---|---|---|
| 1.0 | 3.7 | 92.8% |
| 50.0 | 3.1 | 96.0% |
| 1.0 | 27.7 | 78.1% |
| 50.0 | 27.7 | 98% |
Percent mass transferred from the device through a small 5.64mm aperture and into a catching tray for low/high fluid viscosities and velocities.
In general, higher speeds were associated with reduced mass transfer, possibly due to increased cannula movement and splatter off the aperture edge. Qualitatively, we observed that the device ejected fluid in a cohesive stream with a narrow diameter, especially compared to the plume emitted from a traditional spray device (see Fig. 1). We observed some slight roughness in the stream and breakup in the tail, but this is likely explained by the cannula's flexibility/inadequate support in this mounting configuration, and may not be an issue in the confines of the nasal cavity.
C. In-Vitro Deposition Testing
Next, we conducted a series of in-vitro deposition tests to determine the range of velocities that would optimally deposit fluid in the olfactory cleft of the human nasal passages. To test this, the mechanism was inserted into a transparent 3D-printed model of the nasal cavity (based on work from [23]) (see Fig. 5b). Optimal delivery was considered to be in the “Goldilocks zone,” which represents the approximate location of the olfactory cleft in the model (see Fig. 5c). If ejected too slowly, the fluid would deposit anterior to the front boundary of the goldilocks zone; too quickly and it would deposit beyond the back boundary. Two fluid viscosities (1cP and 50cP) were tested at each of three different insertion angles (30, 35, 40 degrees from the vertical). The cannula's insertion depth was held constant at 37.5mm. The minimum velocity necessary to reach the Front Boundary (v
 ), the olfactory cleft (v
), the olfactory cleft (v
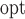 ), and the back boundary (v
), and the back boundary (v
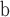 ), for each of the viscosities and deposition angles are presented in Table 3.
), for each of the viscosities and deposition angles are presented in Table 3.
TABLE 3. In-Vitro Deposition Testing.
| Angle (°) | Viscosity (cP) | V
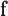 (m/s) (m/s) |
V
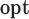 (m/s) (m/s) |
V
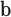 (m/s) (m/s) |
|---|---|---|---|---|
| 30 | 1 | 1.1 | 1.6 | 1.9 |
| 35 | 1 | 1.0 | 1.1-1.9 | 2.2 |
| 40 | 1 | 1.0 | 1.1-1.9 | 2.8 |
| 30 | 50 | 4.7 | 13.9-15.8 | 18.6 |
| 35 | 50 | 4.7 | 11.0-15.8 | 18.6 |
| 40 | 50 | 4.7 | 11.0-15.8 | 18.6 |
Table presents optimal velocities (
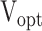 ) for fluids of low/high viscosity across three device angles. The
) for fluids of low/high viscosity across three device angles. The
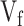 and
and
 columns show the velocity limits at which the deposition was in front of or behind the target area, respectively.
columns show the velocity limits at which the deposition was in front of or behind the target area, respectively.
For velocities within the optimal range, residence times were observed to exceed 10 minutes. Notably, angles of 35-40 degrees permitted a wider range of velocities, but using an angle of 30-degrees did not dramatically shift the optimal velocity. This suggested the device is adaptable to a wide range of angles. Optimal velocities (+/−20%) were found to be 1.6m/s for 1cP fluid and 14.0m/s for 50cP fluid. These optimal velocities may be associated with laminar flow.
A Reynold's number can be computed to quantify whether a fluid exhibits laminar or turbulent flow [24]. The Reynold's number for water at a velocity of
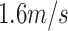 and viscosity of
and viscosity of
 (
(
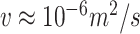 ) is approximately 2,080. Similarly for a fluid of
) is approximately 2,080. Similarly for a fluid of
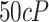 (with the same density as water), the Reynold's number at
(with the same density as water), the Reynold's number at
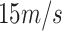 is approximately equal to 390. Both Reynold's numbers fall below 2,300 which is the commonly accepted threshold for laminar flow [24]. The calculations here and visual evidence from the aperture tests support the claim that the idealized deposition velocities using the LFE method operated in the laminar regime.
is approximately equal to 390. Both Reynold's numbers fall below 2,300 which is the commonly accepted threshold for laminar flow [24]. The calculations here and visual evidence from the aperture tests support the claim that the idealized deposition velocities using the LFE method operated in the laminar regime.
In each of the in-human studies below, the fluids themselves have not been altered between LFE and spray conditions. Rather, the LFE device was tuned to eject the fluid at a specific velocity. This velocity was chosen based on the viscosity of the fluid and our in-vitro tests, to maximize the extent of laminar flow.
III. In-Vivo Evaluation Methods
We conducted three proof-of-concept in-vivo imaging studies to visualize delivery to the olfactory cleft (first with technetium-99, and then with methylene blue), and investigate brain activity changes related to olfactory deposition of insulin.
A. Visualizing Olfactory Cleft Delivery with Technetium-99
Participants: Nine healthy participants (ages 19+) with no history of abnormal nasal or sinus symptoms or contraindications for nasal cannulation, Magnetic Resonance Imaging (MRI), or Single-Photon Emission Computed Tomography (SPECT). Participants completed one study visit undergoing magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT) to visualize the deposition of a radiolabeled tracer (technetium-99) in their nasal passages. In total, nine participants were enrolled in the study, and eight completed all study procedures (Participant 1.02 withdrew before completing all imaging procedures). All study procedures were approved by the Horizon Health Network's Human Research Protection Program. Prior to participating in the study, all participants were assessed by a licensed otolaryngologist physician to confirm their eligibility.
1). Study Design
On Day 1 of the study, anatomical (T1-weighted) magnetic resonance images were collected from participants at the Moncton MRI clinic (Moncton, NB, Canada) using a Siemens Skyra VD13 3T scanner. MRI data provided anatomical information regarding soft tissue structure in the nasal cavity and the location of each participant's olfactory region.
On Day 2 of the study, a saline solution including the technetium-99 radiotracer (mean dose 4.6 mCi) was delivered to participants using the LFE method described above. Day 2 study procedures were conducted at the Nuclear Imaging Department of Saint John Regional Hospital (Saint John, NB, Canada). At the time of delivery and for five minutes afterwards, 2D SPECT Flow images were acquired every three seconds using a gamma camera. Flow images were acquired in a single sagittal plane (
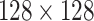 with voxels 4.8mm diameter).
with voxels 4.8mm diameter).
2). Image Analysis
Image analysis was completed in Vivoquant (4.0). SPECT Flow data was resampled to match MRI resolution (
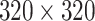 with voxels 1.918mm isotropic) and manually registered to T1 images in x and y space with minimal rotation in the z-plane. This procedure enabled landmarking to nasal passage characteristics minimally visible in the SPECT data alone. Flow data was visually inspected to determine (1) the point in the time series when the bolus was dispensed, (2) the time of cannula removal, (3) the median time point in the series where the bolus initially lodged between points 2 and 4, (4) the time point at which the bolus began additional migration, and (5) the time point of the final image acquired during the five-minute SPECT series. Fig. 6 shows a series of SPECT Flow images for a representative subject at time points 1, 3, and 5. All subjects' SPECT Flow images are presented in Figure S1.
with voxels 1.918mm isotropic) and manually registered to T1 images in x and y space with minimal rotation in the z-plane. This procedure enabled landmarking to nasal passage characteristics minimally visible in the SPECT data alone. Flow data was visually inspected to determine (1) the point in the time series when the bolus was dispensed, (2) the time of cannula removal, (3) the median time point in the series where the bolus initially lodged between points 2 and 4, (4) the time point at which the bolus began additional migration, and (5) the time point of the final image acquired during the five-minute SPECT series. Fig. 6 shows a series of SPECT Flow images for a representative subject at time points 1, 3, and 5. All subjects' SPECT Flow images are presented in Figure S1.
FIGURE 6.
Flow time series for LFE delivery of technetium-99 for participant 1.01. (A) Bolus delivery: t =0s. (B) Median hold time: t =56s. (C) Final frame: t =269s.
B. Visualizing Olfactory Cleft Delivery with Methylene Blue
1). Participants
Five healthy participants (ages 19+) with no history of sinonasal symptoms and no evidence of nasal inflammation were recruited for this study. Prior to recruitment of participants study procedures were approved by Providence Health Care research ethics board, (British Columbia, Canada) and conducted under Health Canada Investigational Testing Authorization #314993. All participants were assessed by a licensed otolaryngologist physician to confirm their eligibility. Participants completed two study visits during which a licensed otolaryngologist physician administered 0.1mL of the visual dye tracer, methylene blue.
2). Study Design
During the first visit, the methylene blue was administered using LFE method (1.5mm cannula; velocity =4.5m/s). During the second visit, methylene blue was administered using a conventional nasal spray device (Pharma systems Item #10272, UPC:063636802714). The Pharma systems spray device was used in these experiments because it was the device normally used by the compounding pharmacy for intranasal delivery. It was the device patients and clinicians were used to at this clinic.
Following delivery, the physician used a nasal endoscope to image the deposition of methylene blue to the olfactory cleft at up to five time points (1, 5, 8, 12, and 15 minutes following delivery). From each image, the physician judged whether at least 50% of the methylene blue fluid was delivered to the olfactory cleft. If <50% of the methylene blue was judged to have deposited in the olfactory cleft, imaging was stopped and that session's trial was completed.
Due to reports from several participants of a tingling sensation following LFE delivery of methylene blue, the physician diluted the methylene blue for the second study visit and limited spray doses to a single attempt. This may have affected the intensity of the dye's color in the images of spray deposition, and makes it difficult to directly compare the LFE and spray conditions. Consequently, deposition images were assessed according the ratio of methylene blue dye deposited in vs. outside of the olfactory cleft in the images, rather than the overall intensity of the dye's color.
C. Visualizing Brain Activity Changes Following LFE Administration of Insulin
1). Participants
Eight healthy participants (ages 19+) with no history of abnormal nasal or sinus symptoms, insulin use, diabetes, metabolic disorder, or any complicating medical conditions, or contraindications for nasal cannulation, MRI, or venous blood sampling were recruited for this study. Additionally, participants reported no previous diagnosis of SARS-CoV-2 with symptoms of anosmia. Study procedures were approved by Horizon Health Network's Human Research Protection Program prior to recruitment of participants. The study was conducted under Health Canada Investigational Testing Authorization #262367. All participants were assessed by a licensed otolaryngologist physician to confirm their eligibility
2). Study Design
Experimental design for this study was based off of work previously conducted by Kullman et al. [25]. An open-label four-arm crossover study design was used to evaluate the insulin brain effects using the novel device and a standard nasal spray. In condition A (n =4), undiluted solution of 80 IU (0.16 mL) prepared from U500 insulin (Entuzity, Eli Lilly, Canada) was delivered to the olfactory cleft using the LFE method (described above). Under this condition, 40 IU (0.08 mL) of human insulin was delivered directly to the olfactory region by a licensed otolaryngologist physician to each nostril. In condition B (n =4), undiluted solution containing 15% MucoLox (#30-4782, PCCA, Canada) (a mucoadhesive polymer) and 80IU (0.16 mL) insulin was delivered to the olfactory region using the LFE method. MucoLox was predicted to increase residence time at the deposition site. For condition C (n =8), a total of 1.6 mL insulin and sterile water mixture was prepared for nasal spray delivery. The nasal spray bottle (Pharma systems Item #10272, UPC:063636 802714) contained 80IU of U500 insulin mixed with a sterile water solution. The spray was delivered over four minutes with two puffs per nostril every minute. The condition sequences AC, CA, BC, and CB comprised the four arms of the study.
Participants were randomly assigned to one of two blocks. In Block 1, participants were randomly assigned by alternating sequence to one of the A arms (AC or CA). Block 1 participants did not receive MucoLox when the LFE method was used. In Block 2, participants were randomly assigned by alternating sequence to one of the B arms (BC or CB). Block 2 participants did receive MucoLox when the LFE method was used. For a given participant, appointments for each condition were scheduled at a minimum of 14 days apart to ensure no carry-over effects from the previous condition.
3). MRI Acquisition and Processing
Detailed methods for MRI acquisition, preprocessing, and denoising are included in the Supplementary Information. In summary, three
 -minute runs of resting-state fMRI were collected from each participant (one at baseline, another 15 minutes after insulin administration, and a third 60 minutes after insulin administration). fMRI images were preprocessed using the open-source, standardized fMRIPrep pipeline (v22.0.2). The fMRI data was then denoised using standard pipelines in the CONN Toolbox (v22.a) and SPM (v12.7).
-minute runs of resting-state fMRI were collected from each participant (one at baseline, another 15 minutes after insulin administration, and a third 60 minutes after insulin administration). fMRI images were preprocessed using the open-source, standardized fMRIPrep pipeline (v22.0.2). The fMRI data was then denoised using standard pipelines in the CONN Toolbox (v22.a) and SPM (v12.7).
4). FMRI ALFF Measures
Amplitude of low frequency fluctuations (ALFF) maps, characterizing low-frequency blood oxygenation level dependent (BOLD) signal variability at each voxel, were estimated as the root mean square (RMS) of the BOLD signal after denoising and band-pass filtering between 0.008 Hz and 0.09 Hz. ALFF can be conceptualized as a measure of the spectral power (or amplitude) of BOLD signal within the filtered frequency band, providing a proxy measure for brain activity at each region.
5). Group-Level Analyses
Un-thresholded ALFF maps are displayed in Fig. 8 for the spray and LFE conditions. Warm colors in these maps highlight brain regions with higher ALFF signal (increased brain activity) in the 15 min. or 60 min. scans compared to baseline. Cool colors in these maps highlight brain regions with lower ALFF signal (decreased brain activity) in the 15 min. or 60 min. scans compared to baseline. Green-colored regions show little to no change between baseline and the 15 or 60 min. follow-up scans.
FIGURE 8.
Group average fMRI results for Block 1 demonstrate a qualitatively greater reduction in prefrontal brain activity associated with insulin delivery via LFE vs. conventional nasal spray. LFE = Laminar Fluid Ejection. ALFF = amplitude of low-frequency fluctuations. (Note: due to the small sample size, statistical comparisons were not conducted.)
IV. Results
A. Visualizing Olfactory Cleft Delivery with Technetium-99
We investigated whether the LFE method could deliver fluid to the olfactory cleft in humans using a radiolabeled tracer, technetium-99.
In seven out of eight participants imaging results demonstrated delivery of the radiotracer to the cribriform area (Fig. 6 presents images from a representative participant). The radiotracer was detected in the cribriform area without any movement for at least 1.5 minutes in six of those seven participants. Additionally, clearance of the radiotracer was minor for the duration of the study for six out of those seven cases. In the two cases where sub-optimal outcomes were reached, it was the result of (i) bolus delivery anterior and superior to the cribriform (Participant 1.07), and (ii) bolus that cleared quickly after reaching the target, possibly due to the bolus remaining well intact as it directly impacted the cribriform (Participant 1.05). See Table 4 for a summary of the results in each participant.
TABLE 4. Visualization of Delivery to the Cribriform Area Using Technetium-99.
| ID | Cribriform Reached? | Hold Time (s) |
|---|---|---|
| 1.01 | Yes | 112 |
| 1.03 | Yes | 100 |
| 1.04 | Yes | 98 |
| 1.05 | Yes | 18 |
| 1.06 | Yes | 129 |
| 1.07 | No | 63 |
| 1.08 | Yes | 118 |
| 1.09 | Yes | 136 |
Results from in-human tests of the LFE method using radiolabeled dye (technetium-99). Hold-time describes the amount of time (in seconds) that the deposited bolus remained stationary before beginning to migrate.
B. Visualizing Olfactory Cleft Delivery with Methylene Blue
To confirm that the device could deliver fluid to the olfactory cleft and to assess delivery at a higher resolution, a second pilot study was conducted using methylene blue dye. Four of the five participants demonstrated successful delivery of >50% of the methylene blue to the olfactory cleft using the LFE method. In each of the five participants, the conventional nasal spray failed to deliver at least 50% of the dye to the olfactory cleft. See Fig. 7 for representative images of methylene blue delivery using the novel device and the conventional nasal spray device.
FIGURE 7.
Comparison of methylene blue deposition to the olfactory cleft using a conventional nasal spray, and the LFE method. O.C. = olfactory cleft (marked with dashed line).
C. Visualizing Brain Activity Changes Following LFE Administration of Insulin
In a third experiment, we examined changes in brain activation (measured with functional magnetic resonance imaging (fMRI)) after laminar fluid ejection of insulin to the olfactory cleft. Delivery of insulin using a conventional nasal spray was used as a control condition. In a second study block, the experiment was repeated in new participants with the addition of a mucoadhesive polymer (MucoLox) to the LFE doses of insulin. MucoLox was predicted to increase residence time at the deposition site.
1). Block 1 (No MucoLox)
Four subjects were assigned to Block 1 of the study, receiving insulin via conventional nasal spray and the LFE method. ALFF maps at 15 min. and 60 min. post insulin administration compared to baseline are shown in Fig. 8. Results suggested that delivery of insulin via both spray and the LFE method were associated with a qualitative reduction in brain activity in prefrontal regions after 15 minutes. After 60 minutes, the reduction in prefrontal brain activity was qualitatively even more intense in the LFE condition compared to the spray condition. (see Fig. 8). (Note: due to the small sample size, statistical comparisons were not conducted.)
2). Block 2 (Yes MucoLox)
Four subjects were assigned to Block 2 of the study, receiving insulin via conventional spray and a mixture of insulin and 15% MucoLox via the novel LFE method. ALFF maps at 15 min. and 60 min. post insulin administration are shown in Figure S2. Results demonstrated some minor deactivation in prefrontal cortex across conditions, but this is notably diminished compared to the results from Block 1. (Note: due to the small sample size, statistical comparisons were not conducted.)
V. Discussion
We have presented in-vitro and in-vivo tests of laminar fluid ejection (LFE), a novel method for intranasal drug delivery targeting the central nervous system. In-vitro tests included aperture testing and deposition testing to determine the optimal device parameters for producing an intact flow of compound targeting the olfactory cleft. In-vivo tests included imaging with technetium-99 and methylene blue to confirm deposition in the olfactory cleft. We also used fMRI to test if LFE-administration of insulin was associated with regional changes in brain activity. Table 6 summarizes the key questions, testing methods, and results from each of the experiments we conducted. Altogether, the results demonstrated that the LFE method efficiently deposits compound at the olfactory cleft, offering a promising opportunity for noninvasive delivery of pharmaceuticals directly to the central nervous system.
TABLE 5. Visualization of Delivery to the Olfactory Cleft Using Methylene Blue.
| ID | LFE Method | Nasal Spray | ||
|---|---|---|---|---|
| Attempts Made | Olfactory Residence Time (min.) | Attempts Made | Olfactory Residence Time (min.) | |
| 2.01 | 1 | 15 | 1 | 0 |
| 2.02 | 2 | 8 | 1 | 0 |
| 2.03 | 1 | 5 | 1 | 0 |
| 2.04 | 5 | 0 | 1 | 0 |
| 2.05 | 1 | 1 | 1 | 0 |
Results from in-human tests of the LFE method using the dye methylene blue.
TABLE 6. Summary of Key Questions and Results.
| Experiment | Method | Key Question | Key Findings |
|---|---|---|---|
| Method Development | Aperture Testing | What velocity is required to eject a laminar flow? | Low viscosity fluids (1cP) benefit from a lower velocity (3m/s), while a higher viscosity fluid (50cP) was delivered successfully through the aperture at both low (3m/s) and high (27m/s) velocities. |
| Deposition Testing (3D model of nasal cavities) | What fluid velocity and device angle are required to reach the olfactory cleft? | Olfactory delivery was achieved at all tested angles (30-40°). Optimal velocities (+/-20%) were found to be 1.6m/s for 1cP fluid and 14.0m/s for 50cP fluid. | |
| Human Deposition Testing – Proof of Concept | Technetium-99 Imaging | Can the device deliver a targeted dose to the cribriform area in humans? | Yes, the technetium-99 tracer was successfully delivered to the cribriform area in seven out of eight participants, with a hold time of 1.5-2 minutes and a portion of the dose remaining after conclusion of imaging (5 minutes). |
| Human Deposition Testing – Detailed Imaging | Methylene-Blue Imaging | With more detailed imaging, can we confirm that the olfactory cleft is reached? | Yes, the olfactory cleft was reached in four out of five participants, with a residence time of 1-15 minutes. |
| Brain Activity Response Testing | Functional MRI | Does LFE delivery of a drug (insulin) elicit a change in brain activity? | Yes, in this pilot study LFE administration of insulin was associated with deactivation in medial prefrontal cortex, possibly lasting longer than similar prefrontal deactivation elicited by a traditional spray device. |
A. Interpreting in-Vitro Results
We conducted in-vitro tests to determine the optimal device parameters for producing a laminar flow that could target the olfactory cleft. During these experiments, we were able to pre-set the velocity at which fluid was ejected from the device. Knowing the viscosity of the fluid ahead of time, we could set the ejection velocity to a value such that the Reynolds number of the resulting flow would be within the domain of laminar flow.
Aperture testing demonstrated successful creation of laminar flow at both low and high viscosities. Deposition testing allowed us to determine the optimal angle and velocity for reaching the olfactory cleft in a 3D-printed model of the human nasal anatomy. By demonstrating successful olfactory deposition at low and high viscosities, we propose that the LFE method can be used for a wide range of pharmaceutical formulations, by simply adjusting the desired ejection velocity.
B. Interpreting in-Vivo Results
Across three pilot experiments conducted in humans, we have presented preliminary, qualitative results suggesting that the LFE method can reach the olfactory cleft efficiently. First, imaging with Technetium-99 demonstrated that the device can deliver an intact bolus to the cribriform area. Moreover, the bolus remained intact at the cribriform area for upwards of two minutes before beginning to migrate. Even at the end of five minutes of imaging, large portions of the initial bolus were observed to remain at the cribriform. Notably, results from the technetium-99 study are qualitative. Residence times varied considerably across participants, possibly due to differences in nasal anatomy. Future LFE devices must consider the challenges associated with variations in nasal anatomy. Because we acquired technetium-99 images using a 2D gamma-camera, we cannot quantify the ratio of the dose delivered to the olfactory cleft (a 3D structure). Future studies using PET-imaging may allow us to perform these types of quantifications in humans and animals.
Using methylene blue, we were able to visualize in detail the deposition of fluid in the olfactory cleft using the LFE method. Interestingly, the LFE method yielded such a high concentration of deposition at the olfactory cleft, that some subjects reported a tingling sensation in response to the methylene blue dye. This is a normal response to exposure of high concentrations of methylene blue. According to participant feedback, the sensation was not associated with the insertion or placement of the cannula. Out of an abundance of caution, the physician diluted the methylene blue for the second study visit and limited spray doses to a single attempt. While we cannot precisely quantify the ratio of the dose delivered in the nasal endoscope images, successful deposition was defined as a physician qualitatively judging at least 50% of the dye in the image to be deposited at the olfactory cleft. Qualitatively, focal deposition from the LFE method greatly contrasted the diffuse delivery of sprayed compound throughout the nasal cavities.
Using fMRI, we observed that LFE administration of insulin was associated with sustained deactivation of prefrontal cortex. Importantly, the small sample size employed in this study precluded rigorous statistical analyses, and these fMRI results are qualitative in nature. This effect was “blocked” with the introduction of MucoLox, a mucoadhesive polymer meant to increase the residence time of compounds on mucosal surfaces such as the nasal cavities. While we cannot precisely know where in the nasal passages the dose was delivered in the fMRI study, it's possible that doses with MucoLox failed to reach the olfactory target. Moreover, MucoLox may have reduced diffusion of the insulin into the brain, keeping it adhered to the outer mucus membrane. While we cannot make concrete statistical claims with this small sample size, we find the magnitude of brain activity changes following LFE administration of insulin quite encouraging. (The effect size of changes in brain activity was medium-to-high: Block 1 ALFF following LFE in Left Anterior Cingulate Cortex showed Cohen's d =1.27, Hedge's g =0.591). We note that the prefrontal regions exhibiting decreased brain activity in our study mirror the regions that showed increased uptake of intranasal insulin in a recent study of non-human primates [26]. The olfactory bulb is located inferior of orbitofrontal cortex and innervates medial-prefrontal/limbic regions. This may explain why we observed effects of insulin in these regions. Future studies using other drugs could explain whether the regional specificity of brain activity changes we observed were due to the nature of the drug or the nature of delivery (e.g. using cocaine, which is known to act on basal ganglia circuitry).
C. Mixed Results with Spray Devices
Despite great promise for delivering pharmaceuticals to the brain, drug and device trials employing conventional intranasal spray devices have yielded mixed results. While one review on research in animals and in smaller clinical studies suggested that intranasal administration of insulin may improve memory in cognitively impaired older adults [27], a review of more recent large-scale trials of intranasal insulin in Alzheimer's disease noted mixed results, and emphasized the need for further optimization of intranasal devices [28]. Studies testing intranasal delivery of similarly sized neuropeptides (e.g. vasopressin, estradiol, melatonin), have also shown conflicting results [29], [30], [31], [31], and [32].
One explanation for these shortcomings could be the inconsistent, imprecise, and unreliable dosing to which spray devices are prone [33]. When small droplets are sprayed into the nasal passages, they are faced with an arduous journey to reach the olfactory cleft. During inhalation, the nasal valve is constricted, further limiting airflow to the upper nasal passages [34]. Droplets that do make it through the nasal valve are often caught up in eddies and deposited in the nasal turbinates where they exit the nasal passages via mucociliary clearance, greatly limiting the ability of spray devices to deliver drugs to the brain [8].
A variety of other devices have been developed to improve intranasal drug delivery, including devices that deliver both powders and fluids (for a recent comprehensive review, see [17]). Examples of device mechanisms include bi-directional flow (activated by blowing into a mouthpiece to close the soft palate and isolate the nasal cavity while providing positive pressure) [35], gas propellent [33], a spring mechanism with integrated backflow metered dispenser [36], and droplets dispensed in an elliptical plume [37]. Still, these devices primarily use a high-force discharge with non-targeted plume geometry.
D. Broader Potential Benefits of Targeted Olfactory Drug Delivery
Our laminar fluid ejection method releases a steady, cohesive stream of fluid, rather than atomizing or aerosolizing the compound into a spray. During atomization, fluid is propelled through a small orifice, subjecting it to immense shear forces. Due to their large and complex molecular structure, modern biopharmaceuticals such as protein formulations and monoclonal antibodies are particularly susceptible to damage from the atomization process [21], [38]. Similarly, lipid nanoparticles (LPNs) are of great interest as a delivery vehicle to help larger compounds pass through the blood brain barrier. However, several studies have highlighted that when atomized by spray devices and nebulizers, LPNs face intense shear forces that can cause them to degrade or be destroyed [39], [40], [41]. By avoiding the formation of a spray plume, the LFE method may increase the yield of intact compound that is deposited at the olfactory cleft. By keeping the drug compound intact and depositing it directly to the olfactory cleft, the LFE method reduces the overall necessary dose. This improves the efficiency of delivery and can save valuable resources for compounds that are expensive and time-consuming to generate.
Perhaps most importantly, intranasal devices are limited in the volume of medication that can be administered in a single dose. A review of the use of intranasal devices in emergency departments and out-of-hospital settings found that while intranasal devices may be particularly useful for children, their limited volume makes them inappropriate for use in adults, who typically require larger doses [2]. The review specifically cited ketamine and dexmedetomidine as compounds for which intranasal administration is particularly ineffective in adults. Administering repeated doses or large volumes of medication intranasally can result in medication dripping back out of the nostrils or being swallowed or inhaled. Not only is this uncomfortable for patients, but swallowing medication can reduce bioavailability (e.g. via first-pass metabolism), and inhaling medication can cause side effects ranging from mild (e.g. coughing) to severe (e.g. infection) [3], [42]. In our fMRI study of insulin, we demonstrated that the LFE method can elicit brain activity responses with a 10-fold decrease in volume of fluid when compared to a spray device (0.1mL vs. 1mL). This suggests that the LFE method may be useful for intranasally administering larger doses of medication in adult patient populations where this was not previously feasible.
E. Future Directions
In these first tests of the LFE method, the main goal was to assess whether the device could deliver fluid to the olfactory cleft. A major advantage of intranasal devices is that they can be self-administered at home, offering increased comfort and lower costs to patients. Ongoing work by our group includes human factors studies to design a handheld device that patients can use at home to self-administer the LFE method (see Fig. 4b,c). Desired characteristics of an LFE device include portability, at-home use, and repeatability in device positioning achieved during self-administration. The prototype device that we used for these proof-of-concept studies was limited due to its large size, rigidity, and the requirement of a physician for administration. Updates to the LFE device include a flexible canula tip and comfortable introducer. This will facilitate insertion of the device past the nasal valve, help to account for variations in nasal anatomy, and allow medication to be deposited directly into the upper nasal cavities (see Fig. 4b,c). With careful consideration for the design, size, and flexibility of the device, future work could allow patients to self-administer medications focally to the olfactory cleft using the LFE method.
Ongoing experiments using the LFE method will test a range of pharmaceutical compounds beyond insulin. Additional small-molecule compounds of interest include ketamine, naloxone, diazepam, and ondansetron. These small-molecule compounds have been used previously as intranasal medications, showing mixed-results using spray devices, including inability to deliver an adequate dose to adults [2], [4]. Further testing with larger molecules (e.g. neuropeptides, lipid nanoparticles, biologics, etc.) is of great interest, as targeted deposition at the olfactory cleft may enhance their blood-brain-barrier-permeability [43].
F. Conclusion
We presented a novel method for olfactory drug delivery. While conventional nasal sprays suffer from inconsistent dosing and unreliable deposition, the laminar fluid ejection method deposits a steady continuous flow of medication directly to the olfactory cleft. Focal targeting of the olfactory cleft offers a promising opportunity for direct delivery of pharmaceuticals to the central nervous system.
Supplementary Materials
Acknowledgment
Aspects of the device used to implement the high-precision fluid ejection method are covered by U.S. Patent No. 11,497,862 and pending applications. This work was funded by Rocket Science Health. All authors have received salary and/or consulting fees from the funding source.
References
- [1].Khan A. R., Liu M., Khan M. W., and Zhai G., “Progress in brain targeting drug delivery system by nasal route,” J. Controlled Release, vol. 268, pp. 364–389, Dec. 2017, doi: 10.1016/j.jconrel.2017.09.001. [DOI] [PubMed] [Google Scholar]
- [2].Rech M. A., Barbas B., Chaney W., Greenhalgh E., and Turck C., “When to pick the nose: Out-of-hospital and emergency department intranasal administration of medications,” Ann. Emergency Med., vol. 70, no. 2, pp. 203–211, Aug. 2017, doi: 10.1016/j.annemergmed.2017.02.015. [DOI] [PubMed] [Google Scholar]
- [3].Keller L.-A., Merkel O., and Popp A., “Intranasal drug delivery: Opportunities and toxicologic challenges during drug development,” Drug Del. Transl. Res., vol. 12, no. 4, pp. 735–757, Apr. 2022, doi: 10.1007/s13346-020-00891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gálvez V., et al. , “Repeated intranasal ketamine for treatment-resistant depression–the way to go? Results from a pilot randomised controlled trial,” J. Psychopharmacol., vol. 32, no. 4, pp. 397–407, Apr. 2018, doi: 10.1177/0269881118760660. [DOI] [PubMed] [Google Scholar]
- [5].Lagerstrom P. A., Laminar Flow Theory. Princeton, NJ, USA: Princeton Univ. Press, 1996. Accessed: Mar. 26, 2024. [Online]. Available: https://press.princeton.edu/books/paperback/9780691025988/laminar-flow-theory [Google Scholar]
- [6].Ghori M. U., Mahdi M. H., Smith A. M., and Conway B. R., “Nasal drug delivery systems: An overview,” Am. J. Pharmacol. Sci., vol. 3, no. 5, pp. 110–119, Dec. 2015. [Google Scholar]
- [7].Djupesland P. G., “Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review,” Drug Del. Transl. Res., vol. 3, no. 1, pp. 42–62, Feb. 2013, doi: 10.1007/s13346-012-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marttin E., Schipper N. G. M., Verhoef J. C., and Merkus F. W. H. M., “Nasal mucociliary clearance as a factor in nasal drug delivery,” Adv. Drug Del. Rev., vol. 29, nos. 1–2, pp. 13–38, Jan. 1998, doi: 10.1016/S0169-409X(97)00059-8. [DOI] [PubMed] [Google Scholar]
- [9].Gizurarson S., “The relevance of nasal physiology to the design of drug absorption studies,” Adv. Drug Del. Rev., vol. 11, no. 3, pp. 329–347, Sep. 1993, doi: 10.1016/0169-409X(93)90015-V. [DOI] [Google Scholar]
- [10].Gizurarson S., “Anatomical and histological factors affecting intranasal drug and vaccine delivery,” Current Drug Del., vol. 9, no. 6, pp. 566–582, Oct. 2012, doi: 10.2174/156720112803529828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Damm M., et al. , “Intranasal volume and olfactory function,” Chem. Senses, vol. 27, no. 9, pp. 831–839, Nov. 2002, doi: 10.1093/chemse/27.9.831. [DOI] [PubMed] [Google Scholar]
- [12].López-Elizalde R., Campero A., Sánchez-Delgadillo T., Lemus-Rodríguez Y., López-González M., and Godínez-Rubí M., “Anatomy of the olfactory nerve: A comprehensive review with cadaveric dissection,” Clin. Anatomy, vol. 31, no. 1, pp. 109–117, Jan. 2018, doi: 10.1002/ca.23003. [DOI] [PubMed] [Google Scholar]
- [13].Purves D., et al. , Neuroscience, 6th ed., London, U.K.: Oxford Univ. Press, 2017. Accessed: Jan. 15, 2024. [Online]. Available: https://global.oup.com/ushe/product/neuroscience-9781605353807 [Google Scholar]
- [14].Illum L., “Is nose-to-brain transport of drugs in man a reality?,” J. Pharmacy Pharmacol., vol. 56, no. 1, pp. 3–17, Jan. 2004, doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- [15].Pardeshi C. V. and Belgamwar V. S., “Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: An excellent platform for brain targeting,” Expert Opinion Drug Del., vol. 10, no. 7, pp. 957–972, Jul. 2013, doi: 10.1517/17425247.2013.790887. [DOI] [PubMed] [Google Scholar]
- [16].Djupesland P. G., Mahmoud R. A., and Messina J. C., “Accessing the brain: The nose may know the way,” J. Cerebral Blood Flow Metabolism, vol. 33, no. 5, pp. 793–794, May 2013, doi: 10.1038/jcbfm.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Trevino J. T., Quispe R. C., Khan F., and Novak V., “Non-invasive strategies for nose-to-brain drug delivery,” J. Clin. Trials, vol. 10, no. 7, p. 439, 2020. [PMC free article] [PubMed] [Google Scholar]
- [18].Crowe T. P. and Hsu W. H., “Evaluation of recent intranasal drug delivery systems to the central nervous system,” Pharmaceutics, vol. 14, no. 3, p. 629, Mar. 2022, doi: 10.3390/pharmaceutics14030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Erdő F., Bors L. A., Farkas D., Bajza Á., and Gizurarson S., “Evaluation of intranasal delivery route of drug administration for brain targeting,” Brain Res. Bull., vol. 143, pp. 155–170, Oct. 2018, doi: 10.1016/j.brainresbull.2018.10.009. [DOI] [PubMed] [Google Scholar]
- [20].Gänger S. and Schindowski K., “Tailoring formulations for intranasal nose-to-brain delivery: A review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa,” Pharmaceutics, vol. 10, no. 3, p. 116, Aug. 2018, doi: 10.3390/pharmaceutics10030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Röhm M., et al. , “A comprehensive screening platform for aerosolizable protein formulations for intranasal and pulmonary drug delivery,” Int. J. Pharm., vol. 532, no. 1, pp. 537–546, Oct. 2017, doi: 10.1016/j.ijpharm.2017.09.027. [DOI] [PubMed] [Google Scholar]
- [22].Gao M., Shen X., and Mao S., “Factors influencing drug deposition in the nasal cavity upon delivery via nasal sprays,” J. Pharm. Invest., vol. 50, no. 3, pp. 251–259, May 2020, doi: 10.1007/s40005-020-00482-z. [DOI] [Google Scholar]
- [23].Brüning J., et al. , “Characterization of the airflow within an average geometry of the healthy human nasal cavity,” Sci. Rep., vol. 10, no. 1, p. 3755, Feb. 2020, doi: 10.1038/s41598-020-60755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Warhaft Z., The Engine and the Atmosphere: An Introduction to Engineering. Cambridge, U.K.: Cambridge Univ. Press, 1997. [Google Scholar]
- [25].Kullmann S., et al. , “Dose-dependent effects of intranasal insulin on resting-state brain activity,” J. Clin. Endocrinol. Metab., vol. 103, no. 1, pp. 253–262, Jan. 2018, doi: 10.1210/jc.2017-01976. [DOI] [PubMed] [Google Scholar]
- [26].Smith K., Fan J., Marriner G. A., Gerdes J., Kessler R., and Zinn K. R., “Distribution of insulin in primate brain following nose-to-brain transport,” Alzheimer's Dementia, Transl. Res. Clin. Interv., vol. 10, no. 1, Jan. 2024, Art. no. e12459, doi: 10.1002/trc2.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Freiherr J., et al. , “Intranasal insulin as a treatment for Alzheimer's disease: A review of basic research and clinical evidence,” CNS Drugs, vol. 27, no. 7, pp. 505–514, Jul. 2013, doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hallschmid M., “Intranasal insulin for Alzheimer's disease,” CNS Drugs, vol. 35, no. 1, pp. 21–37, Jan. 2021, doi: 10.1007/s40263-020-00781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van den Berg M. P., Verhoef J. C., Romeijn S. G., and Merkus F. W. H. M., “Uptake of estradiol or progesterone into the CSF following intranasal and intravenous delivery in rats,” Eur. J. Pharm. Biopharm., vol. 58, no. 1, pp. 131–135, Jul. 2004, doi: 10.1016/j.ejpb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- [30].Al-Ghananeem A. M., Traboulsi A. A., Dittert L. W., and Hussain A. A., “Targeted brain delivery of 17β-estradiol via nasally administered water soluble prodrugs,” AAPS PharmSciTech, vol. 3, no. 1, pp. 40–47, Mar. 2002, doi: 10.1208/pt030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van den Berg M. P., Merkus P., Romeijn S. G., Verhoef J. C., and Merkus F. W. H. M., “Hydroxocobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans,” J. Drug Target., vol. 11, no. 6, pp. 325–331, Jul. 2003, doi: 10.1080/10611860310001640075. [DOI] [PubMed] [Google Scholar]
- [32].van den Berg M. P., Merkus P., Romeijn S. G., Verhoef J. C., and Merkus F. W. H. M., “Uptake of melatonin into the cerebrospinal fluid after nasal and intravenous delivery: Studies in rats and comparison with a human study,” Pharm. Res., vol. 21, no. 5, pp. 799–802, May 2004, doi: 10.1023/B:PHAM.0000026431.55383.69. [DOI] [PubMed] [Google Scholar]
- [33].Craft S., “Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial,” Arch. Neurol., vol. 69, no. 1, p. 29, Jan. 2012, doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cole P., “The four components of the nasal valve,” Amer. J. Rhinol., vol. 17, no. 2, pp. 107–110, Mar. 2003, doi: 10.1177/194589240301700208. [DOI] [PubMed] [Google Scholar]
- [35].Djupesland P. G. and Skretting A., “Nasal deposition and clearance in man: Comparison of a bidirectional powder device and a traditional liquid spray pump,” J. Aerosol Med. Pulm. Drug Del., vol. 25, no. 5, pp. 280–289, Oct. 2012, doi: 10.1089/jamp.2011.0924. [DOI] [PubMed] [Google Scholar]
- [36].Omiya K., Nakadate Y., and Schricker T., “Intranasal insulin and cerebrospinal fluid glucose levels,” J. Anesthesia, vol. 37, no. 5, pp. 818–819, Oct. 2023, doi: 10.1007/s00540-023-03233-0. [DOI] [PubMed] [Google Scholar]
- [37].Wingrove J., et al. , “Characterisation of nasal devices for delivery of insulin to the brain and evaluation in humans using functional magnetic resonance imaging,” J. Controlled Release, vol. 302, pp. 140–147, May 2019, doi: 10.1016/j.jconrel.2019.03.032. [DOI] [PubMed] [Google Scholar]
- [38].Respaud R., et al. , “Effect of formulation on the stability and aerosol performance of a nebulized antibody,” mAbs, vol. 6, no. 5, pp. 1347–1355, Sep. 2014, doi: 10.4161/mabs.29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Miao H., et al. , “Optimization of formulation and atomization of lipid nanoparticles for the inhalation of mRNA,” Int. J. Pharm., vol. 640, Jun. 2023, Art. no. 123050, doi: 10.1016/j.ijpharm.2023.123050. [DOI] [PubMed] [Google Scholar]
- [40].Kim J., et al. , “Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation,” ACS Nano, vol. 16, no. 9, pp. 14792–14806, Sep. 2022, doi: 10.1021/acsnano.2c05647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cortez-Jugo C., Qi A., Rajapaksa A., Friend J. R., and Yeo L. Y., “Pulmonary monoclonal antibody delivery via a portable microfluidic nebulization platform,” Biomicrofluidics, vol. 9, no. 5, Apr. 2015, Art. no. 052603, doi: 10.1063/1.4917181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chung S., Peters J. M., Detyniecki K., Tatum W., Rabinowicz A. L., and Carrazana E., “The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters,” Epilepsy Behav. Rep., vol. 21, Jan. 2023, Art. no. 100581, doi: 10.1016/j.ebr.2022.100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Djupesland P. G., Messina J. C., and Mahmoud R. A., “The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview,” Therapeutic Del., vol. 5, no. 6, pp. 709–733, Jun. 2014, doi: 10.4155/tde.14.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








