Abstract
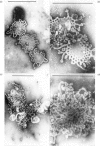
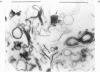
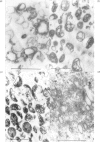
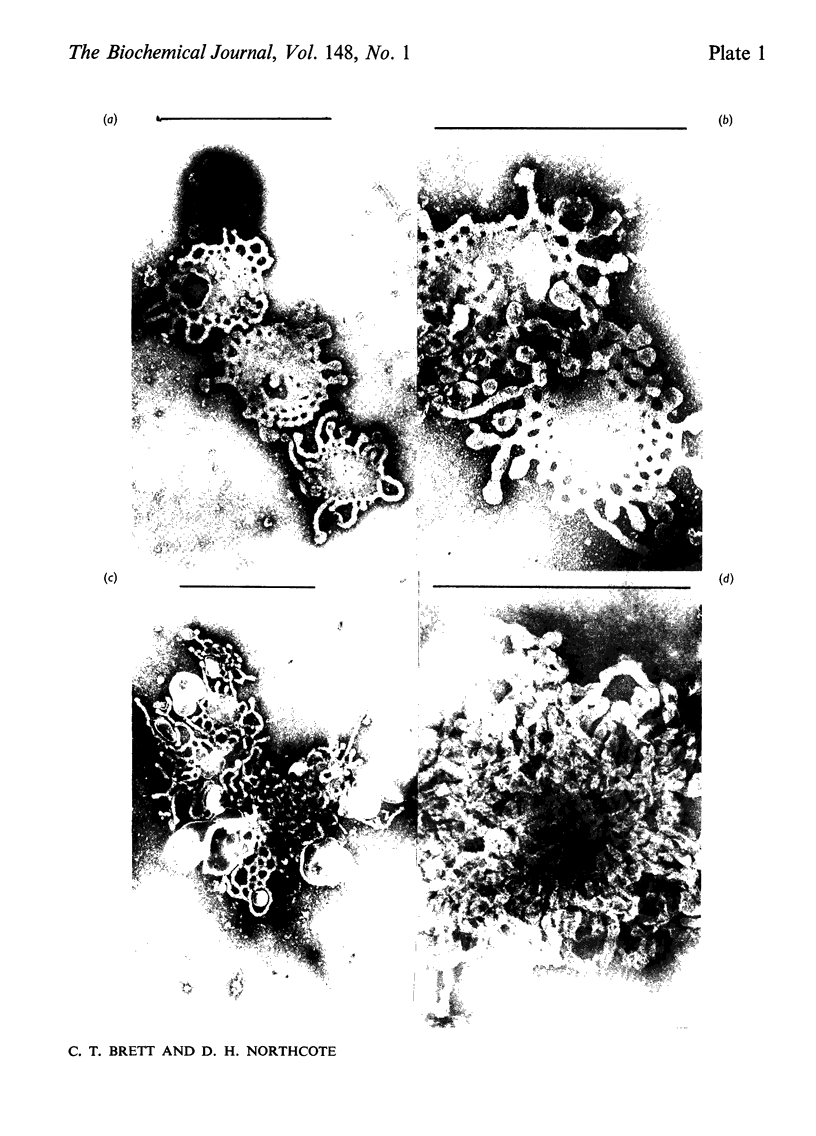
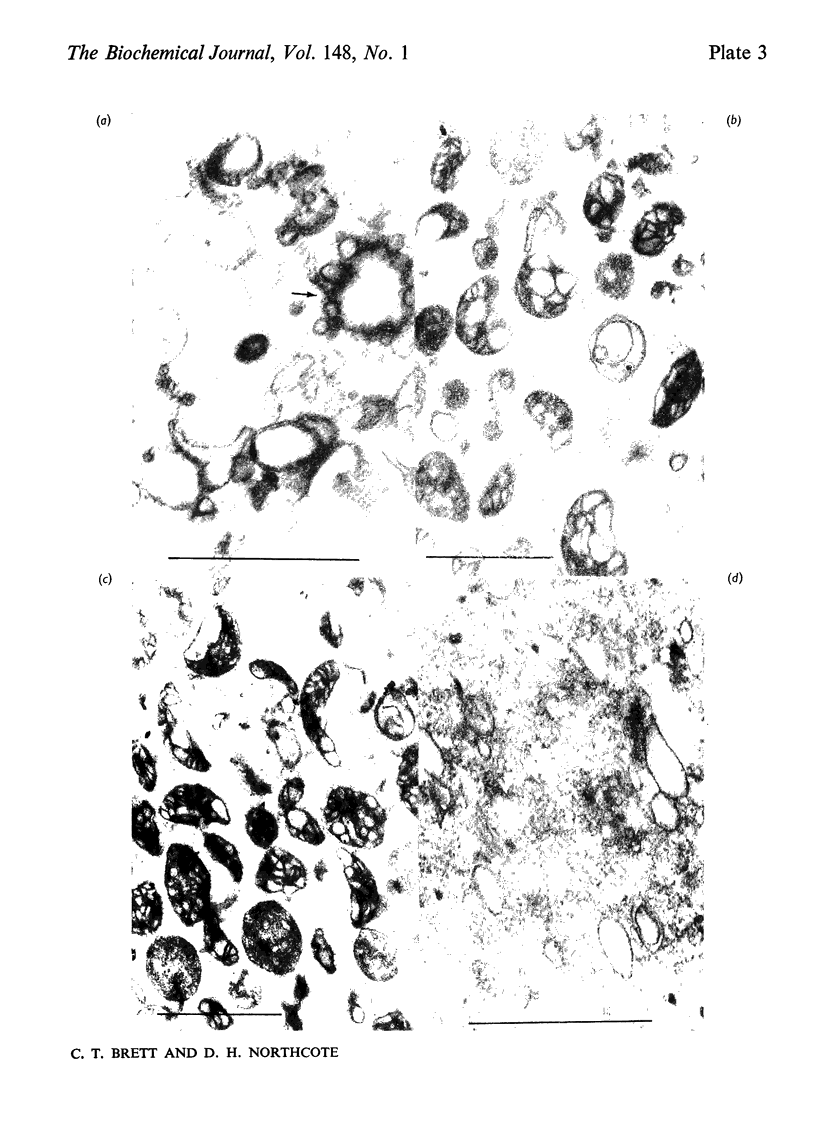
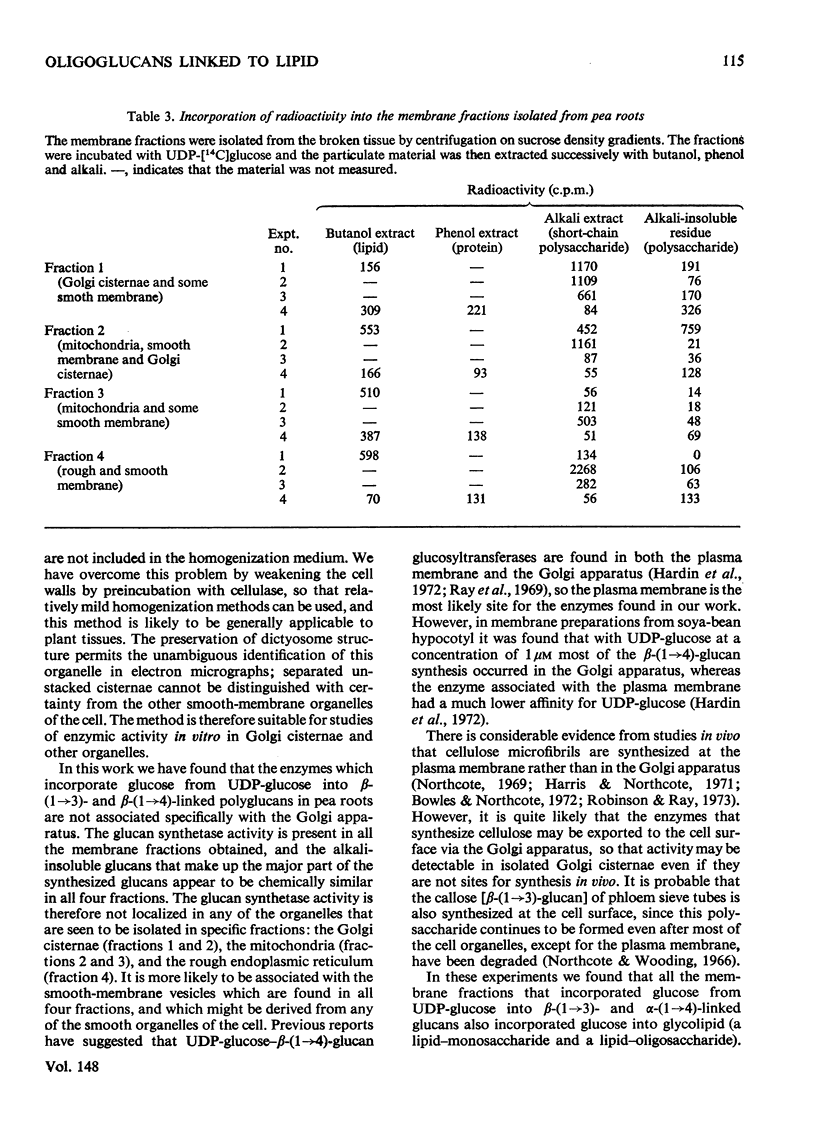
Membrane fractions were obtained from peas roots by using a method that permitted the isolation of a fraction rich in relatively intact dictyosome stacks. No chemical fixatives were used. The method involved incubation of the roots with cellulase, followed by gentle homogenization and sucrose-density-gradient fractionation of the homogenate. The fractions were characterized by electron microscopy. All fractions were enzymically active in incorporating glucose from UDP-glucose into water-insoluble glycolipids containing both single glucose residues and glucose oligosaccharides. Some or all of the linkages of glucose to lipid were through phosphate esters. A substance containing glucose oligosaccharides attached to or very strongly adsorbed on to protein was also formed. The membrane fractions also incorporated glucose from UDP-glucose into alkali-soluble and alkali-insoluble beta-glucans, which like the oligosaccharides contained beta(1leads to 3) and beta-(1leads to4) linkages. The distribution of the enzymic activities and the chemical properties of the lipid-linked and protein-linked oligosaccharides suggest that they may be intermediates in beta-glucan synthesis. The synthetic activity is associated with smooth-membrane vesicles which may be derived from the plasma membrane.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER H. A., ELBEIN A. D., HASSID W. Z. THE SYNTHESIS OF CELLULOSE BY ENZYME SYSTEMS FROM HIGHER PLANTS. J Biol Chem. 1964 Dec;239:4056–4061. [PubMed] [Google Scholar]
- Babczinski P., Tanner W. Involvement of dolicholmonophosphate in the formation of specific mannosyl-linkages in yeast glycoproteins. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1119–1124. doi: 10.1016/0006-291x(73)90808-5. [DOI] [PubMed] [Google Scholar]
- Behrens N. H., Carminatti H., Staneloni R. J., Leloir L. F., Cantarella A. I. Formation of lipid-bound oligosaccharides containing mannose. Their role in glycoprotein synthesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3390–3394. doi: 10.1073/pnas.70.12.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Northcote D. H. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochem J. 1972 Dec;130(4):1133–1145. doi: 10.1042/bj1301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLVIN J. R. Synthesis of cellulose from ethanol-soluble precursors in green plants. Can J Biochem Physiol. 1961 Dec;39:1921–1926. doi: 10.1139/o61-212. [DOI] [PubMed] [Google Scholar]
- COLVIN J. R. Synthesis of cellulose in ethanol extracts of Acetobacter xylinum. Nature. 1959 Apr 18;183(4668):1135–1136. doi: 10.1038/1831135a0. [DOI] [PubMed] [Google Scholar]
- Clark A. F., Villemez C. L. An artificial mannosyl acceptor for GDP-D-mannose: lipid phosphate transmannosylase from Phaseolus aureus. FEBS Lett. 1973 May 15;32(1):84–86. doi: 10.1016/0014-5793(73)80743-4. [DOI] [PubMed] [Google Scholar]
- Clark A. F., Villemez C. L. The Formation of beta, 1 --> 4 Glucan from UDP-alpha-d-Glucose Catalyzed by a Phaseolus aureus Enzyme. Plant Physiol. 1972 Sep;50(3):371–374. doi: 10.1104/pp.50.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Beasley C. A., Ordin L. Utilization of nucleoside diphosphate glucoses in developing cotton fibers. Plant Physiol. 1974 Feb;53(2):149–153. doi: 10.1104/pp.53.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Biosynthesis of a cell wall glucomannan in mung bean seedlings. J Biol Chem. 1969 Mar 25;244(6):1608–1616. [PubMed] [Google Scholar]
- FEINGOLD D. S., NEUFELD E. F., HASSID W. Z. Synthesis of a beta-1, 3-linked glucan by extracts of Phaseolus aureus seedlings. J Biol Chem. 1958 Oct;233(4):783–788. [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of acidic glycolipids in cotton fibers. Possible intermediates in cell wall synthesis. Biochem Biophys Res Commun. 1972 Nov 15;49(4):930–939. doi: 10.1016/0006-291x(72)90301-4. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of mannosyl- and glucosyl-phosphoryl-polyprenols in cotton fibers. J Biol Chem. 1973 Apr 25;248(8):2858–2867. [PubMed] [Google Scholar]
- García R. C., Recondo E., Dankert M. Polysaccharide biosynthesis in Acetobacter xylinum. Enzymatic synthesis of lipid diphosphate and monophospate sugars. Eur J Biochem. 1974 Mar 15;43(1):93–105. doi: 10.1111/j.1432-1033.1974.tb03389.x. [DOI] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H., Morré D. J., Lembi C. A. Enhancement of RNA polymerase activity by a factor released by auxin from plasma membrane. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3146–3150. doi: 10.1073/pnas.69.11.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Polysaccharide formation in plant Golgi bodies. Biochim Biophys Acta. 1971 Apr 20;237(1):56–64. doi: 10.1016/0304-4165(71)90029-8. [DOI] [PubMed] [Google Scholar]
- Kauss H. A plant mannosyl-lipid acting in reversible transfer of mannose. FEBS Lett. 1969 Sep;5(1):81–84. doi: 10.1016/0014-5793(69)80298-x. [DOI] [PubMed] [Google Scholar]
- Kemp J., Loughman B. C. Cyclitol glucosides and their role in the synthesis of a glucan from uridine diphosphate glucose in Phaseolus aureus. Characterization of some cyclitol glucosides and their synthesis. Biochem J. 1974 Jul;142(1):153–159. doi: 10.1042/bj1420153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine R. A., Elbein A. D. Steryl glucosides in Phaseolus aureus. Use of gas-liquid chromatography and mass spectrometry for structural identification. Biochemistry. 1971 Jun 22;10(13):2547–2553. doi: 10.1021/bi00789a020. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Scher M. G. Metabolism and function of polyisoprenol sugar intermediates in membrane-associated reactions. Biochim Biophys Acta. 1972 Aug 4;265(3):417–441. doi: 10.1016/0304-4157(72)90015-9. [DOI] [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H., CHAMBERS J. E. GLUTARALDEHYDE STABILIZATION AS AN AID TO GOLGI APPARATUS ISOLATION. Exp Cell Res. 1965 Jun;38:672–675. doi: 10.1016/0014-4827(65)90392-7. [DOI] [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H. ISOLATION OF THE GOLGI APPARATUS FROM PLANT CELLS. J Cell Biol. 1964 Nov;23:295–305. doi: 10.1083/jcb.23.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote D. H. The synthesis and metabolic control of polysaccharides and lignin during the differentiation of plant cells. Essays Biochem. 1969;5:89–137. [PubMed] [Google Scholar]
- OLAITAN S. A., NORTHCOTE D. H. Polysaccharides of Chlorella pyrenoidosa. Biochem J. 1962 Mar;82:509–519. doi: 10.1042/bj0820509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Staneloni R., Cantarella A. I., Leloir L. F., Behrens N. H., Carminatti H., Levy J. A. Further studies on a glycolipid formed from dolichyl-D-glucosyl monophosphate. Carbohydr Res. 1973 Feb;26(2):393–400. doi: 10.1016/s0008-6215(00)84527-9. [DOI] [PubMed] [Google Scholar]
- Péaud-Lenoël C., Axelos M. Structural features of the beta-glucans enzymatically synthesized from uridine diphosphate glucose by wheat seedlings. FEBS Lett. 1970 Jun 8;8(4):224–228. doi: 10.1016/0014-5793(70)80270-8. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. I. Characterization of mannan-14C formed enzymatically from mannosyl-1-phosphoryl-undecaprenol. J Biol Chem. 1969 May 25;244(10):2777–2789. [PubMed] [Google Scholar]
- Storm D. L., Hassid W. Z. The Role of a d-Mannosyl-Lipid as an Intermediate in the Synthesis of Polysaccharide in Phaseolus aureus Seedlings. Plant Physiol. 1972 Oct;50(4):473–476. doi: 10.1104/pp.50.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Hassid W. Z. Solubilization and Separation of Uridine Diphospho-d-glucose: beta-(1 --> 4) Glucan and Uridine Diphospho-d-glucose:beta-(1 --> 3) Glucan Glucosyltransferases from Coleoptiles of Avena sativa. Plant Physiol. 1971 Jun;47(6):740–744. doi: 10.1104/pp.47.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemez C. L. Characterization of intermediates in plant cell wall biosynthesis. Biochem Biophys Res Commun. 1970 Aug 11;40(3):636–641. doi: 10.1016/0006-291x(70)90951-4. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lucas J. J., Lennarz W. J. Evidence for xylosyl lipids as intermediates in xylosyl transfers in hen oviduct membranes. Biochem Biophys Res Commun. 1974 Jan 23;56(2):343–350. doi: 10.1016/0006-291x(74)90848-1. [DOI] [PubMed] [Google Scholar]