Abstract
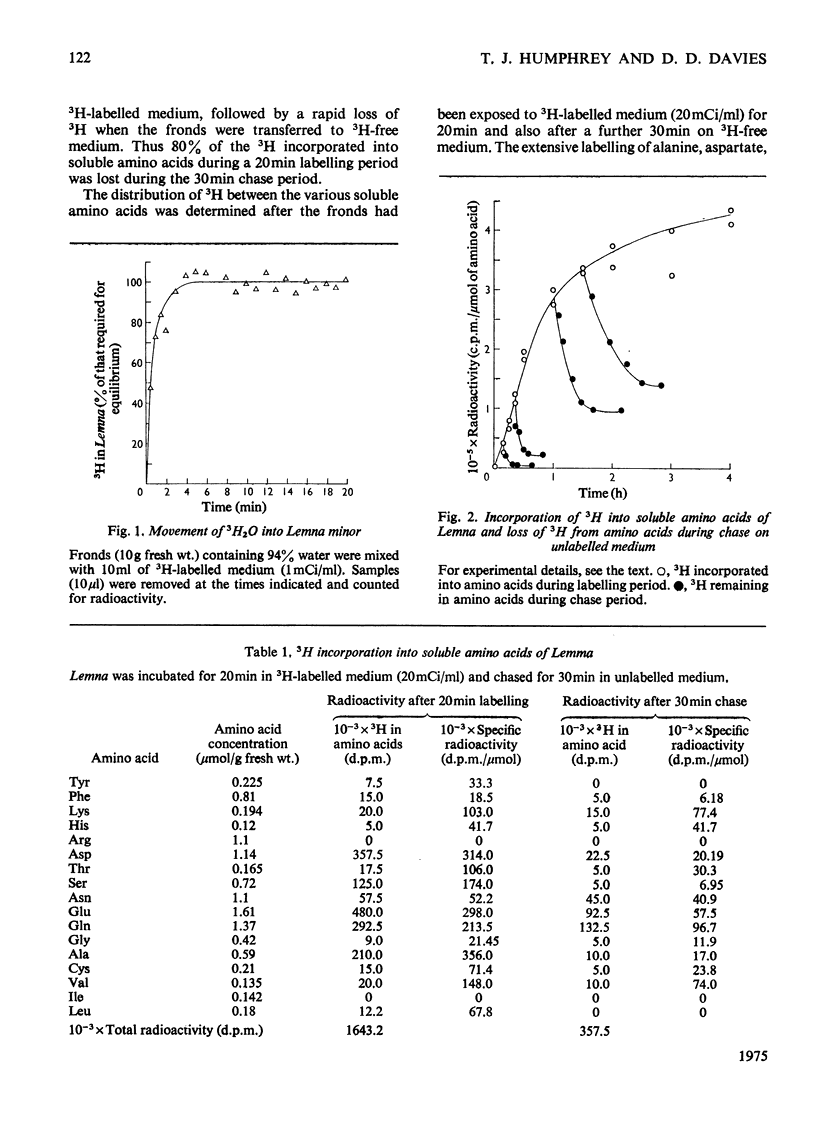
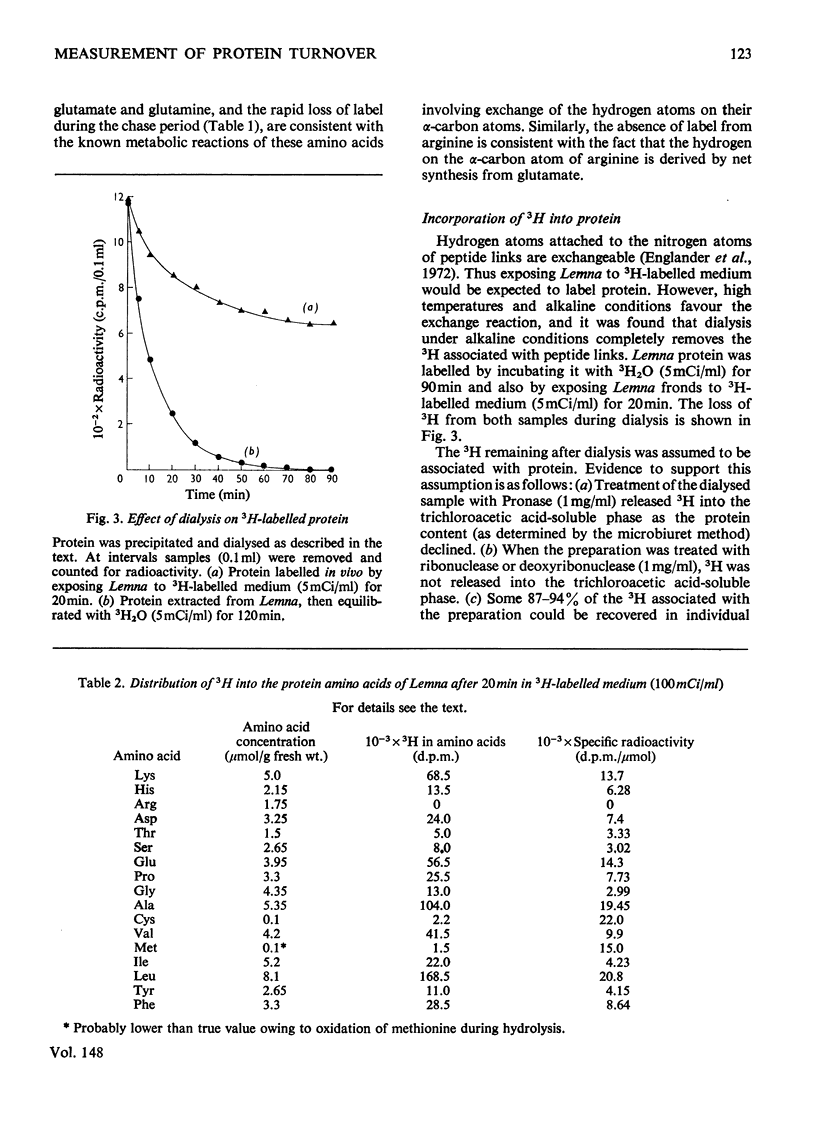
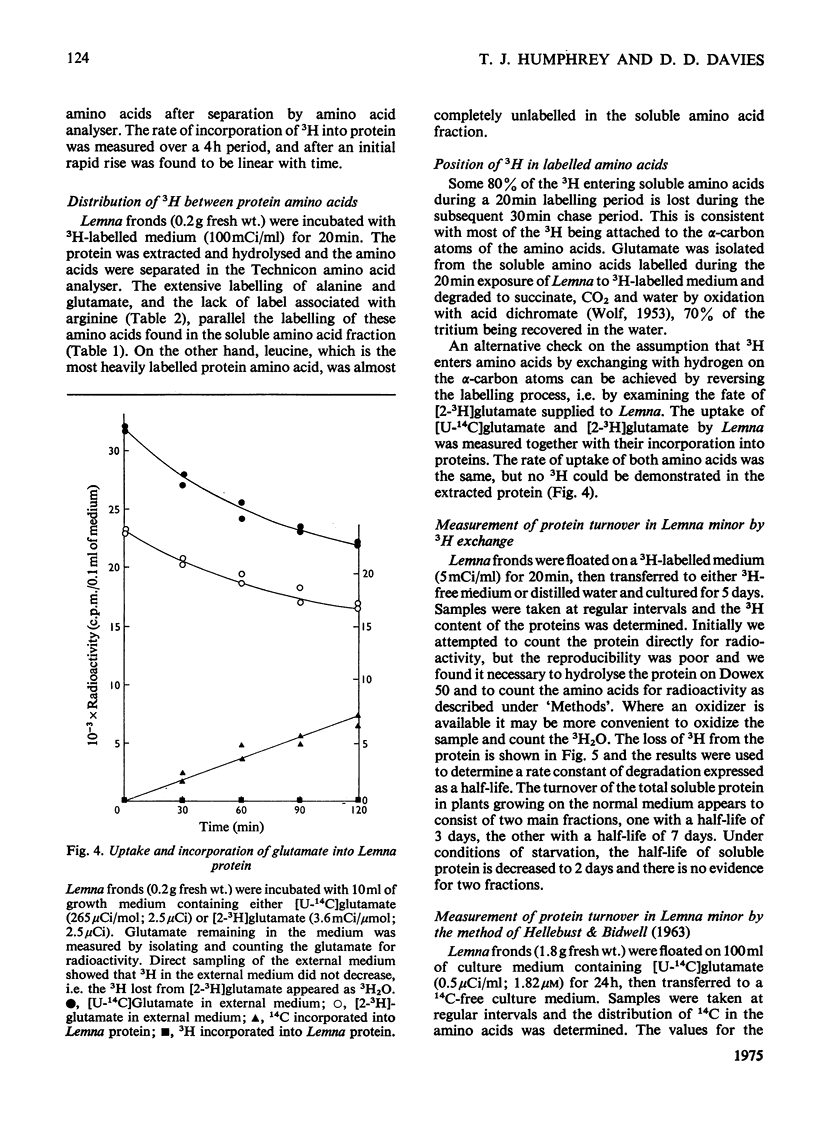
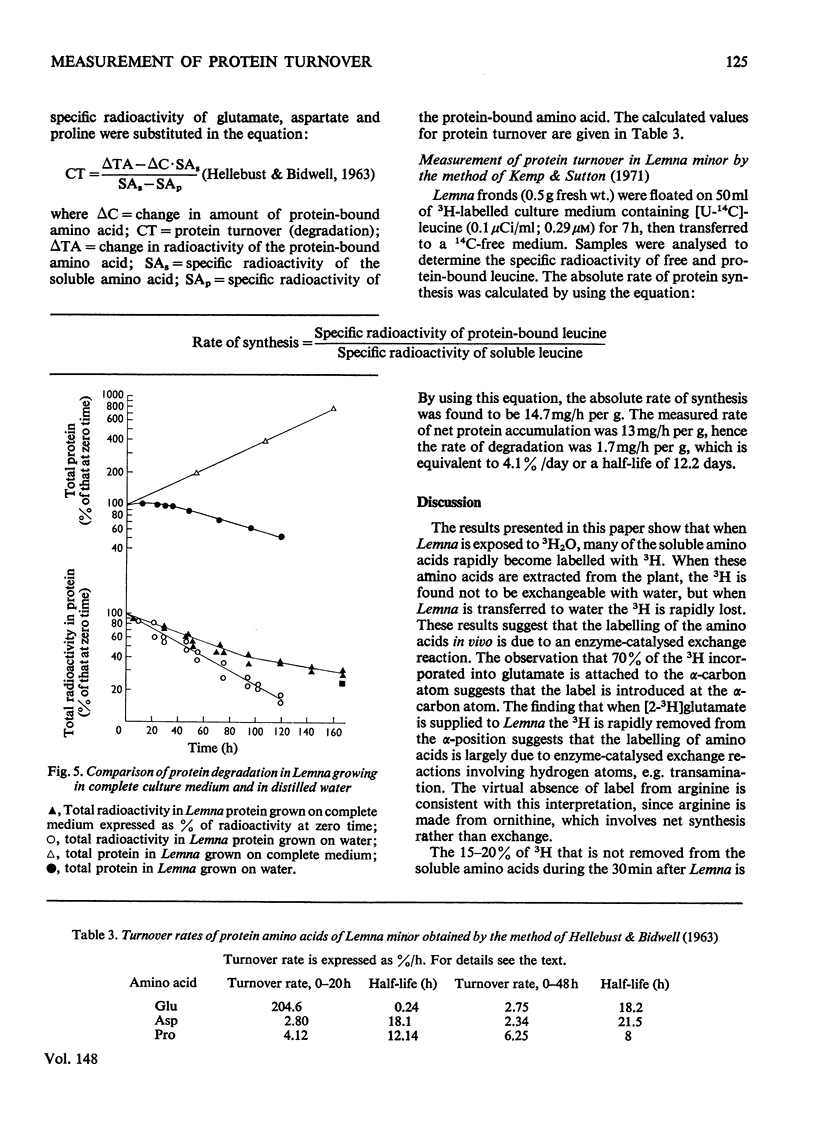
A new technique for the determination of rate constants of protein degradation is described. By using the method, half-lives of total soluble protein of Lemna minor during growth on full culture medium and distilled water were measured. The method involves incubating Lemna on a growth medium containing 3H2O. After a short exposure (20 min) to 3H-labelled culture medium, 3H was found in soluble amino acids, especially aspartate, glutamate, glutamine and alanine. After transfer to a 3H-free medium for 30 min, 80% of the 3H originally present in soluble amino acids was lost. These results suggest that 3H enters and leaves amino acids at the alpha-carbon atom, a conclusion supported by the observed labelling of glutamates. The exchange of H and 3H on the alpha-carbon atom is catalysed by transaminases and the speed of this exchange ensures that when the 3H2O is removed, the 3H in free amino acids is rapidly lost, thereby eliminating problems connected with metabolic pools and recycling. After an exposure of 20 min to 3H-labelled medium all protein amino acids, except for arginine, were found to be radioactive. The loss of radioactivity from protein amino acids was used to measure protein degradation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- BIDWELL R. G., STEWARD F. C., YEMM E. W. Protein metabolism, respiration and growth; a synthesis of results from the use of 14C-labelled substrates and tissue cultures. Nature. 1956 Oct 6;178(4536):734–contd. doi: 10.1038/178734a0. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Englander S. W., Downer N. W., Teitelbaum H. Hydrogen exchange. Annu Rev Biochem. 1972;41:903–924. doi: 10.1146/annurev.bi.41.070172.004351. [DOI] [PubMed] [Google Scholar]
- HILTON M. A., BARNES F. W., Jr, HENRY S. S., ENNS T. Mechanisms in enzymatic transamination; rate of exchange of the hydrogen of aspartate. J Biol Chem. 1954 Aug;209(2):743–754. [PubMed] [Google Scholar]
- Joy K. W. Nitrogen metabolism of Lemna minor. I. Growth, nitrogen sources and amino acid inhibition. Plant Physiol. 1969 Jun;44(6):845–848. doi: 10.1104/pp.44.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D., Sutton D. W. Protein metabolism in cultured plant tissues. Calculation of an absolute rate of protein synthesis, accumulation, and degradation in tobacco callus in vivo. Biochemistry. 1971 Jan 5;10(1):81–88. doi: 10.1021/bi00777a013. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A. The effect of leucine on the biosynthesis of leucine in maize root tips. Plant Physiol. 1965 Jan;40(1):149–155. doi: 10.1104/pp.40.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Trewavas A. Determination of the Rates of Protein Synthesis and Degradation in Lemna minor. Plant Physiol. 1972 Jan;49(1):40–46. doi: 10.1104/pp.49.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. The Turnover of Nucleic Acids in Lemna minor. Plant Physiol. 1970 Jun;45(6):742–751. doi: 10.1104/pp.45.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF G. The metabolism of alpha-C14-histidine in the intact rat. I. Radioactivity in amino acids from protein. J Biol Chem. 1953 Feb;200(2):637–645. [PubMed] [Google Scholar]


