Abstract
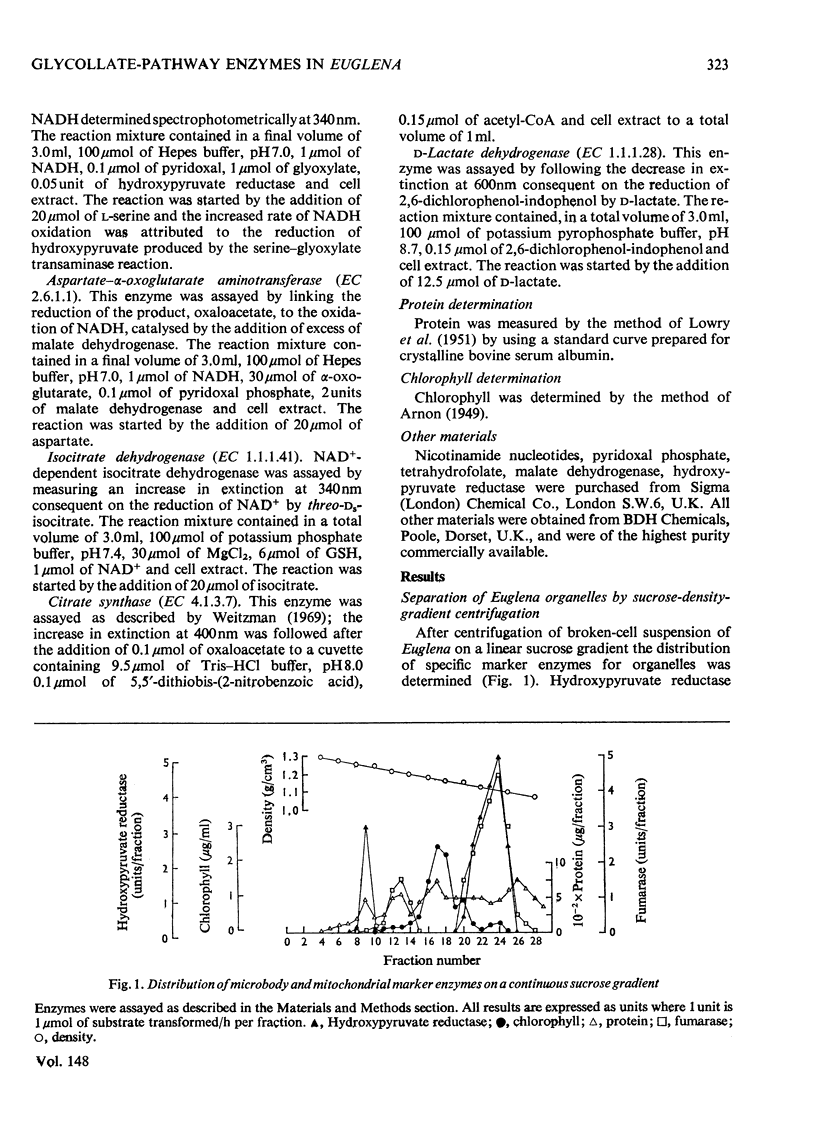
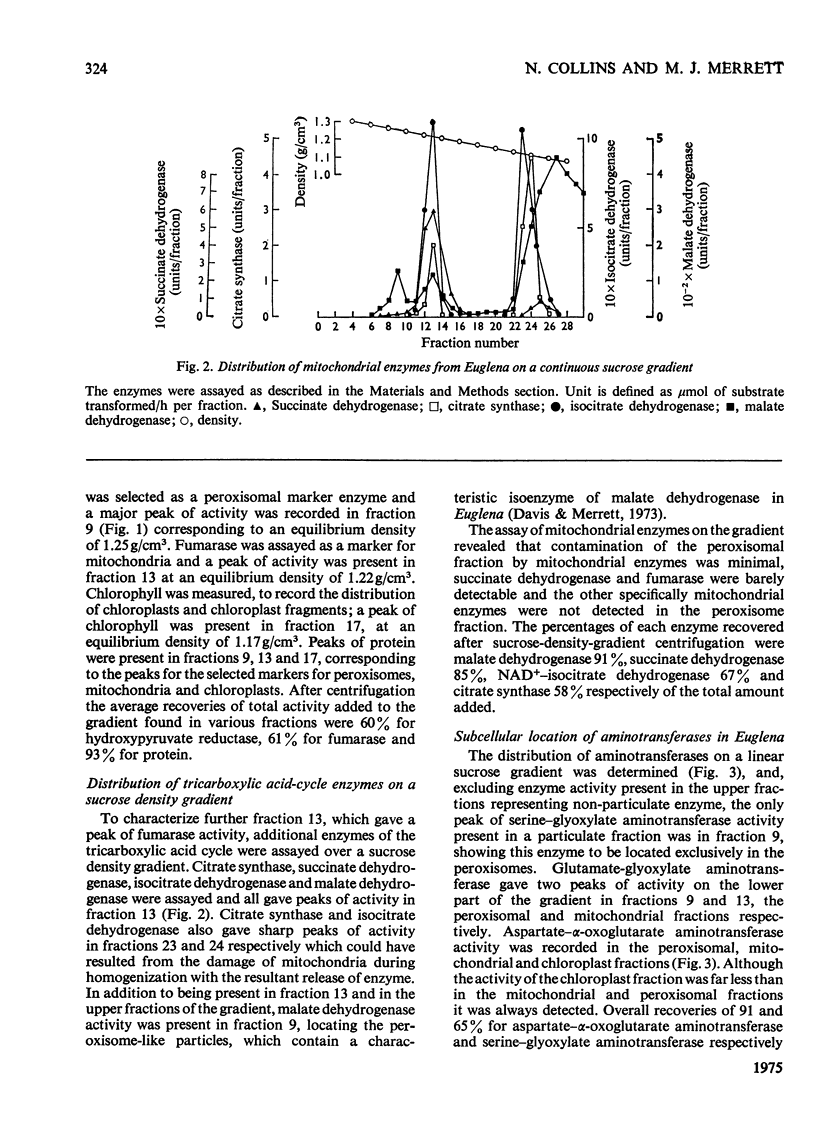
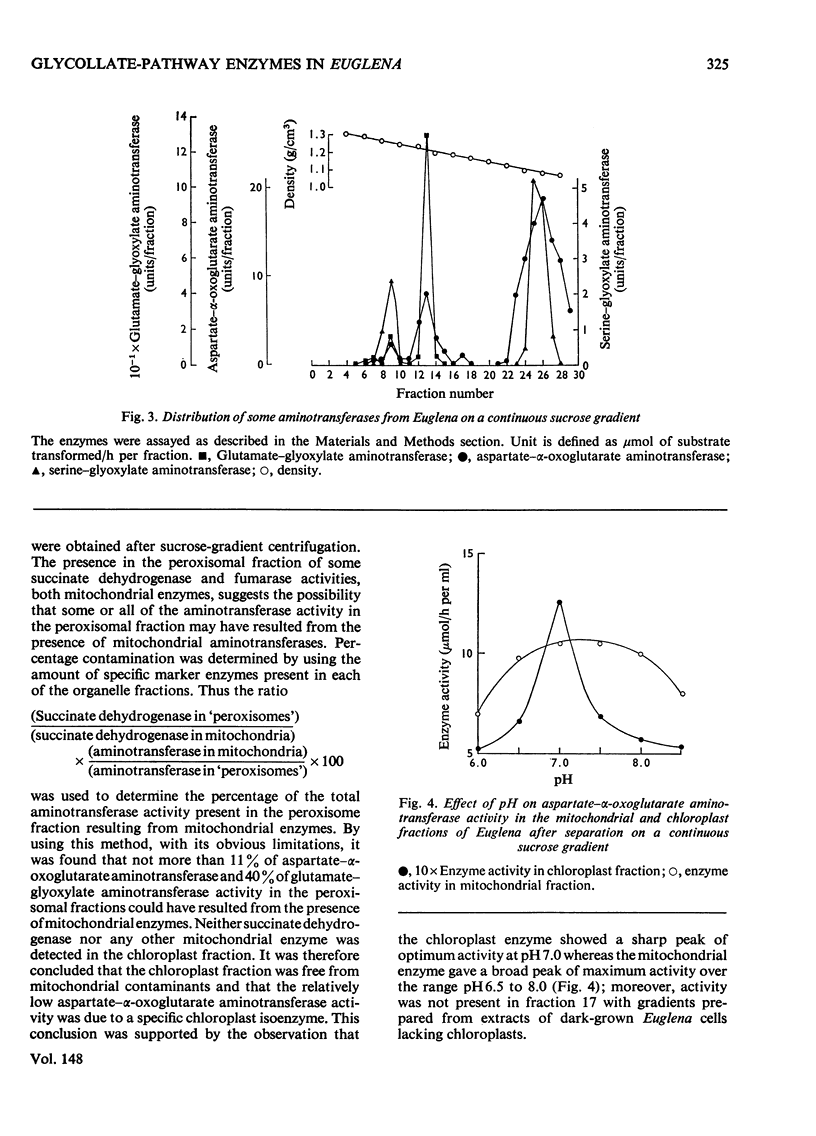
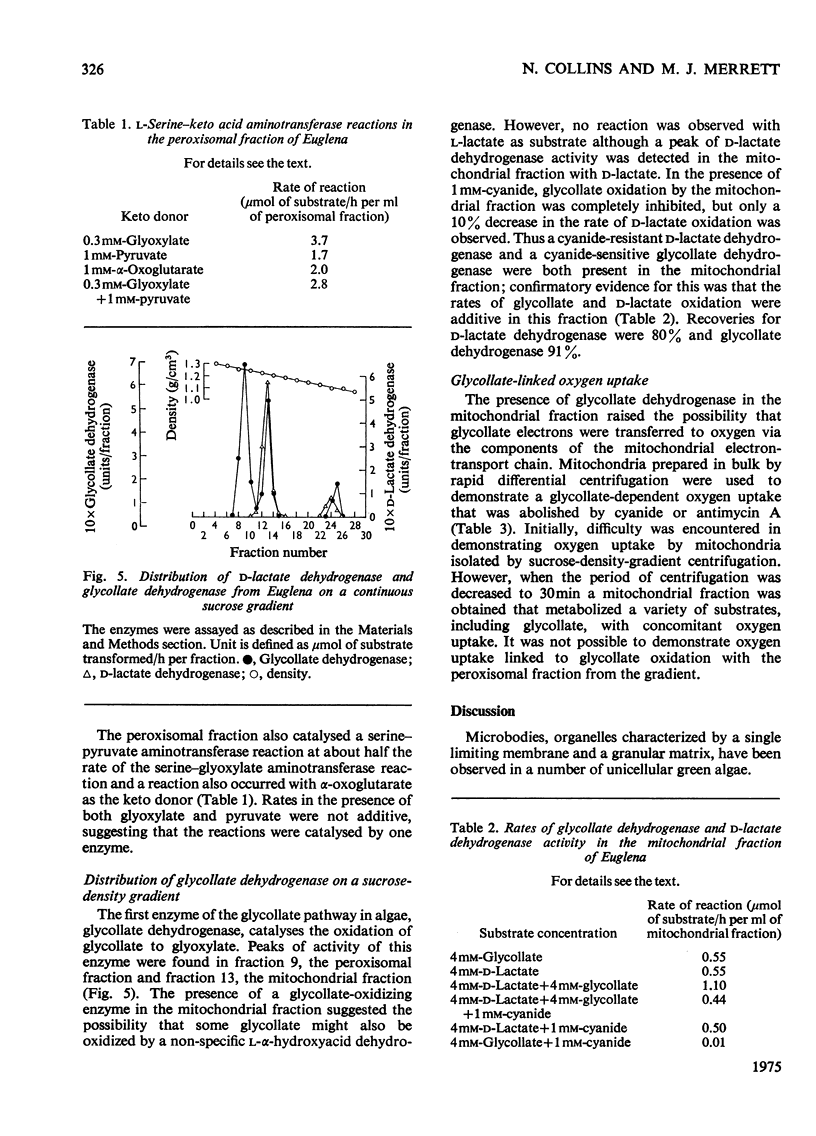
Isolation of organelles from broken-cell suspensions of phototrophically grown Euglena gracilis Klebs was achieved by isopycnic centrifugation on sucrose gradients. 2. Equilibrium densities of 1.23g/cm3 for peroxisome-like particles, 1.22g/cm3 for mitochondria and 1.17g/cm3 for chloroplasts were recorded. 3. The enzymes glycollate dehydrogenase, glutamate-glyoxylate aminotransferase, serineglyoxylate aminotransferase, aspartate-alpha-oxoglutarate aminotransferase, hydroxy pyruvate reductase and malate dehydrogenase were present in peroxisome-like particles. 4. Unlike higher plants glycollate dehydrogenase and glutamate-glyoxylate aminotransferase were present in the mitochondria of Euglena. 5. Rates of glycollate and D-lactate oxidation were additive in the mitochondria, and, although glycollate dehydrogenase was inhibited by cyanide, D-lactate dehydrogenase activity was unaffected. 6. Glycollate oxidation was linked to O2 uptake in mitochondria but not in peroxisome-like particles. This glycollate-dependent O2 uptake was inhibited by antimycin A or cyanide. 7. The physiological significance of glycollate metabolism in Euglena mitochondria is discussed, with special reference to its role in photorespiration in algae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock B. L., Wilkinson D. A., King J. Glyoxylate aminotansferases from oat leaves. Can J Biochem. 1970 Apr;48(4):486–492. doi: 10.1139/o70-078. [DOI] [PubMed] [Google Scholar]
- Brody M., White J. E. Environmental factors controlling enzymatic activity in microbodies and mitochondria of Euglena gracilis. FEBS Lett. 1972 Jun 15;23(2):149–152. doi: 10.1016/0014-5793(72)80327-2. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Lord J. M., Merrett M. J. Fractionation of the proteins of plant microbodies. Biochem J. 1974 Dec;144(3):559–566. doi: 10.1042/bj1440559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin W. J., Nelson E. B., Tolbert N. E. Glycolate pathway in green algae. Plant Physiol. 1970 Sep;46(3):386–391. doi: 10.1104/pp.46.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégin-Heick N. The localization of enzymes of intermediary metabolism in Astasia and Euglena. Biochem J. 1973 Jun;134(2):607–616. doi: 10.1042/bj1340607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Lord J. M., Merrett M. J. The glycollate oxidising enzyme of algae. FEBS Lett. 1969 Dec 30;5(5):341–342. doi: 10.1016/0014-5793(69)80352-2. [DOI] [PubMed] [Google Scholar]
- Davis B., Merrett M. J. Malate dehydrogenase isoenzymes in division synchronized cultures of euglena. Plant Physiol. 1973 Jun;51(6):1127–1132. doi: 10.1104/pp.51.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. E., Newcomb E. H. Microbody-like organelles in leaf cells. Science. 1969 Mar 21;163(3873):1353–1355. doi: 10.1126/science.163.3873.1353. [DOI] [PubMed] [Google Scholar]
- Graves L. B., Jr, Hanzely L., Trelease R. N. The occurrence and fine structural characterization of microbodies in Euglena gracilis. Protoplasma. 1971;72(2):141–152. doi: 10.1007/BF01279047. [DOI] [PubMed] [Google Scholar]
- Graves L. B., Jr, Trelease R. N., Grill A., Becker W. M. Localization of glyoxylate cycle enzymes in glyoxysomes in Euglena. J Protozool. 1972 Aug;19(3):527–532. doi: 10.1111/j.1550-7408.1972.tb03521.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. The intracellular localization of glycollate oxidoreductase in Euglena gracilis. Biochem J. 1971 Sep;124(2):275–281. doi: 10.1042/bj1240275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. The pathway of glycollate utilization in Chlorella pyrenoidosa. Biochem J. 1970 May;117(5):929–937. doi: 10.1042/bj1170929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- Pulich W. M., Ward C. H. Physiology and Ultrastructure of an Oxygen-resistant Chlorella Mutant under Heterotrophic Conditions. Plant Physiol. 1973 Feb;51(2):337–344. doi: 10.1104/pp.51.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Malate dehydrogenase in leaf peroxisomes. Biochim Biophys Acta. 1969 Mar 18;178(1):11–20. doi: 10.1016/0005-2744(69)90127-2. [DOI] [PubMed] [Google Scholar]
- de Duve C. The peroxisome: a new cytoplasmic organelle. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):71–83. [PubMed] [Google Scholar]


