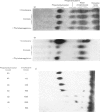
Abstract
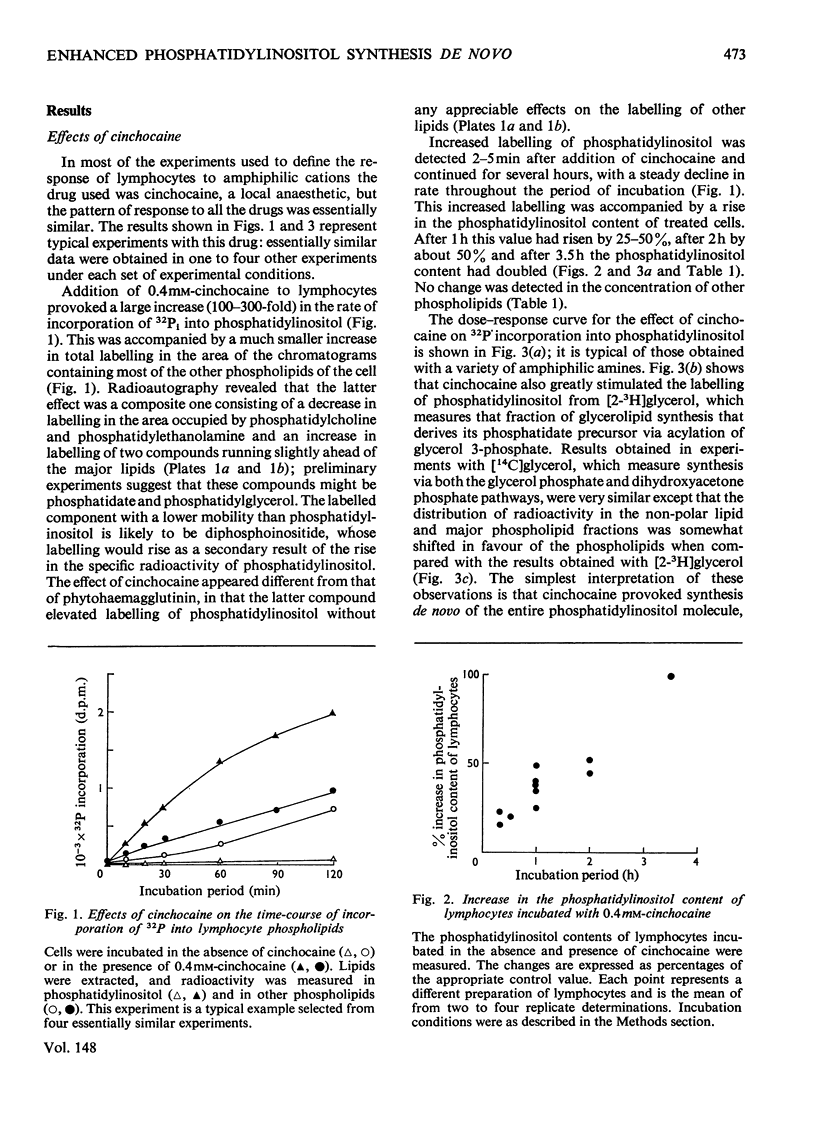
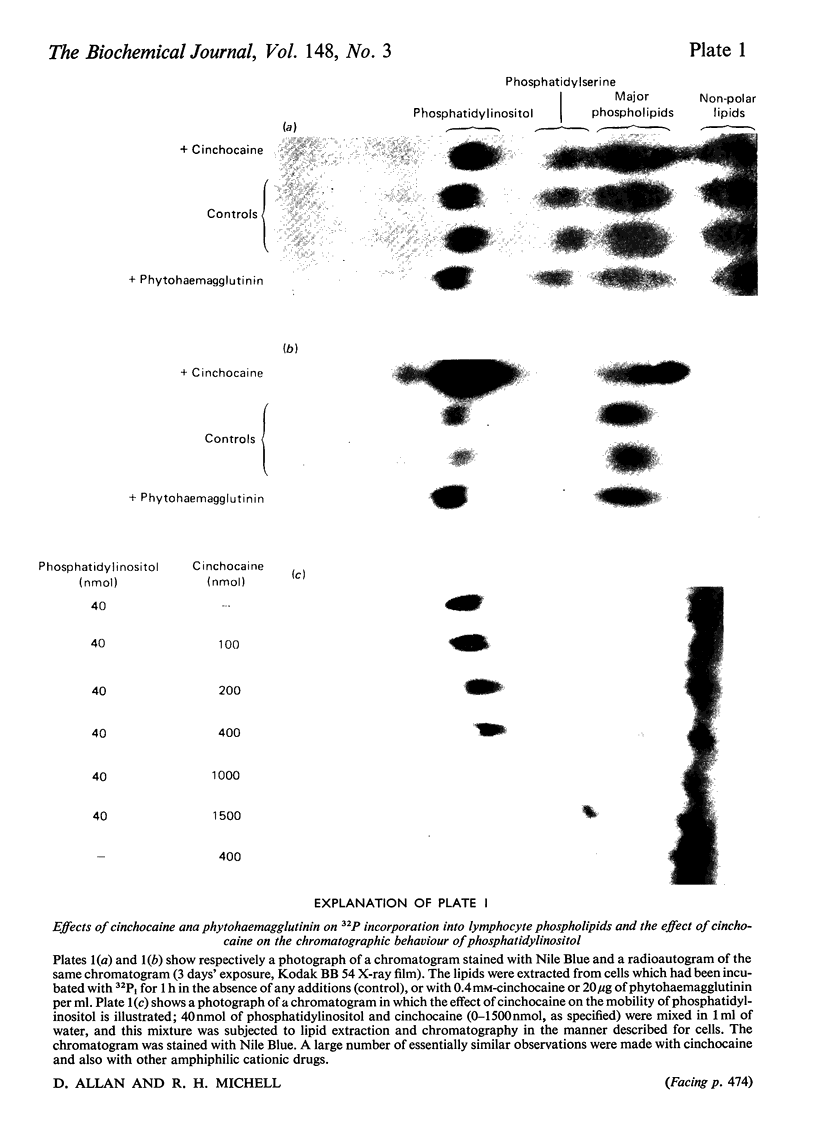
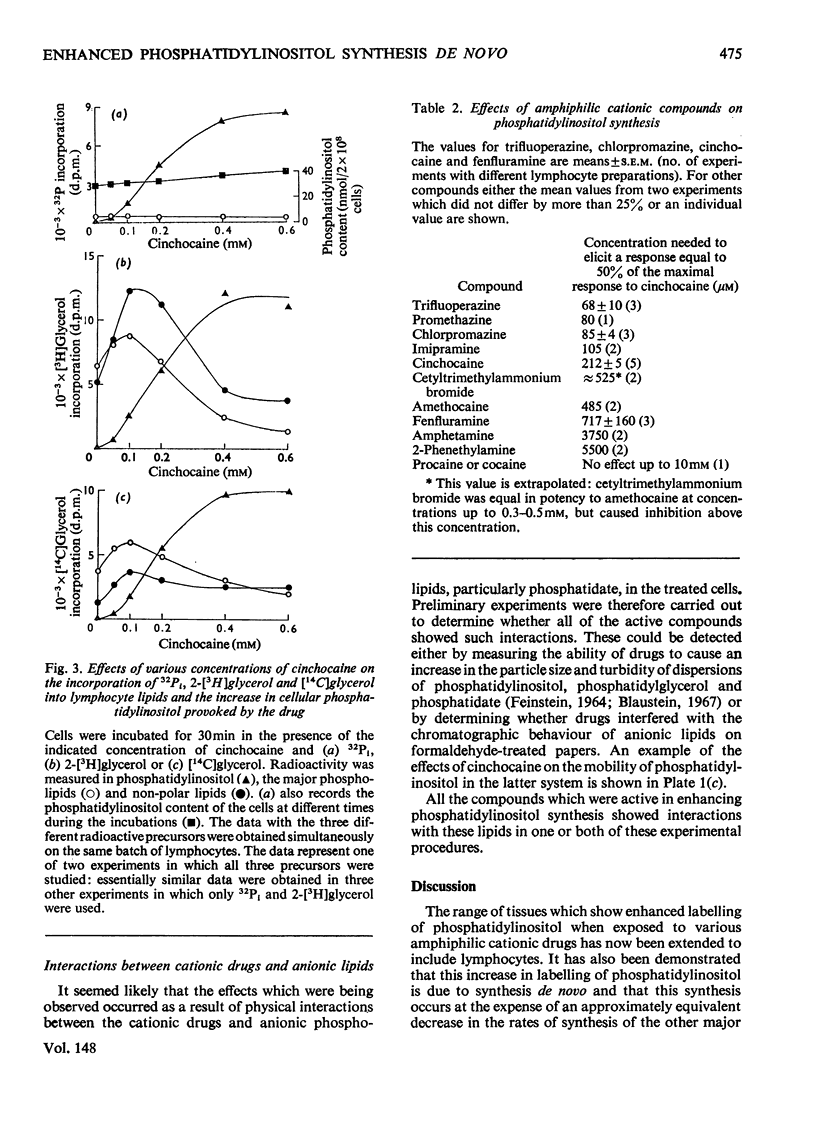
A variety of amphiphilic cations caused very large increases in the rates of incorporation of Pi and glycerol into phosphatidylinositol in pig mesenteric small lymphocytes. This synthesis de novo of phosphatidylinositol led to a doubling of the phosphatidylinositol concentration in the cells within 3.5 h. The increase in synthesis of phosphatidylinositol labelled with [3H]- or [14C]-glycerol was matched by an approximately equivalent decrease in incorporation of glycerol into phosphatidylcholine, phosphatidylethanolamine and triacylglycerol. Amphilic cations which produced these effects included, in order of decreasing effectiveness, trifluoperazine (half-maximal effect at about 70 mum) greater than chlorpromazine approximately promethazine approximately imipramine greater than cinchocaine greater than amethocaine approximately cetyltrimethylammonium greater than fenfluramine greater than amphetamine greater than 2-phenethylamine greater than cocaine approximately procaine; the most effective compounds were those with the largest and most hydrophobic non-polar substituents. The response to cations was not changed by varying the extracellular Ca2+ concentration in the range 10 nm-1mm. The active amphiphilic cations interacted with anionic phospholipids causing aggregation of aqueous dispersions and/or changes in chromatographic behaviour. These results indicate that amphiphilic cations redirect glycerolipid synthesis de novo, probably owing to inhibition of phosphatidate phosphohydrolase, so that phosphatidylinositol synthesis is increased at the expense of other glycerolipids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Michell R. H. Phosphatidylinositol cleavage catalysed by the soluble fraction from lymphocytes. Activity at pH5.5 and pH7.0. Biochem J. 1974 Sep;142(3):591–597. doi: 10.1042/bj1420591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Phosphatidylinositol cleavage in lymphocytes. Requirement for calcium ions at a low concentration and effects of other cations. Biochem J. 1974 Sep;142(3):599–604. doi: 10.1042/bj1420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROSSARD M., QUASTEL J. H. Effects of morphine and Tofranil on the incorporation of phosphate (32P) into phospolipids of rat brain slices. Biochem Pharmacol. 1963 Jul;12:766–768. doi: 10.1016/0006-2952(63)90054-6. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Phospholipids as ion exchangers: implications for a possible role in biological membrane excitability and anesthesia. Biochim Biophys Acta. 1967 Sep 9;135(4):653–668. doi: 10.1016/0005-2736(67)90096-x. [DOI] [PubMed] [Google Scholar]
- Brindley D. N., Bowley M. Drugs affecting the synthesis of glycerides and phospholipids in rat liver. The effects of clofibrate, halofenate, fenfluramine, amphetamine, cinchocaine, chlorpromazine, demethylimipramine, mepyramine and some of their derivatives. Biochem J. 1975 Jun;148(3):461–469. doi: 10.1042/bj1480461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole W. P., Simon E. J. Effects of levorphanol on phospholipid metabolism in the giant axon of the squid. J Neurochem. 1974 Jan;22(1):183–185. doi: 10.1111/j.1471-4159.1974.tb12197.x. [DOI] [PubMed] [Google Scholar]
- Eichberg J., Hauser G. Stimulation by local anesthetics of the metabolism of acidic phospholipids in the rat pineal gland. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1460–1467. doi: 10.1016/0006-291x(74)90362-3. [DOI] [PubMed] [Google Scholar]
- Eichberg J., Shein H. M., Schwartz M., Hauser G. Stimulation of 32 P i incorporation into phosphatidylinositol and phosphatidyglycerol by catecholamines and -adrenergic receptor blocking agents in rat pineal organ cultures. J Biol Chem. 1973 May 25;248(10):3615–3622. [PubMed] [Google Scholar]
- FEINSTEIN M. B. REACTION OF LOCAL ANESTHETICS WITH PHOSPHOLIPIDS. A POSSIBLE CHEMICAL BASIS FOR ANESTHESIA. J Gen Physiol. 1964 Nov;48:357–374. doi: 10.1085/jgp.48.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L. E., Salway J. G. Letter: The effect of local anaesthetics on the incorporation of 32P into the phosphoinositides of rabbit vagus nerve. J Pharm Pharmacol. 1973 Sep;25(9):745–747. doi: 10.1111/j.2042-7158.1973.tb10058.x. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. Breakdown of phosphatidylinositol provoked by muscarinic cholinergic stimulation of rat parotid-gland fragments. Biochem J. 1974 Sep;142(3):583–590. doi: 10.1042/bj1420583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. A membrane-bound activity catalysing phosphatidylinositol breakdown to 1,2-diacylglycerol, D-myoinositol 1:2-cyclic phosphate an D-myoinositol 1-phosphate. Properties and subcellular distribution in rat cerebral cortex. Biochem J. 1973 Mar;131(3):433–442. doi: 10.1042/bj1310433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. Phosphatidylinositol metabolism in cells receiving extracellular stimulation. FEBS Lett. 1973 Apr 1;31(1):1–10. doi: 10.1016/0014-5793(73)80061-4. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. Stimulation by acetylcholine of phosphatidylinositol labelling. Subcellular distribution in rat cerebral-cortex slices. Biochem J. 1972 Mar;126(5):1141–1147. doi: 10.1042/bj1261141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther P. J., Waller R. E. Physical hazards. Postgrad Med J. 1975;51 (Suppl 2):suppl 51–9. [PubMed] [Google Scholar]
- Levey G. S., Roth J., Pastan I. Effect of propranolol and phentolamine on canine and bovine responses to TSH. Endocrinology. 1969 May;84(5):1009–1015. doi: 10.1210/endo-84-5-1009. [DOI] [PubMed] [Google Scholar]
- Magee W. L., Berry J. F., Strickland K. P., Rossiter R. J. Labelling of phospholipids from inorganic [P]phosphate in brain preparations. Effect of acetylcholine, chlorpromazine and azacyclonol. Biochem J. 1963 Jul;88(1):45–52. doi: 10.1042/bj0880045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maino V. C., Green N. M., Crumpton M. J. The role of calcium ions in initiating transformation of lymphocytes. Nature. 1974 Sep 27;251(5473):324–327. doi: 10.1038/251324b0. [DOI] [PubMed] [Google Scholar]
- Maino V. C., Hayman M. J., Crumpton M. J. Relationship between enhanced turnover of phosphatidylinositol and lymphocyte activation by mitogens. Biochem J. 1975 Jan;146(1):247–252. doi: 10.1042/bj1460247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa Y., Osawa T., Inoue K., Nojima S. Effects of various mitogens on the phospholipid metabolism of human peripheral lymphocytes. Biochim Biophys Acta. 1973 Dec 20;326(3):339–344. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Mulé S. J. Morphine and the incorporation of Pi32 into brain phospholipids of nontolerant, tolerant and abstinent guinea pigs. J Pharmacol Exp Ther. 1967 Apr;156(1):92–100. [PubMed] [Google Scholar]
- Onaya T., Solomon D. H. Effects of chlorpromazine and propranolol on in vitro thyroid activation by thyrotropin, long-acting thyroid stimulator and dibutyryl cyclic-AMP. Endocrinology. 1969 Dec;85(6):1010–1017. doi: 10.1210/endo-85-6-1010. [DOI] [PubMed] [Google Scholar]
- Salway J. G., Hughes I. E. An investigation of the possible role of phosphoinositides as regulators of action potentials by studying the effect of electrical stimulation, tetrodotoxin and cinchocaine on phosphoinositide labelling by 32 P in rabbit vagus. J Neurochem. 1972 May;19(5):1233–1240. doi: 10.1111/j.1471-4159.1972.tb01449.x. [DOI] [PubMed] [Google Scholar]
- Siddle K., Hales C. N. The action of local anaesthetics on lipolysis and on adenosine 3':5'-cyclic monophosphate content in isolated rat fat-cells. Biochem J. 1974 Aug;142(2):345–351. doi: 10.1042/bj1420345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Good J. J., Barclay M., Reggio R. B. Quantitative analysis of simple lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1968 Jan 10;152(1):10–19. doi: 10.1016/0005-2760(68)90003-9. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. M., Hales C. N. Effect of adrenaline on 32 P incorporation into rat fat-cell phospholipids. Biochem J. 1972 Jul;128(3):531–541. doi: 10.1042/bj1280531. [DOI] [PMC free article] [PubMed] [Google Scholar]