Abstract
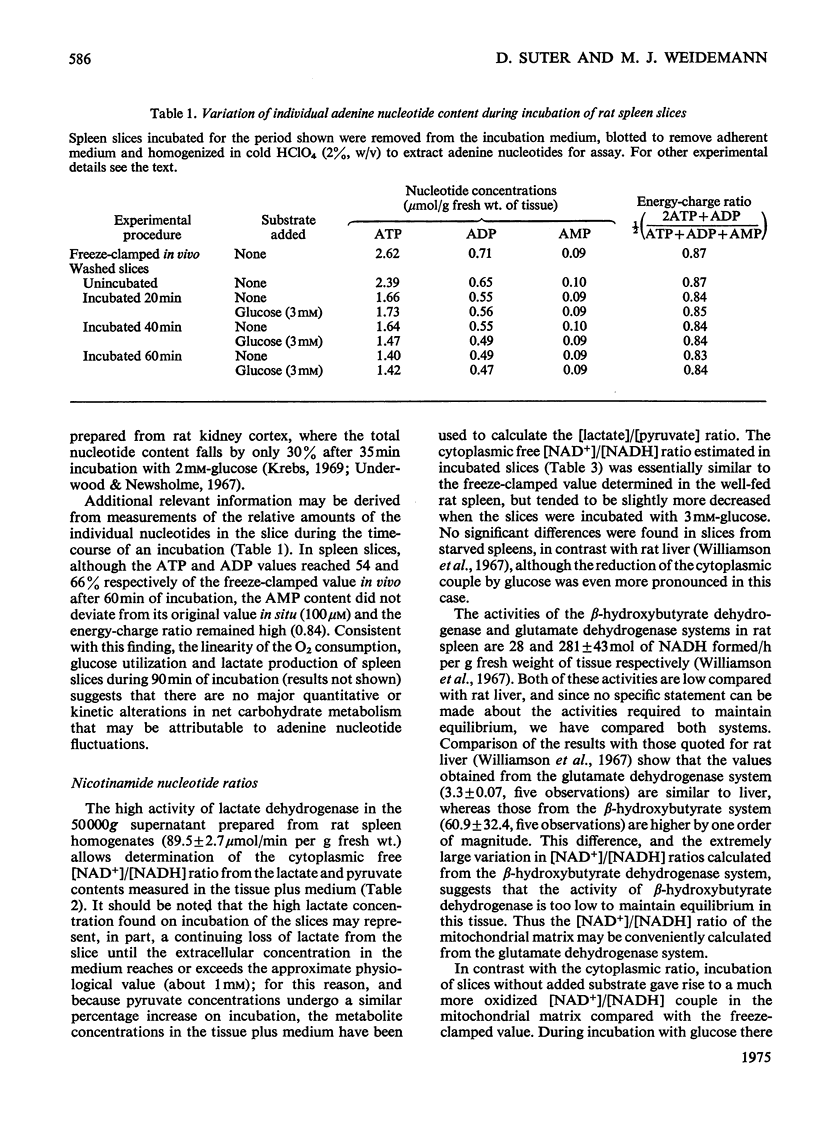
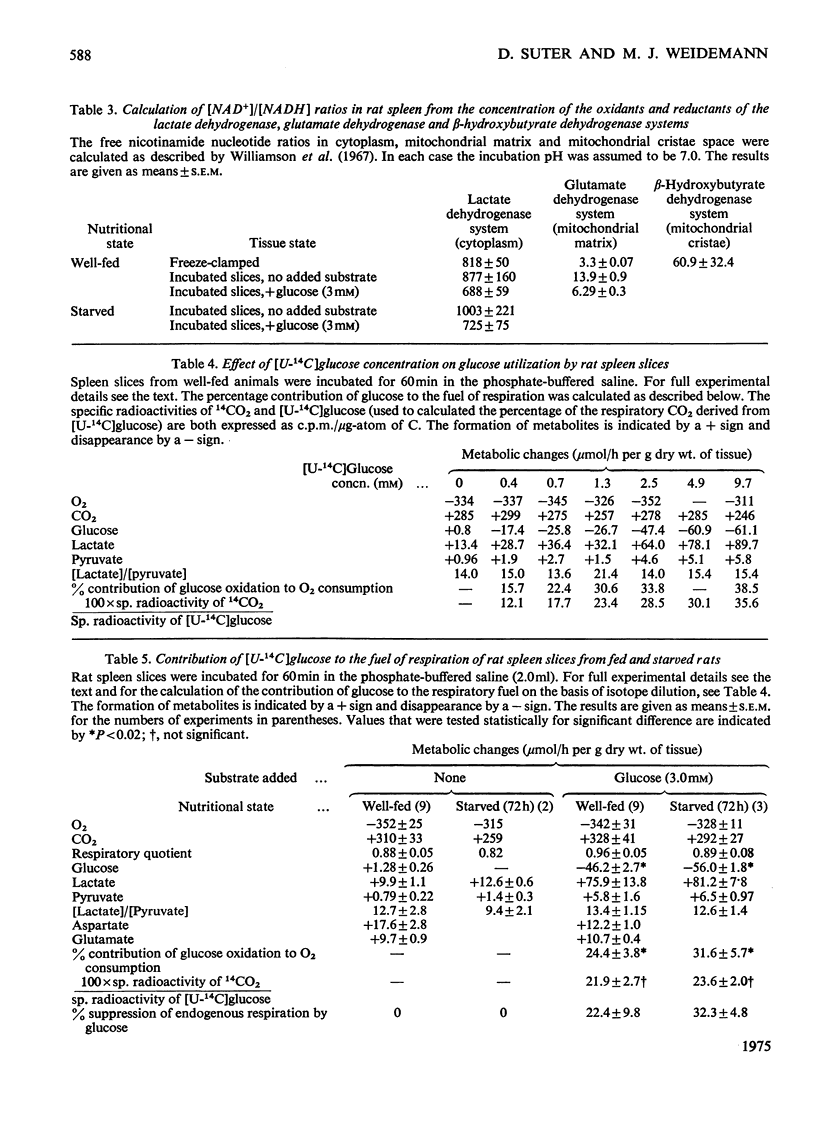
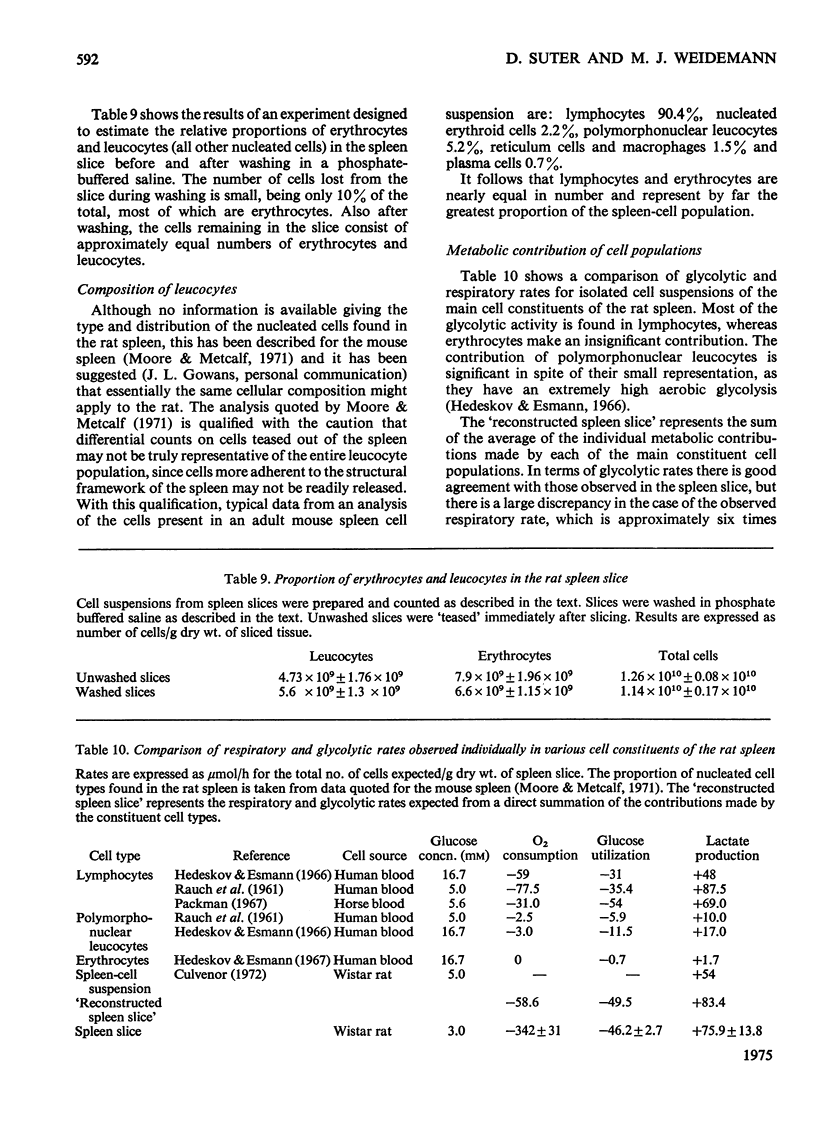
When washed spleen slices from fed rats are incubated with 3 mm-[U-14C]glucose, the rate of glucose utilization (46.2 mumol/h per g dry wt.) is sufficient to account, theoretically, for 80% of the O2 consumption. Measurement of net lactate production, however, and the fate of the radioactive carbon, indicates that the contribution of glucose to the respiratory fuel of the tissue is only 25-30% whereas 60-70% of the glucose utilized is converted into lactate. At saturating glucose concentrations (above 5 mm) its contribution to the respiratory fuel of the slice is increased to a maximum value of 34-39%. Only 2% of the glucose utilized is metabolized via the oxidative steps of the pentose phosphate pathway. Starvation for 72 h marginally increases both the rate of glucose utilization (by 21%) and its net contribution to the respiratory fuel (by 29%). Insulin, glucagon, adrenaline and adenosine 3':5'-cyclic monophosphate have no significant effect on either the rate of glucose utilization or on the pattern of radioactive isotope distribution. The uptake of glucose is increased by only 20%, whereas the production of lactate doubles when slices are incubated under anaerobic conditions. In assessing the suitability of spleen slices for metabolic studies, the only serious major perturbation, compared with the freeze-clamped organ, is an elevated mitochondrial [NAD+]/[NADH] ratio (connected with increased endogenous NH3 production) that is partially restored to normal values on incubation with glucose. Equal proportions of erythrocytes and leucocytes are found in the washed spleen slice. Metabolic contributions of the constituent cell populations in the washed slice are calculated and it is concluded that lymphocytes account for the major part of the glycolytic metabolism (80-90%), whereas the contribution of erythrocytes is insignificant.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK W. S. Occurrence and control of the phosphogluconate oxidation pathway in normal and leukemic leukocytes. J Biol Chem. 1958 May;232(1):271–283. [PubMed] [Google Scholar]
- BLACK L., BERENBAUM M. C. FACTORS AFFECTING THE DYE EXCLUSION TEST FOR CELL VIABILITY. Exp Cell Res. 1964 Jun;35:9–13. doi: 10.1016/0014-4827(64)90066-7. [DOI] [PubMed] [Google Scholar]
- BRADY T. G., O'DONOVAN C. I. A STUDY OF THE TISSUE DISTRIBUTION OF ADENOSINE DEAMINASE IN SIX MAMMAL SPECIES. Comp Biochem Physiol. 1965 Jan;14:101–120. doi: 10.1016/0010-406x(65)90011-3. [DOI] [PubMed] [Google Scholar]
- BUSCH H. Studies on the metabolism of acetate-1-C in tissues of tumor-bearing rats. Cancer Res. 1953 Nov;13(11):789–794. [PubMed] [Google Scholar]
- Bartley W., Dean B. Extraction and estimation of glycogen and oligosaccharides from rat heart. Anal Biochem. 1968 Oct 24;25(1):99–108. doi: 10.1016/0003-2697(68)90084-5. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Wang P., McCall C. E. Hexose monophosphate shunt activity and oxygen consumption during phagocytosis: temporal sequence. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1434–1436. doi: 10.3181/00379727-140-36690. [DOI] [PubMed] [Google Scholar]
- Dickens F., Greville G. D. Metabolism of normal and tumour tissue: Respiration in fructose and in sugar-free media. Biochem J. 1933;27(3):832–841. doi: 10.1042/bj0270832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gevers W., Krebs H. A. The effects of adenine nucleotides on carbohydrate metabolism in pigeon-liver homogenates. Biochem J. 1966 Mar;98(3):720–735. doi: 10.1042/bj0980720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLAM R. J., KREBS H. A. THE METABOLISM OF GLUTAMATE IN HOMOGENATES AND SLICES OF BRAIN CORTEX. Biochem J. 1963 Sep;88:566–578. doi: 10.1042/bj0880566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeskov C. J. Early effects of phytohaemagglutinin on glucose metabolism of normal human lymphocytes. Biochem J. 1968 Nov;110(2):373–380. doi: 10.1042/bj1100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeskov C. J., Esmann V. Major metabolic pathways of glucose in normal human lymphocytes and the effect of cortisol. Biochim Biophys Acta. 1967 Nov 28;148(2):372–383. doi: 10.1016/0304-4165(67)90133-x. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J., Esmann V. Respiration and glycolysis of normal human lymphocytes. Blood. 1966 Aug;28(2):163–174. [PubMed] [Google Scholar]
- KIRSTEN E., GEREZ C., KIRSTEN R. [An enzymatic microdetermination method for ammonia, specifically for extracts of animal tissues and fluids. Determination of NH4 ions in blood]. Biochem Z. 1963;337:312–319. [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Krebs H. A., De Gasquet P. Inhibition of gluconeogenesis by alpha-oxo acids. Biochem J. 1964 Jan;90(1):149–154. doi: 10.1042/bj0900149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Some aspects of the regulation of fuel supply in omnivorous animals. Adv Enzyme Regul. 1972;10:397–420. doi: 10.1016/0065-2571(72)90025-8. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., RANDLE P. J., REGEN D. M. Regulation of glucose uptake by muscle. 3. The effects of insulin, anoxia, salicylate and 2:4-dinitrophenol on membrane transport and intracellular phosphorylation of glucose in the isolated rat heart. Biochem J. 1959 Dec;73:573–579. doi: 10.1042/bj0730573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro A. J., Jackson R. J., Korner A. Studies on the nature of polysomes. Biochem J. 1964 Aug;92(2):289–299. doi: 10.1042/bj0920289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachman L. M. The carbohydrate metabolism and respiration of isolated small lymphocytes. In vitro studies of normal and phytohemagglutinin stimulated cells. Blood. 1967 Dec;30(6):691–706. [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 1. The effects of insulin, anaerobiosis and cell poisons on the uptake of glucose and release of potassium by isolated rat diaphragm. Biochem J. 1958 Nov;70(3):490–500. doi: 10.1042/bj0700490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUCH H. C., LOOMIS M. E., JOHNSON M. E., FAVOUR C. B. In vitro suppression of polymorphonuclear leukocyte and lymphocyte glycolysis by cortisol. Endocrinology. 1961 Mar;68:375–385. doi: 10.1210/endo-68-3-375. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R. J., Bhat K. S. Phagocytosis and leucocyte enzymes in protein-calorie malnutrition. Biochem J. 1972 Mar;127(1):255–259. doi: 10.1042/bj1270255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Burger R., Lowenstein J. M. Adenylate deaminase. I. The effects of adenosine and guanosine triphosphates on activity and the organ distribution of the regulated enzyme. J Biol Chem. 1966 Mar 10;241(5):1244–1245. [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Control of glycolysis and gluconeogenesis in rat kidney cortex slices. Biochem J. 1967 Jul;104(1):300–305. doi: 10.1042/bj1040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLANCE-OWEN J., HURLOCK B. Estimation of plasma-insulin by the rat diaphragm method. Lancet. 1954 Jan 9;266(6802):68–70. doi: 10.1016/s0140-6736(54)90820-x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H. F., Krebs H. A. Lactate production in the perfused rat liver. Biochem J. 1971 Nov;125(1):129–139. doi: 10.1042/bj1250129. [DOI] [PMC free article] [PubMed] [Google Scholar]


