Abstract
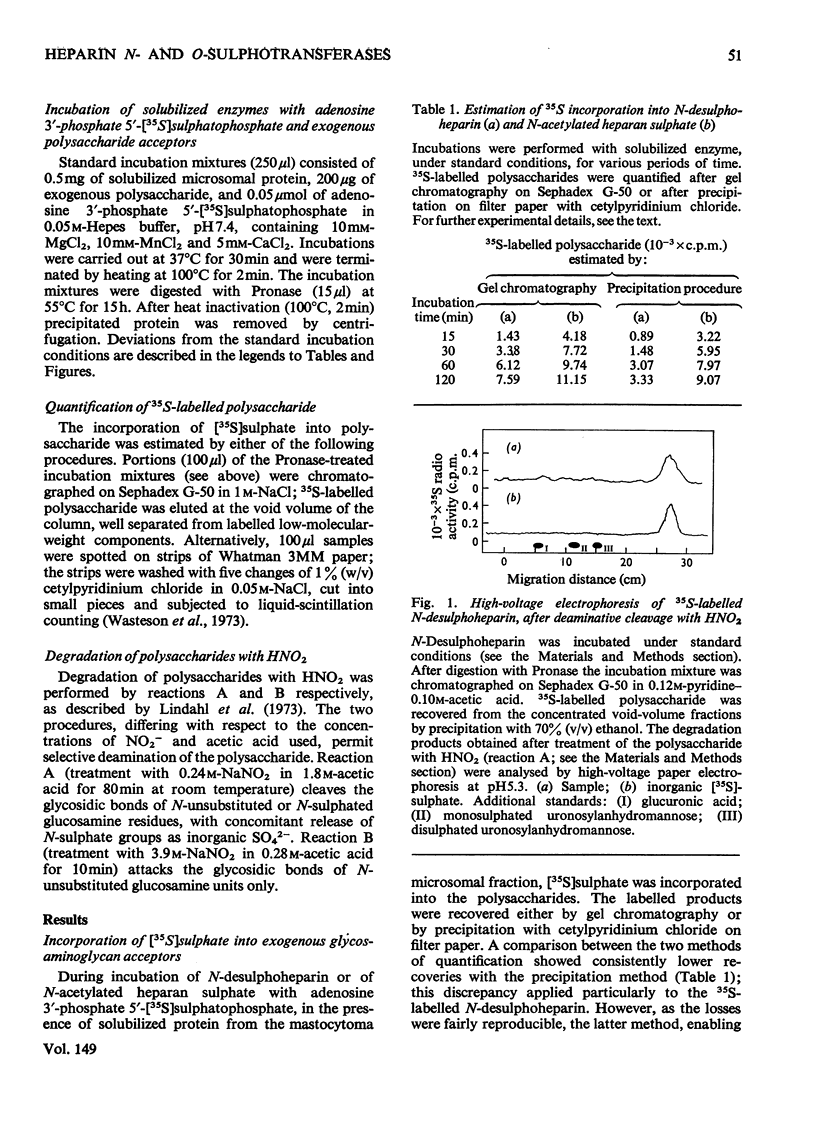
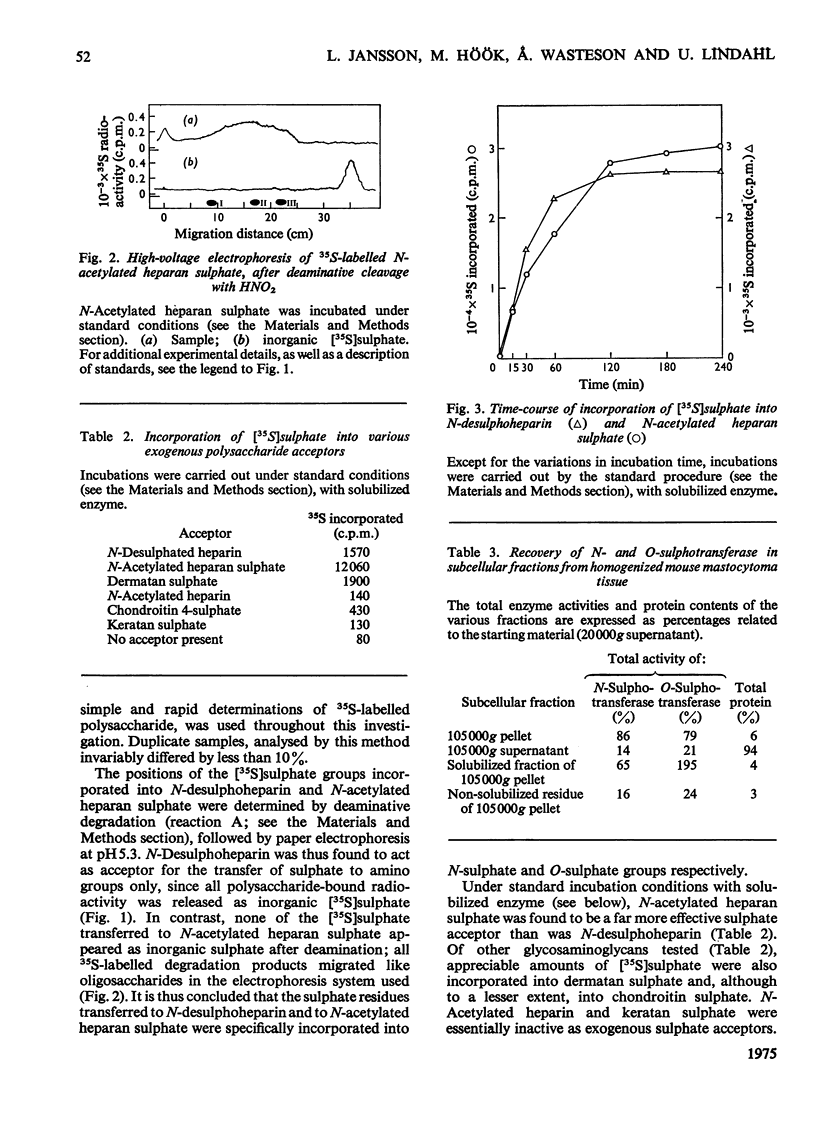
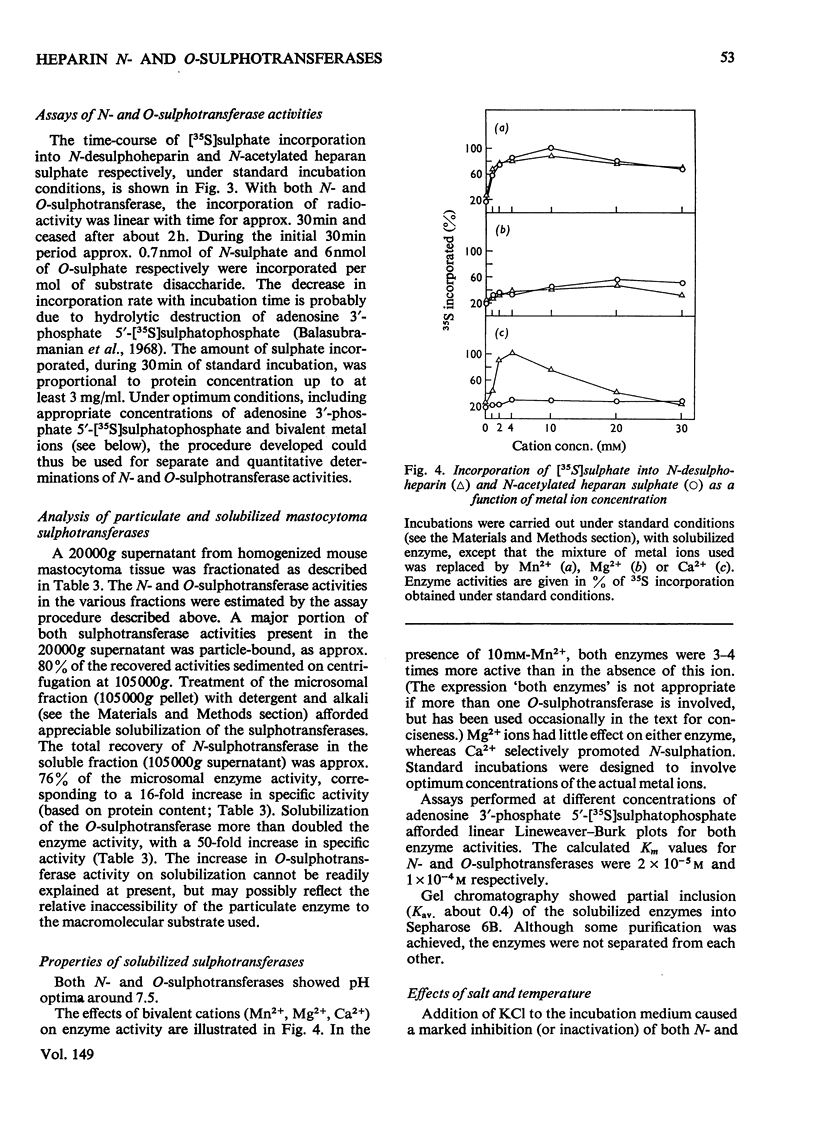
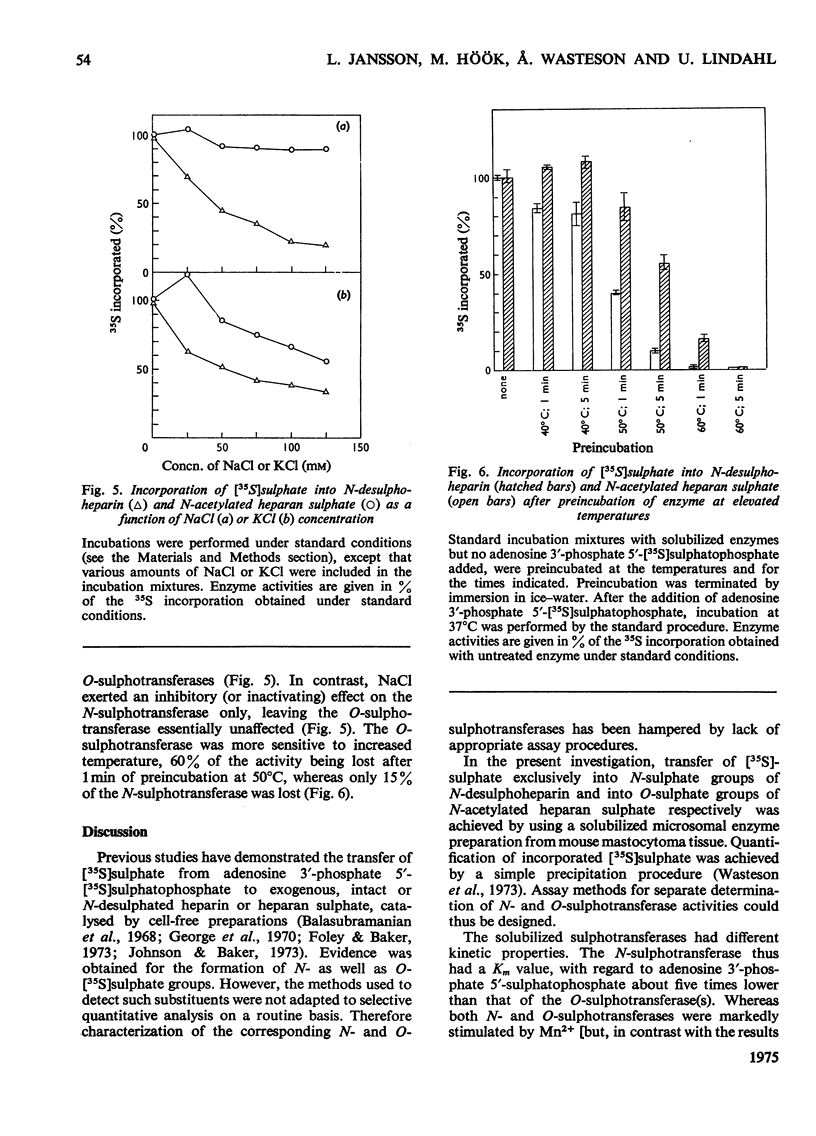
Assay methods were developed enabling separate determination of N- and O-sulphotransferase activities in an enzyme preparation from mouse mastocytoma. N-Desulphoheparin and chemically N-acetylated heparan sulphate were used as specific exogenous sulphate acceptors in the transfer of [35S]sulphate residues from adenosine 3'-phosphate 5'-[35S]sulphatophosphate to amino and hydroxyl groups respectively. The resulting 35S-labelled polysaccharides were isolated as their cetylpyridinium complexes on filter paper. Sulphotransferases were solubilized from a mastocytoma microsomal fraction by treatment with detergent-alkali. The pH optimum for both enzymes was about 7.5 Km with regard to adenosine 3'-phosphate 5'-sulphatophosphate was estimated to be 2 X 10(-5) M for the N-sulphotransferase and 1 X 10(-4) M for the O-sulphotransferase(s). The enzymes required bivalent cations for maximum activity, Mn2+ stimulating both the N- and O-sulphotransferase four- to five-fold, whereas Ca2+ increased the N- but not the O-sulphotransferase activity. The O-sulphotransferase was found to be more sensitive to heat-inactivation, 60% of the activity being lost after 1 min at 50 degrees C, whereas only 15% of the N-sulphotransferase activity was lost. In contrast, the N-sulphotransferase was selectively inhibited (or inactivated) by NaCl; at 0.125 M-NaCl concentration the O-sulphotransferase activity was essentially unaffected, whereas the N-sulphotransferase activity was depressed by 80%. These results strongly indicate that N- and O-sulphate-transfer reactions should be ascribed to different enzymes, or, alternatively, to separate and independent active sites on the same enzyme molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANSETH A., LAURENT T. C. Studies on corneal polysaccharides. I. Separation. Exp Eye Res. 1961 Sep;1:25–38. doi: 10.1016/s0014-4835(61)80005-5. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Balasubramanian A. S., Joun N. S., Marx W. Sulfation of N-desulfoheparin and heparan sulfate by a purified enzyme from mastocytoma. Arch Biochem Biophys. 1968 Dec;128(3):623–636. doi: 10.1016/0003-9861(68)90072-6. [DOI] [PubMed] [Google Scholar]
- Balasubramanian A. S., Spolter L., Rice L. I., Sharon J. B., Marx W. Preparation of 3'-phosphoadenylyl sulfate in substrate quantities using mastocytoma enzymes. Anal Biochem. 1967 Oct;21(1):22–33. doi: 10.1016/0003-2697(67)90078-4. [DOI] [PubMed] [Google Scholar]
- DANISHEFSKY I., STEINER H. INVESTIGATIONS ON THE CHEMISTRY OF HEPARIN. V. DISACCHARIDES OBTAINED AFTER PARTIAL HYDROLYSIS. Biochim Biophys Acta. 1965 Mar 1;101:37–45. doi: 10.1016/0926-6534(65)90028-x. [DOI] [PubMed] [Google Scholar]
- FURTH J., HAGEN P., HIRSCH E. I. Transplantable mastocytoma in the mouse containing histamine, heparin, 5-hydroxytryptamine. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):824–828. doi: 10.3181/00379727-95-23375. [DOI] [PubMed] [Google Scholar]
- Foley T., Baker J. R. Heparan sulphate sulphotransferase. Properties of an enzyme from ox lung. Biochem J. 1973 Sep;135(1):187–192. doi: 10.1042/bj1350187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E., Singh M., Bachhawat B. K. The nature of sulphation of uronic acid-containing glycosaminoglycans catalysed by brain sulphotransferase. J Neurochem. 1970 Feb;17(2):189–200. doi: 10.1111/j.1471-4159.1970.tb02200.x. [DOI] [PubMed] [Google Scholar]
- Helting T. Biosynthesis of heparin. Solubilization, partial separation, and purification of uridine diphosphate-galactose: acceptor galactosyltransferases from mouse mastocytoma. J Biol Chem. 1971 Feb 10;246(3):815–822. [PubMed] [Google Scholar]
- Hök M., Lindahl U., Iverius P. H. Distribution of sulphate and iduronic acid residues in heparin and heparan sulphate. Biochem J. 1974 Jan;137(1):33–43. doi: 10.1042/bj1370033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. H., Baker J. R. The enzymatic sulphation of heparan sulphate by hen's uterus. Biochim Biophys Acta. 1973 Sep 14;320(2):341–351. doi: 10.1016/0304-4165(73)90314-0. [DOI] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl U., Axelsson O. Identification of iduronic acid as the major sulfated uronic acid of heparin. J Biol Chem. 1971 Jan 10;246(1):74–82. [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Meezan E., Davidson E. A. Mucopolysaccharide sulfation in chick embryo cartilage. II. Characterization of the endogenous acceptor. J Biol Chem. 1967 Nov 10;242(21):4956–4962. [PubMed] [Google Scholar]
- Ogren S., Lindahl U. Degradation of heparin in mouse mastocytoma tissue. Biochem J. 1971 Dec;125(4):1119–1129. doi: 10.1042/bj1251119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINGERTZ N. R. Polysaccharides of neoplastic mast cells. Ann N Y Acad Sci. 1963 Feb 26;103:209–224. doi: 10.1111/j.1749-6632.1963.tb53700.x. [DOI] [PubMed] [Google Scholar]
- SUZUKI S., STROMINGER J. L. Enzymatic sulfation of mucopolysaccharides in hen oviduct. I. Transfer of sulfate from 3'-phosphoadenosine 5'-phosphosulfate to mucopolysaccharides. J Biol Chem. 1960 Feb;235:257–266. [PubMed] [Google Scholar]
- Silbert J. E. Biosynthesis of heparin. 3. Formation of a sulfated glycosaminoglycan with a microsomal preparation from mast cell tumors. J Biol Chem. 1967 Nov 10;242(21):5146–5152. [PubMed] [Google Scholar]
- Wasteson A., Uthne K., Westermark B. A novel assay for the biosynthesis of sulphated polysaccharide and its application to studies on the effects of somatomedin on cultured cells. Biochem J. 1973 Dec;136(4):1069–1074. doi: 10.1042/bj1361069. [DOI] [PMC free article] [PubMed] [Google Scholar]


