Abstract
1. Phenylalanine ammonia-lyase (EC 4.3.1.5) was purified to homogeneity from the acetone-dried powders of the mycelial felts of the plant pathogenic fungus Rhizoctonia solani. 2. A useful modification in protamine sulphate treatment to get substantial purification of the enzyme in a single-step is described. 3. The purified enzyme shows bisubstrate activity towards L-phenylalanine and L-tyrosine. 4. It is sensitive to carbonyl reagents and the inhibition is not reversed by gel filtration. 5. The molecular weight of the enzyme as determined by Sephadex G-200 chromatography and sucrose-density-gradient centrifugation is around 330000. 6. The enzyme is made up of two pairs of unidentical subunits, with a molecular weight of 70000 (alpha) and 90000 (beta) respectively. 7. Studies on initial velocity versus substrate concentration have shown significant deviations from Michaelis-Menten kinetics. 8. The double-reciprocal plots are biphasic (concave downwards) and Hofstee plots show a curvilinear pattern. 9. The apparent Km value increases from 0.18 mM to as high as 5.0 mM with the increase in the concentration of the substrate and during this process the Vmax, increases by 2-2.5-fold. 10. The value of Hill coefficient is 0.5. 11. Steady-state rates of phenylalanine ammonia-lyase reaction in the presence of inhibitors like D-phenylalanine, cinnamic, p-coumaric, caffeic, dihydrocaffeic and phenylpyruvic acid have shown that only one molecule of each type of inhibitor binds to a molecule of the enzyme. These observations suggest the involvement of negative homotropic interactions in phenylalanine ammonia-lyase. 12. The enzyme could not be desensitized by treatment with HgCl2, p-chloromercuribenzoic acid or by repeated freezing and thawing.
Full text
PDF
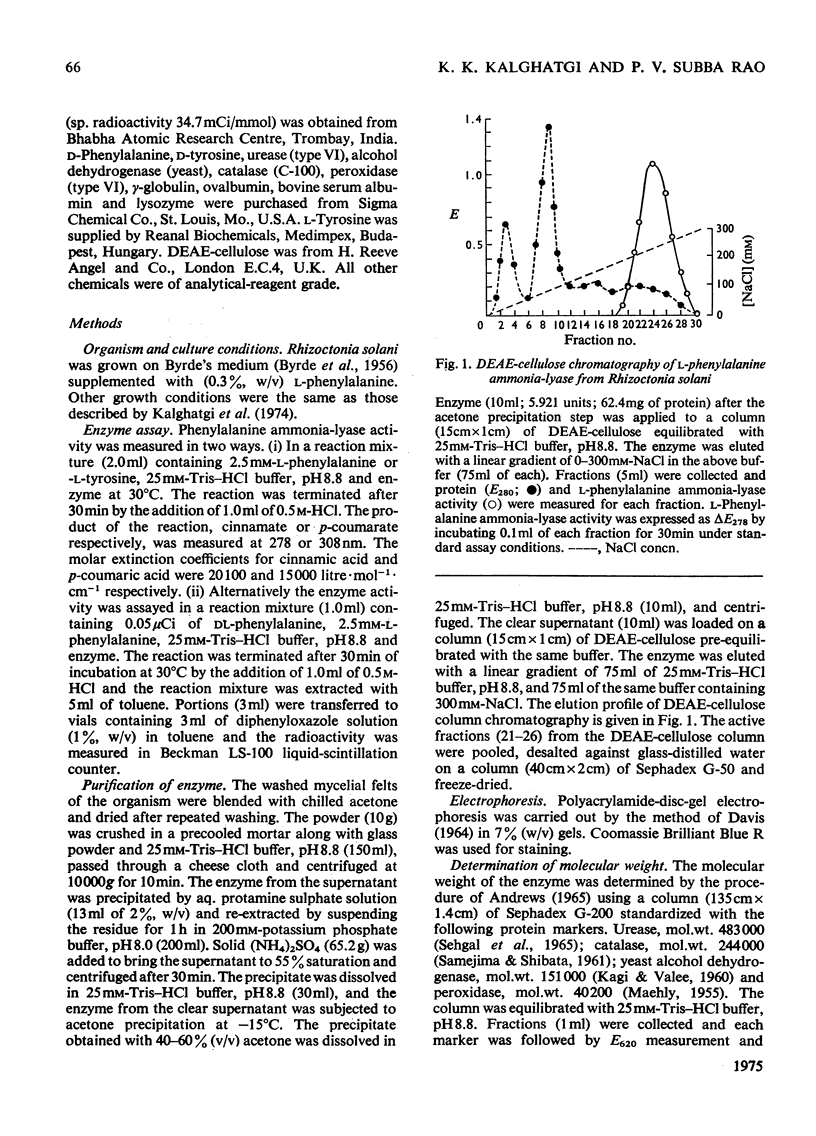

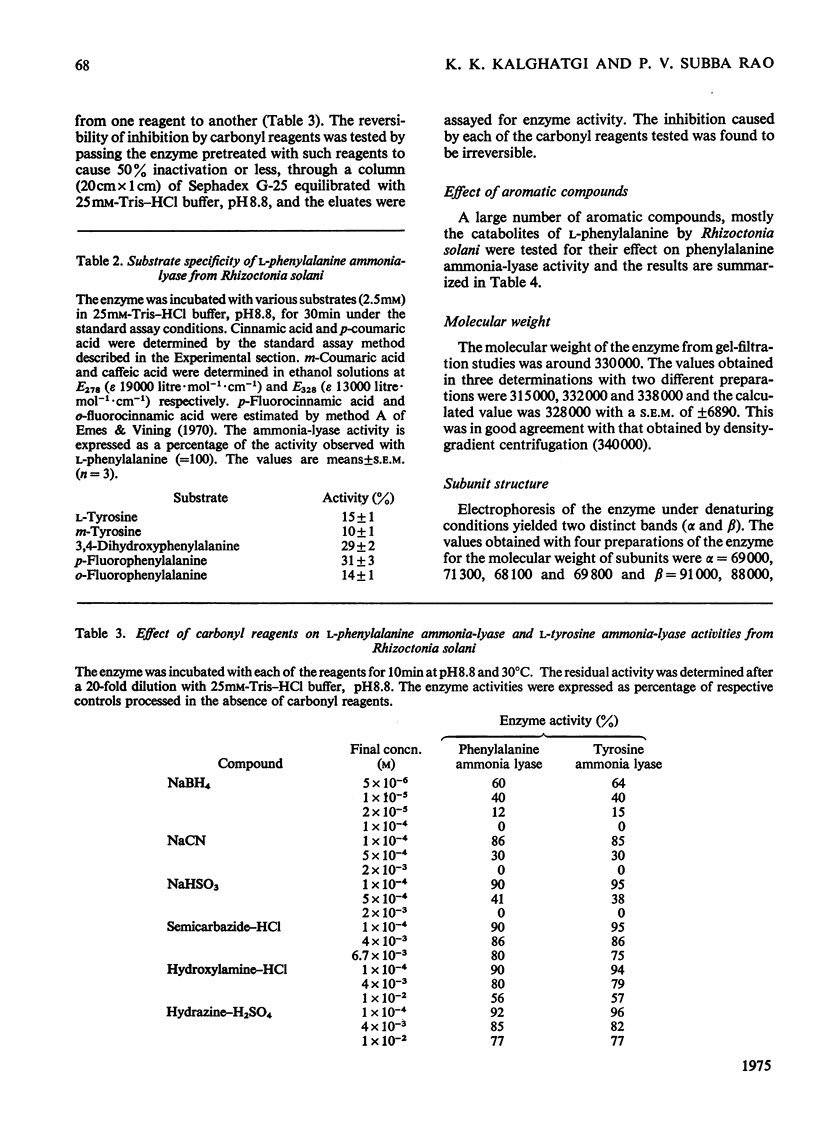
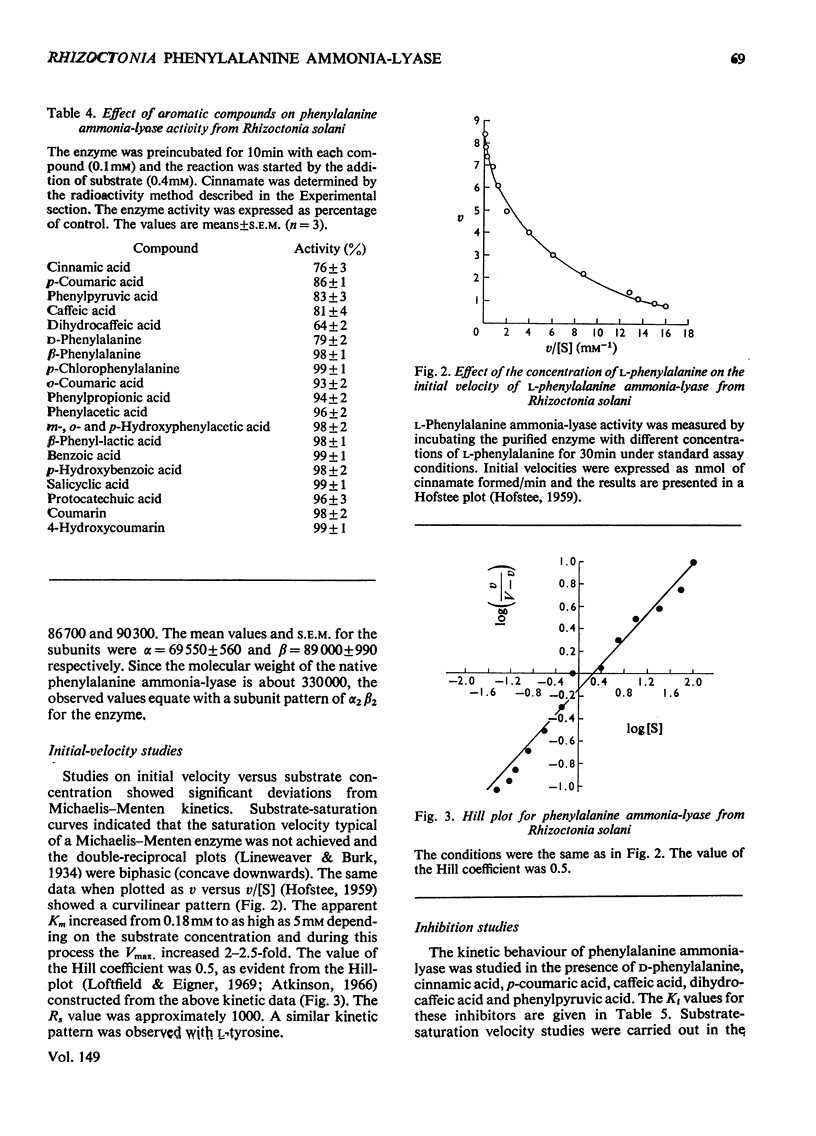
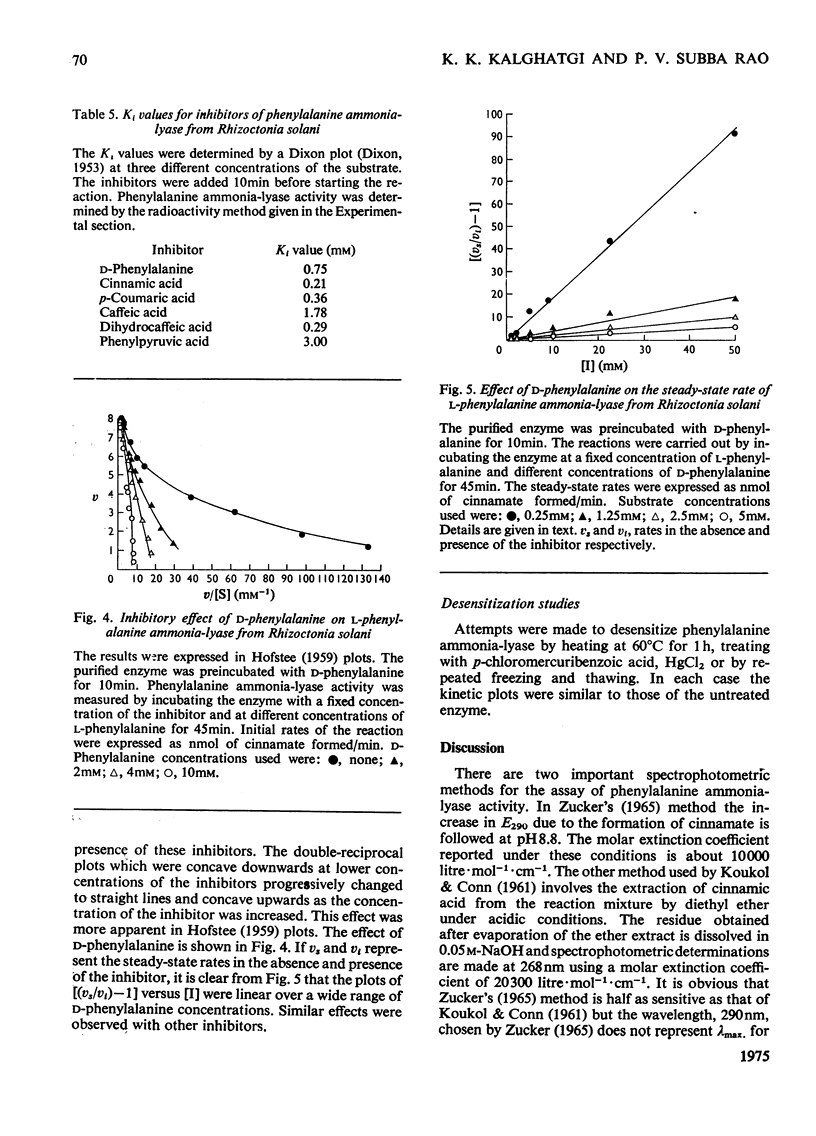


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attridge T. H., Stewart G. R., Smith Harry. End-product inhibition of Pisum phenylalanine ammonia-lyase by the Pisum flavonoids. FEBS Lett. 1971 Sep 15;17(1):84–86. doi: 10.1016/0014-5793(71)80569-0. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- BYRDE R. J., HARRIS J. F., WOODCOCK D. Fungal detoxication, the metabolism of -(2-naphthyloxy)-n-alkylcarboxylic acids by Aspergillus niger. Biochem J. 1956 Sep;64(1):154–160. doi: 10.1042/bj0640154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F. J., Barker R. Examination of the dissociation of multichain proteins in guanidine hydrochloride by membrane osmometry. Biochemistry. 1968 Jun;7(6):2207–2217. doi: 10.1021/bi00846a025. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes A. V., Vining L. C. Partial purification and properties of L-phenylalanine ammonia-lyase from Streptomyces verticillatus. Can J Biochem. 1970 May;48(5):613–622. doi: 10.1139/o70-099. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959 Oct 24;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-Phenylalanine ammonia-lyase. I. Purification and molecular size of the enzyme from potato tubers. Biochemistry. 1968 May;7(5):1896–1903. doi: 10.1021/bi00845a038. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase (maize and potato). Evidence that the enzyme is composed of four subunits. Biochemistry. 1973 Apr 10;12(8):1583–1591. doi: 10.1021/bi00732a019. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase. II. Mechanism and kinetic properties of the enzyme from potato tubers. Biochemistry. 1968 May;7(5):1904–1914. doi: 10.1021/bi00845a039. [DOI] [PubMed] [Google Scholar]
- Havir E. A. l-Phenylalanine Ammonia-Lyase (Maize): Evidence for a Common Catalytic Site for l-Phenylalanine and l-Tyrosine. Plant Physiol. 1971 Aug;48(2):130–136. doi: 10.1104/pp.48.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins D. S. Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential dehydroalanine. J Biol Chem. 1971 May 10;246(9):2977–2985. [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. The role of zinc in alcohol dehydrogenase. V. The effect of metal-binding agents on thestructure of the yeast alcohol dehydrogenase molecule. J Biol Chem. 1960 Nov;235:3188–3192. [PubMed] [Google Scholar]
- KOUKOL J., CONN E. E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem. 1961 Oct;236:2692–2698. [PubMed] [Google Scholar]
- Kalghatgi K. K., Nambudiri A. M., Bhat J. V., Subba Rao P. V. Degradation of L-penylalanine by Rhizoctonia solani. Indian J Biochem Biophys. 1974 Jun;11(2):116–118. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftfield R. B., Eigner E. A. Molecular order of participation of inhibitors (or activators) in biological systems. Science. 1969 Apr 18;164(3877):305–308. doi: 10.1126/science.164.3877.305. [DOI] [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Marsh H. V., Jr, Havir E. A., Hanson K. R. L-Phenylalanine ammonia-lyase. 3. Properties of the enzyme from maize seedlings. Biochemistry. 1968 May;7(5):1915–1918. doi: 10.1021/bi00845a040. [DOI] [PubMed] [Google Scholar]
- Minamikawa T., Uritani I. Phenylalanine ammonia-lyase in sliced sweet potato roots. J Biochem. 1965 May;57(5):678–688. [PubMed] [Google Scholar]
- Nari J., Mouttet C., Fouchier F., Ricard J. Subunit interactions in enzyme catalysis. Kinetic analysis of subunit interactions in the enzyme L-phenylalanine ammonia-lyase. Eur J Biochem. 1974 Feb 1;41(3):499–515. doi: 10.1111/j.1432-1033.1974.tb03291.x. [DOI] [PubMed] [Google Scholar]
- Parkhurst J. R., Hodgins D. S. Yeast phenylalanine ammonia-lyase. Properties of the enzyme from Sporobolomyces pararoseus and its catalytic site. Arch Biochem Biophys. 1972 Oct;152(2):597–605. doi: 10.1016/0003-9861(72)90255-x. [DOI] [PubMed] [Google Scholar]
- Rao P. V., Moore K., Towers G. H. Degradation of aromatic amino acids by fungi. II. Purification and properties of phenylalanine ammonia-lyase from Ustilago hordei. Can J Biochem. 1967 Dec;45(12):1863–1872. doi: 10.1139/o67-219. [DOI] [PubMed] [Google Scholar]
- SAMEJIMA T., SHIBATA K. Denaturation of catalase by formamide and urea related to the subunit make-up of the molecule. Arch Biochem Biophys. 1961 May;93:407–412. doi: 10.1016/0003-9861(61)90286-7. [DOI] [PubMed] [Google Scholar]
- Seghal P. P., Tanis R. J., Naylor A. W. A low molecular-weight form of urease. Biochem Biophys Res Commun. 1965 Sep 8;20(5):550–554. doi: 10.1016/0006-291x(65)90433-x. [DOI] [PubMed] [Google Scholar]
- Sund H., Weber K., Mölbert E. Dissoziation der Rinderleber-Katalase in ihre Untereinheiten. Eur J Biochem. 1967 Jun;1(4):400–410. doi: 10.1111/j.1432-1033.1967.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Hoch F. L. ZINC, A COMPONENT OF YEAST ALCOHOL DEHYDROGENASE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]


