Abstract
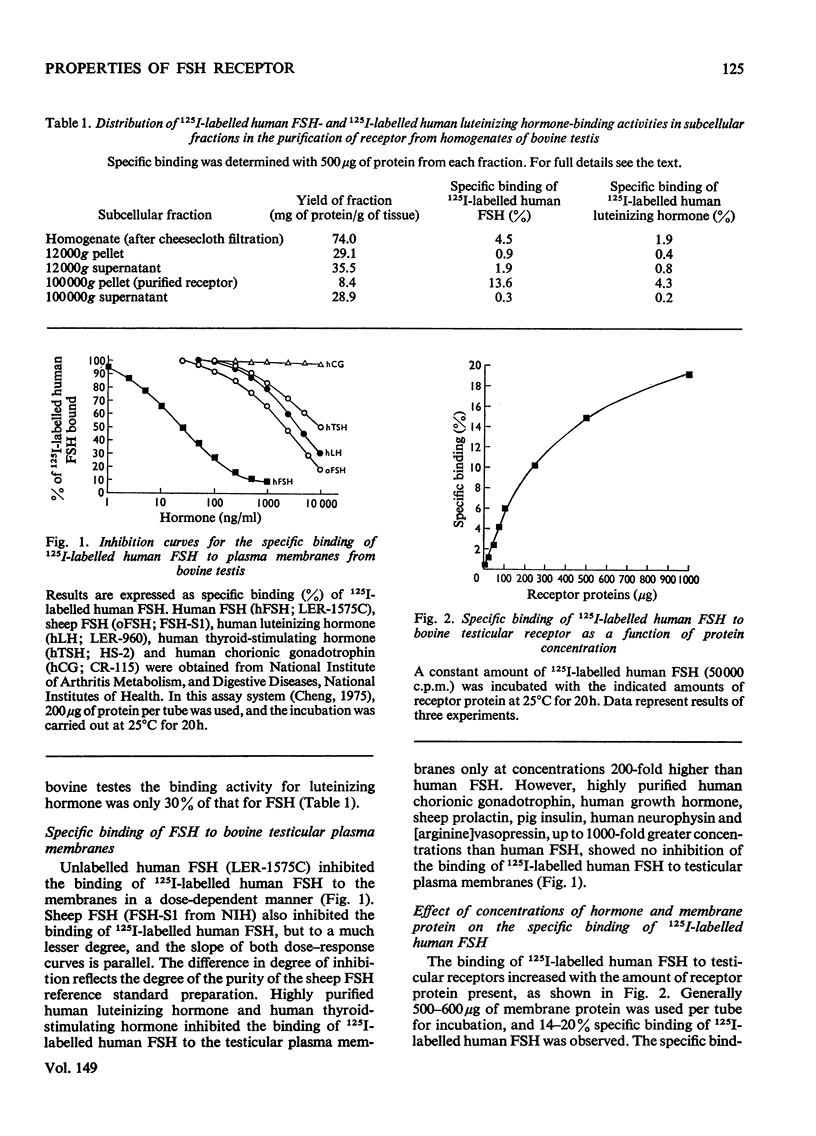
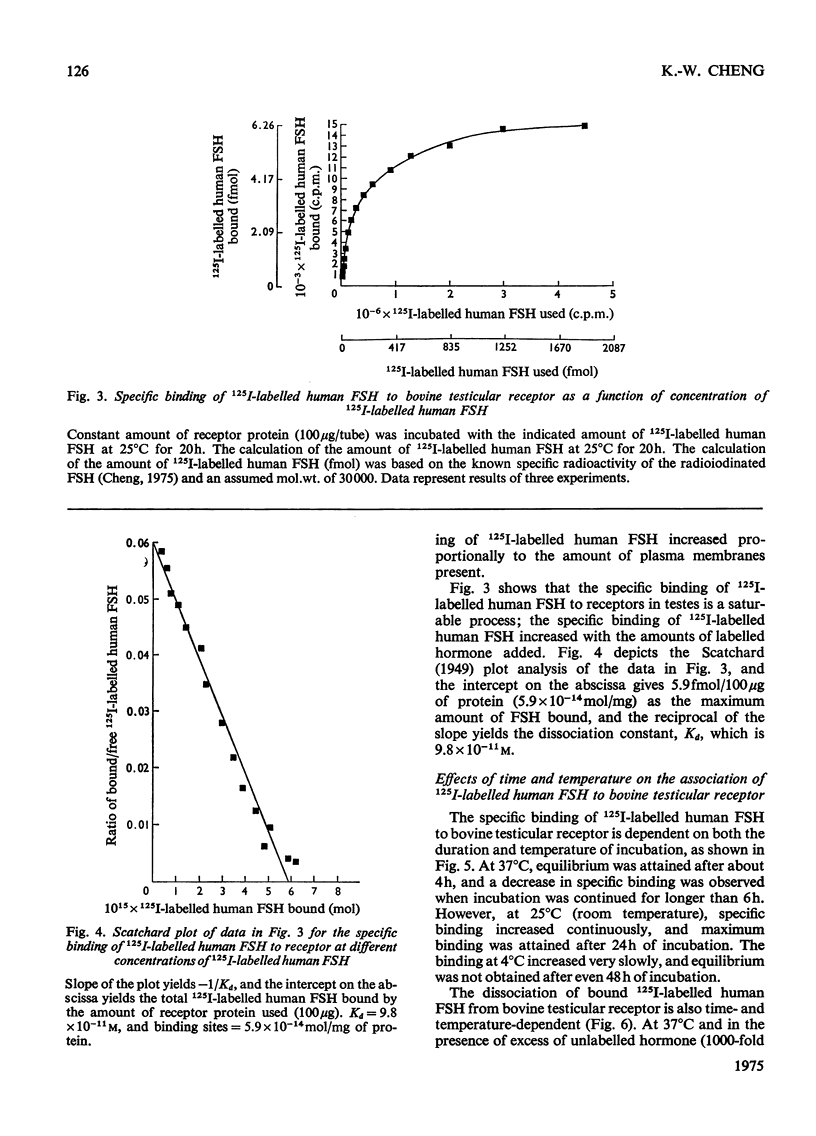
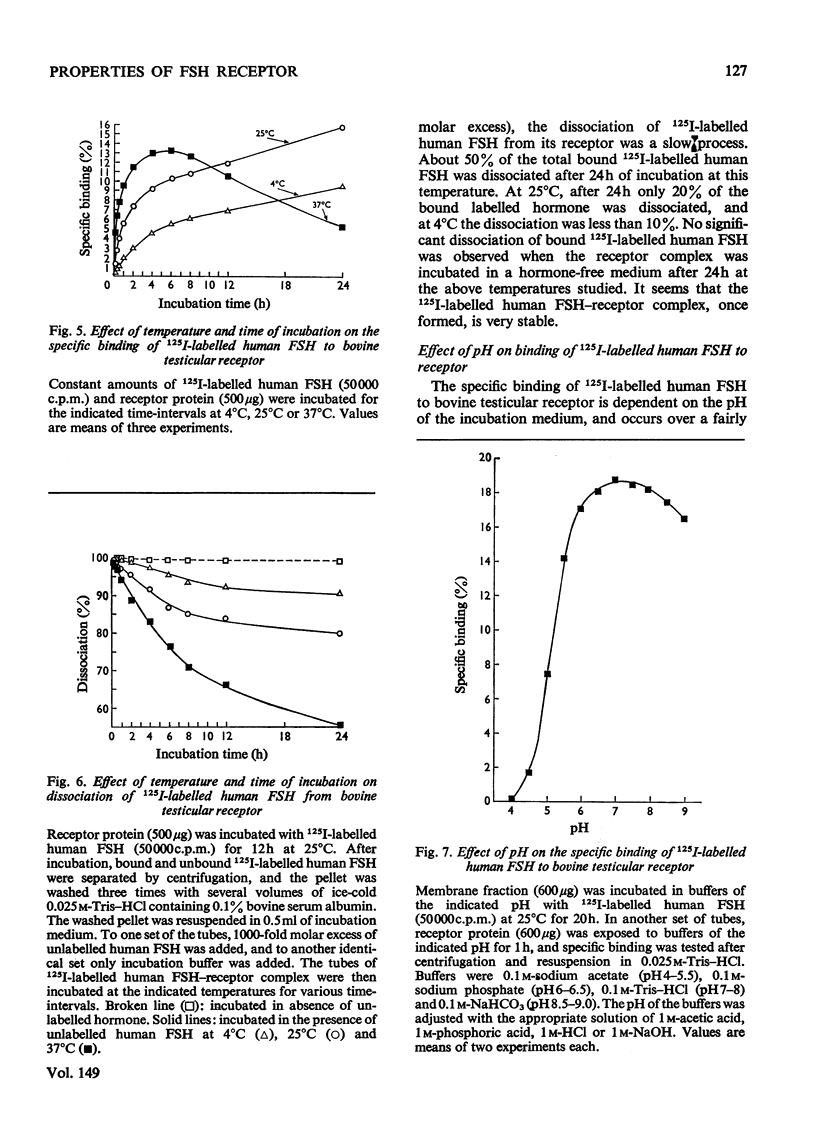
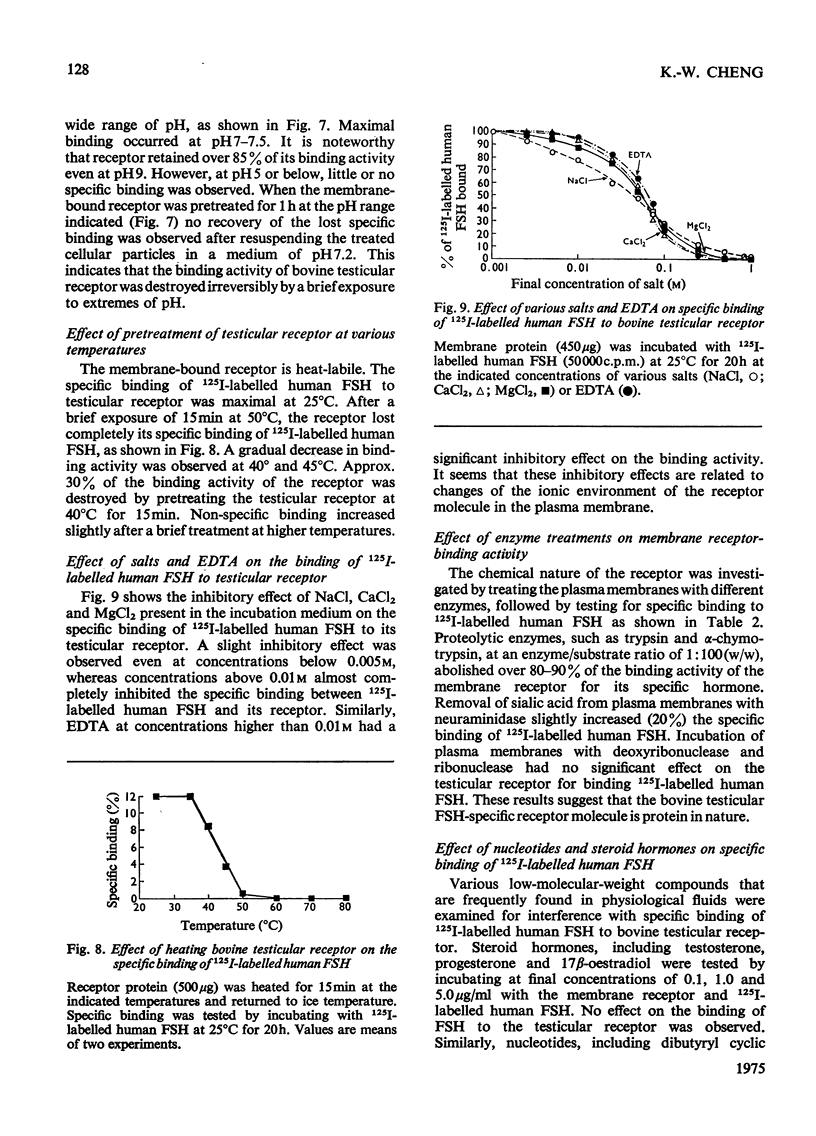
A simple method for preparing plasma membranes from bovine testes is described. Bovine testicular receptor has a high affinity and specificity for 125I-labelled human FSH (follicle-stimulating hormone). The specific binding of 125I-labelled human FSH to the plasma membranes is a saturable process with respect to the amounts of receptor protein and FSH added. The association and dissociation of 125I-labelled human FSH are time- and temperature-dependent, and the binding of labelled human FSH to bovine testicular receptor is strong and not readily reversible. Scatchard [Ann. N.Y. Acad. Sci. (1949) 51, 660-672] analysis indicates a dissociation constant, Kd, of 9.8 X10(-11)M, and 5.9 X 10(-14)mol of binding sites/mg of membrane protein. The testicular membrane receptor is heat-labile. Preheating at 40 degrees C for 15 min destroyed 30% of the binding activity. Specific binding is pH-dependent, with an optimum between pH 7.0 and 7.5. Brief exposure to extremes of pH caused irreversible damage to the receptors. The ionic strength of the incubation medium markedly affects the association of 125I-labelled human FSH with its testicular receptor. Various cations at concentrations of 0.1M inhibit almost completely the binding of 125I-labelled human FSH. Nuclectides and steroid hormones at concentrations of 1mM and 5mu/ml respectively have no effect on the binding of FSH to its receptor. Incubation of membranes with and chymotrypsin resulted in an almost complete loss of binding activity, suggesting that protein moieties are essential for the binding of 125I-labelled human FSH. Binding of 125I-labelled human FSH to bovine testicular receptor does not result in destruction or degradation of the hormone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhalla V. K., Reichert L. E., Jr Properties of follicle-stimulating hormone-receptor interactions. Specific binding of human follicle-stimulating hormone to rat testes. J Biol Chem. 1974 Jan 10;249(1):43–51. [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L., Tsuruhara T. Studies on a radioligand-receptor assay system for luteinizing hormone and chorionic gonadotropin. J Clin Endocrinol Metab. 1971 Jun;32(6):860–863. doi: 10.1210/jcem-32-6-860. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Kammerman S. Characteristics of gonadotropin receptors of porcine granulosa cells during follicle maturation. Endocrinology. 1973 Feb;92(2):531–540. doi: 10.1210/endo-92-2-531. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor of isolated fat cell membranes. J Biol Chem. 1971 Dec 10;246(23):7265–7274. [PubMed] [Google Scholar]
- Cutrecasas P. Perturbation of the insulin receptor of isolated fat cells with proteolytic enzymes. Direct measurement of insulin-receptor interactions. J Biol Chem. 1971 Nov;246(21):6522–6531. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Midgley A. R., Jr, Kleinsmith L. J. Human chorionic gonadotropin binding to rat ovarian tissue in vitro. Proc Soc Exp Biol Med. 1972 Jan;139(1):88–92. doi: 10.3181/00379727-139-36084. [DOI] [PubMed] [Google Scholar]
- De Kretser D. M., Catt K. J., Burger H. G., Smith G. C. Radioautographic studies on the localization of 125I-labelled human luteinizing and growth hormone in immature male rats. J Endocrinol. 1969 Jan;43(1):105–111. doi: 10.1677/joe.0.0430105. [DOI] [PubMed] [Google Scholar]
- De Kretser D. M., Catt K. J., Paulsen C. A. Studies on the in vitro testicular binding of iodinated luteinizing hormone in rats. Endocrinology. 1971 Feb;88(2):332–337. doi: 10.1210/endo-88-2-332. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Fritz I. B. Effects of gonadotropins on cyclic AMP production by isolated seminiferous tubule and interstitial cell preparations. Endocrinology. 1974 Feb;94(2):395–403. doi: 10.1210/endo-94-2-395. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Vernon R. G., Fritz I. B. The effect of gonadotrophins on the 3',5'-AMP levels of seminiferous tubules. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1523–1528. doi: 10.1016/0006-291x(72)90780-2. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Preparation and characterization of plasma membranes from bovine corpus luteum. J Biol Chem. 1973 Jul 25;248(14):5050–5056. [PubMed] [Google Scholar]
- Gospodarowicz D. Properties of the luteinizing hormone receptor of isolated bovine corpus luteum plasma membranes. J Biol Chem. 1973 Jul 25;248(14):5042–5049. [PubMed] [Google Scholar]
- HALL P. F., EIK-NES K. B. The action of gonadotropic hormones upon rabbit testis in vitro. Biochim Biophys Acta. 1962 Oct 8;63:411–422. doi: 10.1016/0006-3002(62)90105-1. [DOI] [PubMed] [Google Scholar]
- Haour F., Saxena B. B. Characterization and solubilization of gonadotropin receptor of bovine corpus luteum. J Biol Chem. 1974 Apr 10;249(7):2195–2205. [PubMed] [Google Scholar]
- Kammerman S., Canfield R. E. The inhibition of binding of iodinated human chorionic gonadotropin to mouse ovary in vitro. Endocrinology. 1972 Feb;90(2):384–389. doi: 10.1210/endo-90-2-384. [DOI] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Patanelli D. J., Tarnoff J., Humes J. L. Testicular adenyl cyclase: stimulation by the pituitary gonadotrophins. Biol Reprod. 1970 Feb;2(1):154–163. doi: 10.1095/biolreprod2.1.154. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y., Coulam C. B., Jiang N. S., Ryan R. J. Receptors for human luteinizing hormone in human corpora luteal tissue. J Clin Endocrinol Metab. 1973 Jan;36(1):148–152. doi: 10.1210/jcem-36-1-148. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. Interaction of ovarian receptors with human luteinizing hormone and human chorionic gonadotropin. Biochemistry. 1973 Nov 6;12(23):4609–4615. doi: 10.1021/bi00747a011. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. The uptake of human luteinizing hormone (hLH) by slices of luteinized rat ovaries. Endocrinology. 1971 Dec;89(6):1515–1523. doi: 10.1210/endo-89-6-1515. [DOI] [PubMed] [Google Scholar]
- Leidenberger F., Reichert L. E., Jr Evaluation of a rat testis homogenate radioligand receptor assay for human pituitary LH. Endocrinology. 1972 Oct;91(4):901–909. doi: 10.1210/endo-91-4-901. [DOI] [PubMed] [Google Scholar]
- Marsh J. M., Savard K. The stimulation of progesterone synthesis in bovine corpora lutea by adenosine 3',5'-monophosphate. Steroids. 1966 Aug;8(2):133–148. doi: 10.1016/0039-128x(66)90088-2. [DOI] [PubMed] [Google Scholar]
- Marsh J. M. The stimulatory effect of luteinizing hormone on adenyl cyclase in the bovine corpus luteum. J Biol Chem. 1970 Apr 10;245(7):1596–1603. [PubMed] [Google Scholar]
- Means A. R., Vaitukaitis J. Peptide hormone "receptors": specific binding of 3 H-FSH to testis. Endocrinology. 1972 Jan;90(1):39–46. doi: 10.1210/endo-90-1-39. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Vaitukaitis J. L., Nieschlag E., Lipsett M. B. Enzymatic radioiodination of gonadotropins. J Clin Endocrinol Metab. 1972 Jan;34(1):23–28. doi: 10.1210/jcem-34-1-23. [DOI] [PubMed] [Google Scholar]
- Murad F., Strauch B. S., Vaughan M. The effect of gonadotropins on testicular adenyl cyclase. Biochim Biophys Acta. 1969 May 6;177(3):591–598. doi: 10.1016/0304-4165(69)90324-9. [DOI] [PubMed] [Google Scholar]
- Rajaniemi H., Vanha-Perttula T. Specific receptor for LH in the ovary: evidence by autoradiography and tissue fractionation. Endocrinology. 1972 Jan;90(1):1–9. doi: 10.1210/endo-90-1-1. [DOI] [PubMed] [Google Scholar]
- Rao C. V. Properties of gonadotropin receptors in the cell membranes of bovine corpus luteum. J Biol Chem. 1974 May 10;249(9):2864–2872. [PubMed] [Google Scholar]
- Rao C. V., Saxena B. B. Gonadotropin receptors in the plasma membranes of rat luteal cells. Biochim Biophys Acta. 1973 Jul 28;313(2):372–389. doi: 10.1016/0304-4165(73)90037-8. [DOI] [PubMed] [Google Scholar]
- Reichert L. E., Jr, Bhalla V. K. Development of a radioligand tissue receptor assay for human follicle-stimulating hormone. Endocrinology. 1974 Feb;94(2):483–491. doi: 10.1210/endo-94-2-483. [DOI] [PubMed] [Google Scholar]
- Rubalcava B., Rodbell M. The role of acidic phospholipids in glucagon action on rat liver adenylate cyclase. J Biol Chem. 1973 Jun 10;248(11):3831–3837. [PubMed] [Google Scholar]
- Schorr I., Rathnam P., Saxena B. B., Ney R. L. Multiple specific hormone receptors in the adenylate cyclase of an adrenocortical carcinoma. J Biol Chem. 1971 Sep 25;246(18):5806–5811. [PubMed] [Google Scholar]
- Steinberger E. Hormonal control of mammalian spermatogenesis. Physiol Rev. 1971 Jan;51(1):1–22. doi: 10.1152/physrev.1971.51.1.1. [DOI] [PubMed] [Google Scholar]
- Tsuruhara T., Van Hall E. V., Dufau M. L., Catt K. J. Ovarian binding of intact and desialytated hcg in vivo and in vitro. Endocrinology. 1972 Aug;91(2):463–469. doi: 10.1210/endo-91-2-463. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J. L., Sherins R., Ross G. T., Hickman J., Ashwell G. A method for the preparation of radioactive FSH with preservation of biologic activity. Endocrinology. 1971 Dec;89(6):1356–1360. doi: 10.1210/endo-89-6-1356. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Tamaoki B. I. Influence of immunization with luteinizing hormone upon the anterior pituitary-gonad system of rats and rabbits, with special reference to histological changes and biosynthesis of luteinizing hormone and steroids. Endocrinology. 1966 Sep;79(3):477–485. doi: 10.1210/endo-79-3-477. [DOI] [PubMed] [Google Scholar]


