Abstract
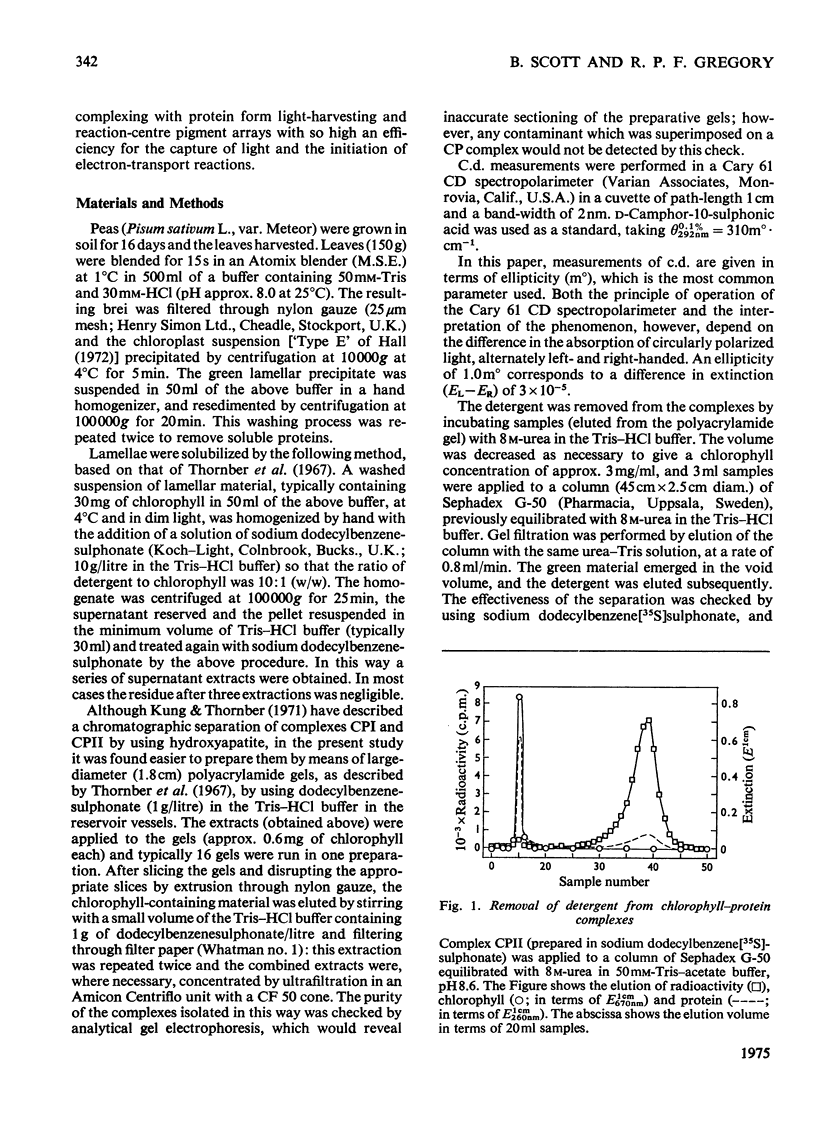
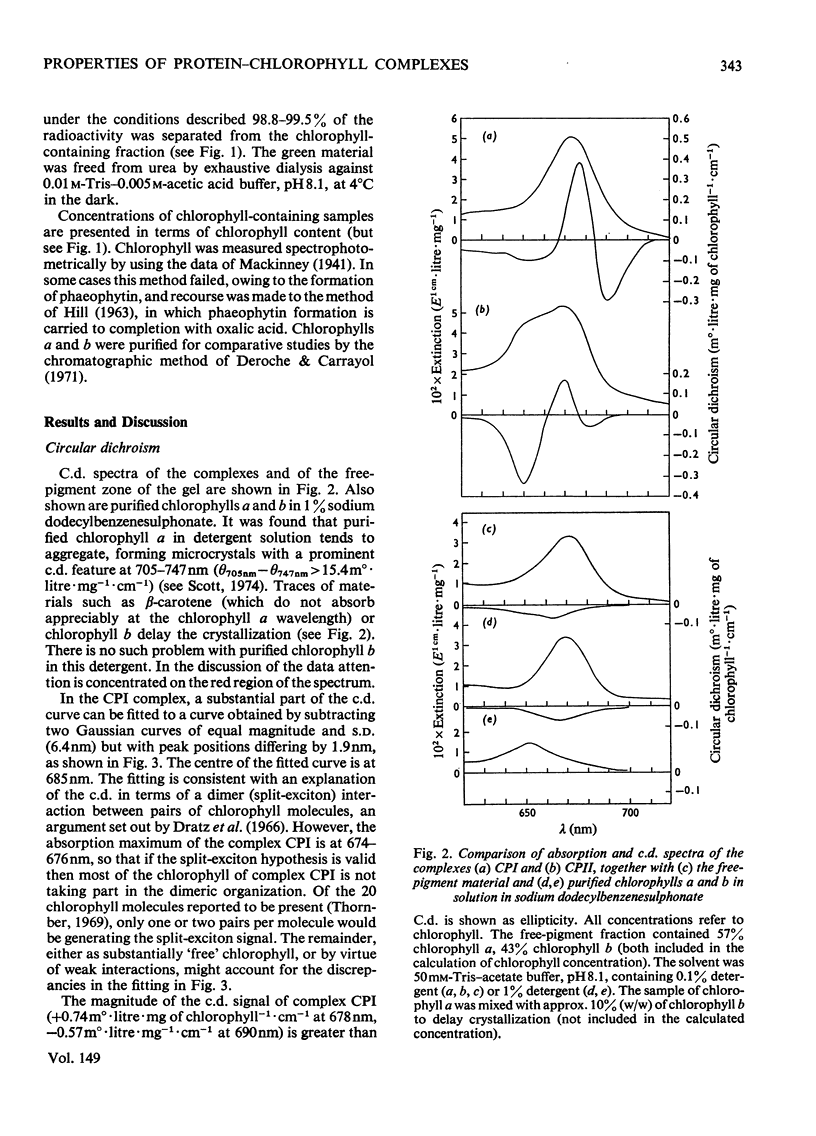
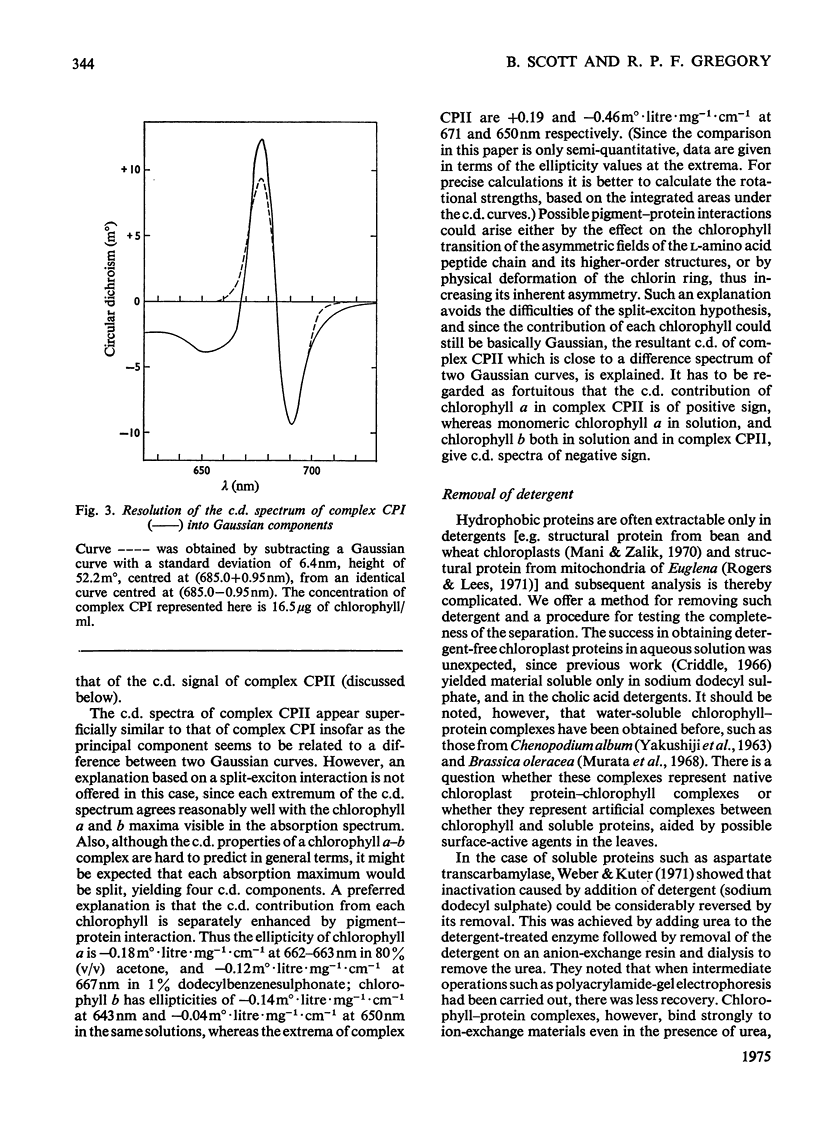
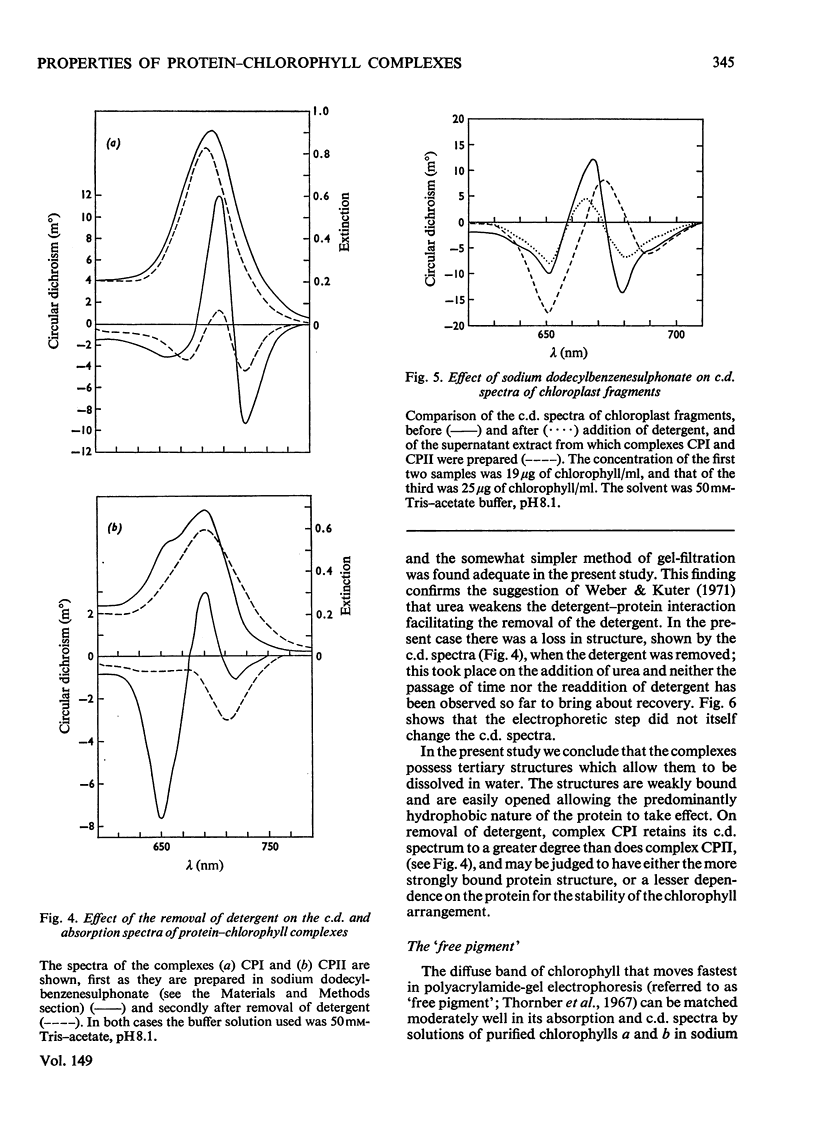
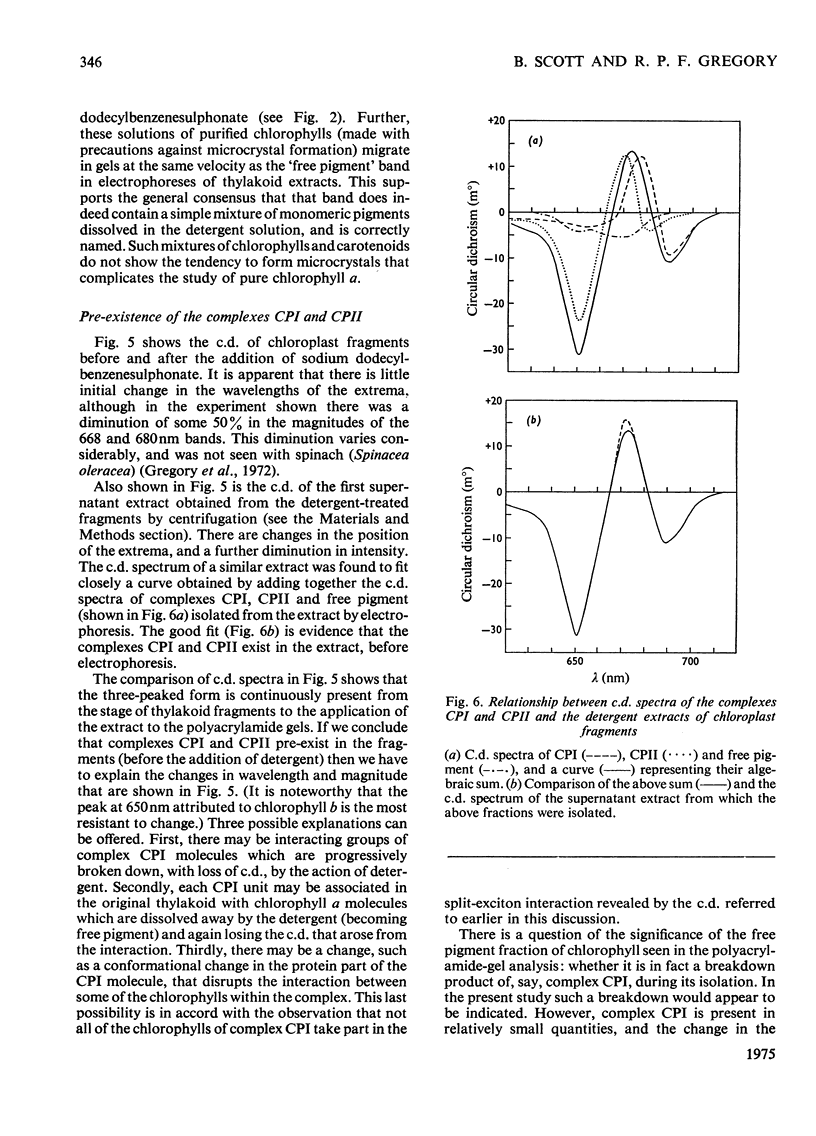
Chlorophyll-protein-detergent complexes were prepared from pea chloroplasts by using sodium dodecylbenzenesulphonate and polyacrylamide-gel electrophoresis. Circular-dichroism spectra showed that complex CPI has a dimeric arrangement of chlorophyll a, with additional weaker interactions. Ellipticities were determined for both complexes and for purified chlorophylls in solution, and it is argued that the circular dichroism of complex CPII is derived from chlorophyll-protein interaction rather than from interaction between chlorophylls a and b. The detergent could be removed from the complexes by using urea and gel filtration, leaving the chlorophyll-protein in solution, although in each case a diminished ellipticity indicated some loss of organization. Three-peaked circular-dichroism spectra of chloroplast fragments before and after addition of detergent were compared with a curve obtained by summing graphically the spectra of complexes CPI, CPII and the free-pigment fraction. There was good correspondence at 650 nm, and the longer-wavelength peaks agreed in form and magnitude, but with discrepancies in position. It was concluded that complexes CPI and CPII pre-exist in the original material, but that there is an environmental effect which is destroyed when the complexes are extracted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dietrich W. E., Jr, Thornber J. P. The P700-chlorophyll -protein of a blue-green alga. Biochim Biophys Acta. 1971 Sep 7;245(2):482–493. doi: 10.1016/0005-2728(71)90164-2. [DOI] [PubMed] [Google Scholar]
- Dratz E. A., Schultz A. J., Sauer K. Chlorophyll-chlorophyll interactions. Brookhaven Symp Biol. 1966;19:303–318. [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P. Photosystem I and II chlorophyll-protein complexes of higher plant chloroplasts. Biochim Biophys Acta. 1971 Nov 2;253(1):285–289. doi: 10.1016/0005-2728(71)90255-6. [DOI] [PubMed] [Google Scholar]
- Mani R. S., Zalik S. Physiochemical studies of bean and wheat chloroplast structural protein. Biochim Biophys Acta. 1970 Jan 20;200(1):132–137. doi: 10.1016/0005-2795(70)90051-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Obata F., Shibata K. Two pigment proteins in spinach chloroplasts. Biochim Biophys Acta. 1966 Feb 7;112(2):223–234. doi: 10.1016/0926-6585(66)90323-2. [DOI] [PubMed] [Google Scholar]
- Thornber J. P. Comparison of a chlorophyll a- protein complex isolated from a blue-green alga with chlorophyll-protein complexes obtained from green bacteria and higher plants. Biochim Biophys Acta. 1969 Feb 25;172(2):230–241. doi: 10.1016/0005-2728(69)90066-8. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Gregory R. P., Smith C. A., Bailey J. L. Studies on the nature of the chloroplast lamella. I. Preparation and some properties of two chlorophyll-protein complexes. Biochemistry. 1967 Feb;6(2):391–396. doi: 10.1021/bi00854a004. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- YAKUSHIJI E., UCHINO K., SUGIMURA Y., SHIRATORI I., TAKAMIYA F. ISOLATION OF WATER-SOLUBLE CHLOROPHYLL PROTEIN FROM THE LEAVES OF CHENOPODIUM ALBUM. Biochim Biophys Acta. 1963 Nov 29;75:293–298. doi: 10.1016/0006-3002(63)90615-2. [DOI] [PubMed] [Google Scholar]


