Abstract
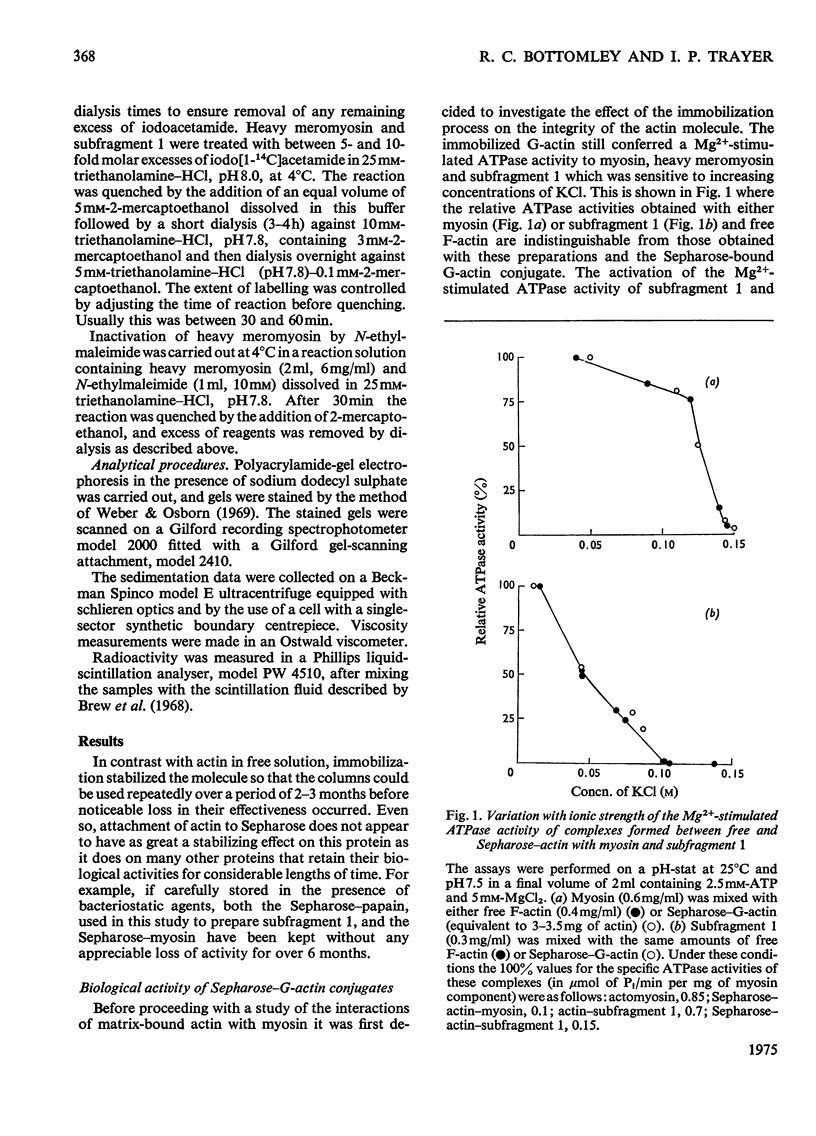
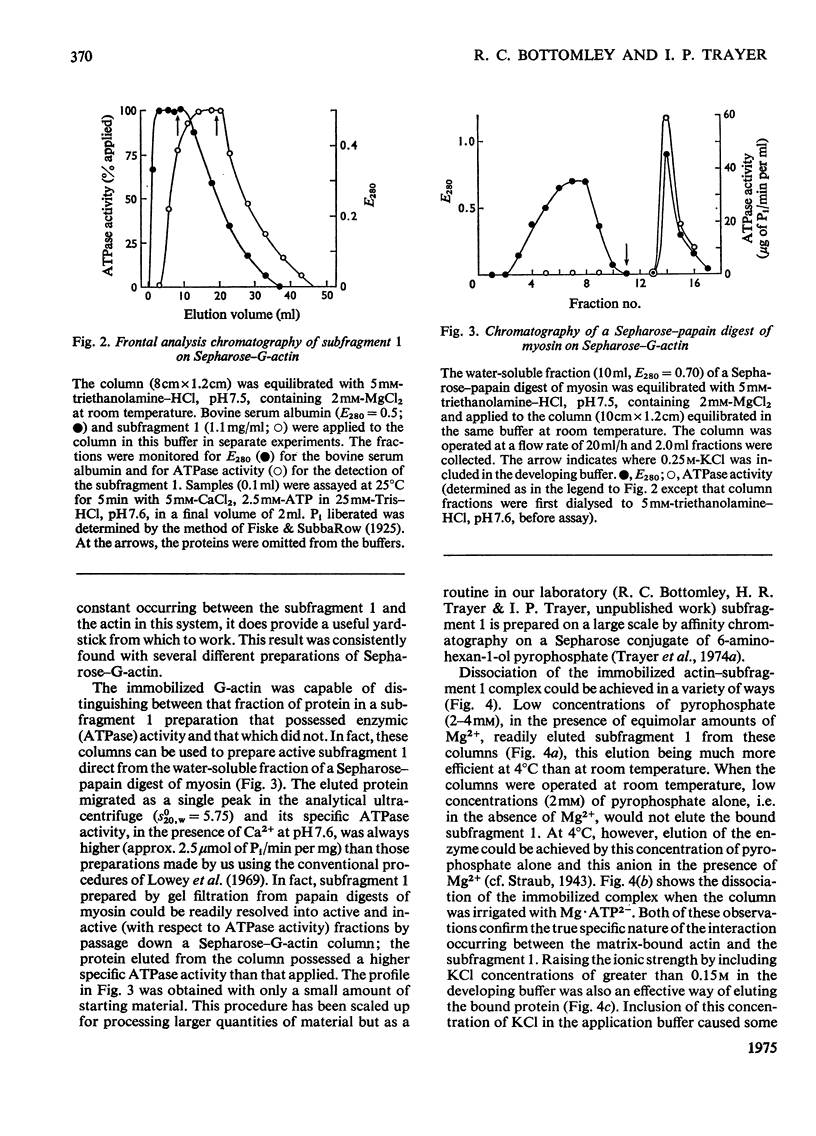
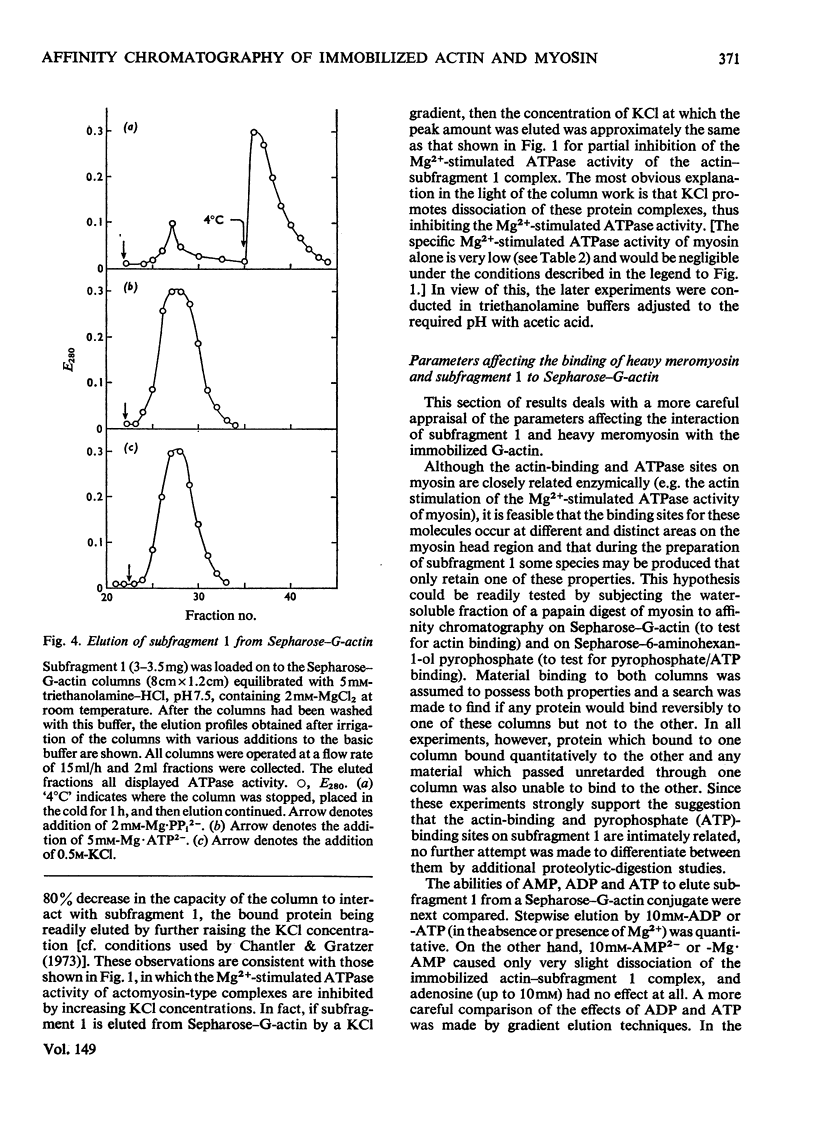
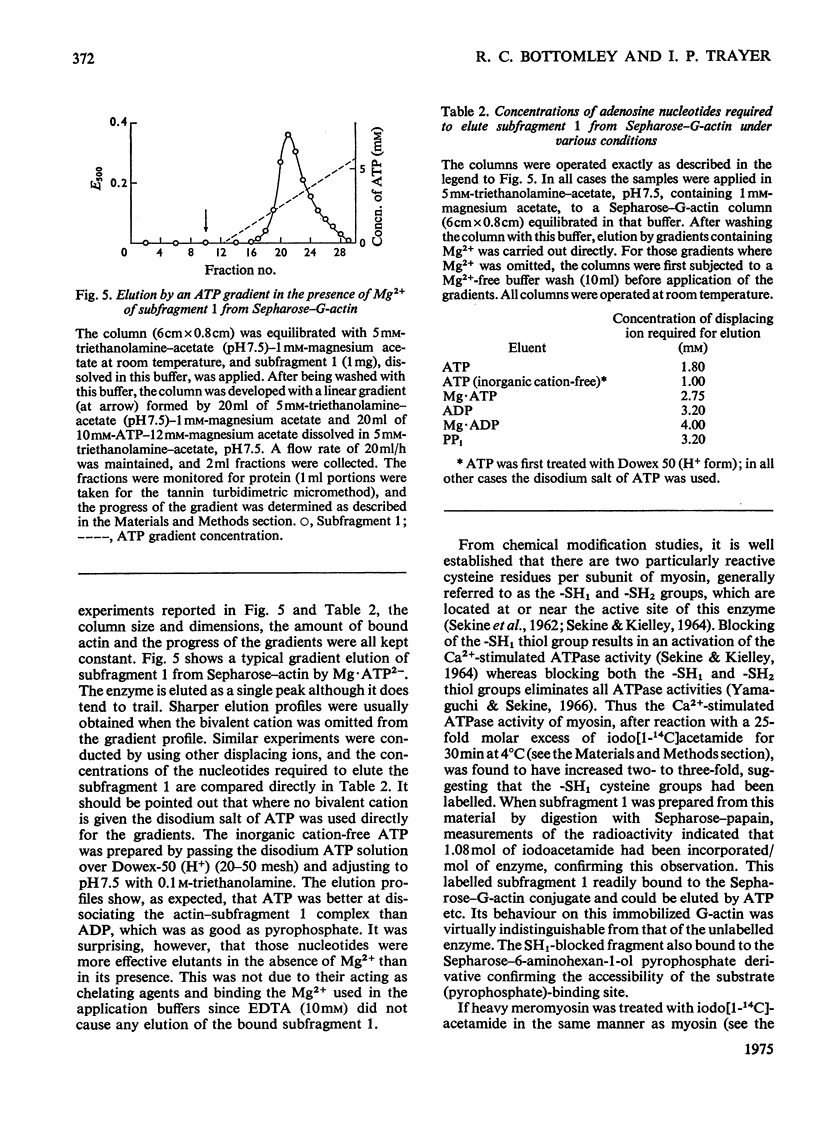
Actin and myosin were immobilized by coupling them to agarose matrices. Both immobilized G-actin and immobilized myosin retain most of the properties of the proteins in free solution and are reliable over long periods of time. Sepharose-F-actin, under the conditions used in this study, has proved unstable and variable in its properties. Sepharose-G-actin columns were used to bind heavy meromyosin and myosin subfragment 1 specifically and reversibly. The interaction involved is sensitive to variation in ionic strength, such that myosin itself is not retained by the columns at the high salt concentration required for its complete solubilization. Myosin, rendered soluble at low ionic strength by polyalanylation, will interact successfully with the immobilized actin. The latter can distinguish between active and inactive fractions of the proteolytic and polyalanyl myosin derivatives, and was used in the preparation of these molecules. The complexes formed between the myosin derivatives and Sepharose-G-actin can be dissociated by low concentrations of ATP, ADP and pyrophosphate in both the presence and the absence of Mg2+. The G-actin columns were used to evaluate the results of chemical modifications of myosin subfragments on their interactions with actin. F-Actin in free solution is bound specifically and reversibly to columns of insolubilized myosin. Thus, with elution by either ATP or pyrophosphate, actin has been purified in one step from extracts of acetone-dried muscle powder.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- BARANY M., BARANY K., GUBA F. Preparation of actin without extraction of myosin. Nature. 1957 Apr 20;179(4564):818–819. doi: 10.1038/179818b0. [DOI] [PubMed] [Google Scholar]
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. The role of alpha-lactalbumin and the A protein in lactose synthetase: a unique mechanism for the control of a biological reaction. Proc Natl Acad Sci U S A. 1968 Feb;59(2):491–497. doi: 10.1073/pnas.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodelius P., Mosbach K. Separation of the isoenzymes of lactate dehydrogenase by affinity chromatography using an immobilized AMP-analogue. FEBS Lett. 1973 Sep 15;35(2):223–226. doi: 10.1016/0014-5793(73)80290-x. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Gratzer W. B. Interaction of myosin with monomeric matrix-bound actin. FEBS Lett. 1973 Aug 1;34(1):10–14. doi: 10.1016/0014-5793(73)80691-x. [DOI] [PubMed] [Google Scholar]
- Cooke R., Morales M. F. Interaction of globular actin with myosin subfragments. J Mol Biol. 1971 Sep 14;60(2):249–261. doi: 10.1016/0022-2836(71)90291-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography of macromolecules. Adv Enzymol Relat Areas Mol Biol. 1972;36:29–89. doi: 10.1002/9780470122815.ch2. [DOI] [PubMed] [Google Scholar]
- Edelman I. S., Hoffer E., Bauminger S., Sela M. Preparation and enzymic characteristics of poly-DL-alanyl myosin. Arch Biochem Biophys. 1968 Feb;123(2):211–221. doi: 10.1016/0003-9861(68)90126-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Dobkin L., Kielley W. W. Binding of actin to heavy meromyosin in the absence of adenosine triphosphate. Biochemistry. 1972 Dec 5;11(25):4657–4660. doi: 10.1021/bi00775a003. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Moos C. Actin activation of heavy meromyosin adenosine triphosphatase. Dependence on adenosine triphosphate and actin concentrations. J Biol Chem. 1970 May 10;245(9):2451–2456. [PubMed] [Google Scholar]
- Eisenberg E., Moos C. The adenosine triphosphatase activity of acto-heavy meromyosin. A kinetic analysis of actin activation. Biochemistry. 1968 Apr;7(4):1486–1489. doi: 10.1021/bi00844a035. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Moos C. The interaction of actin with myosin and heavy meromyosin in solution at low ionic strength. J Biol Chem. 1967 Jun 25;242(12):2945–2951. [PubMed] [Google Scholar]
- Kikuchi M., Noda H., Maruyama K. Interaction of actin with H-meromyosin at low ionic strength. J Biochem. 1969 Jun;65(6):945–952. doi: 10.1093/oxfordjournals.jbchem.a129099. [DOI] [PubMed] [Google Scholar]
- Kondo H., Hayashi H., Mihashi K. Preparation of F-actin: Sepharose 4B matrix. J Biochem. 1972 Sep;72(3):759–760. doi: 10.1093/oxfordjournals.jbchem.a129954. [DOI] [PubMed] [Google Scholar]
- Liu-Osheroff P., Guillory R. J. The enzymic properties of a modified ox heart myosin adenosine triphosphatase on covalent binding to an insoluble cellulose matrix. Biochem J. 1972 Apr;127(2):419–424. doi: 10.1042/bj1270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Actin activation of the myosin ATPase: a kinetic analysis. J Theor Biol. 1974 Feb;43(2):313–328. doi: 10.1016/s0022-5193(74)80063-9. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Transient state phosphate production in the hydrolysis of nucleoside triphosphates by myosin. Biochemistry. 1970 Jul 21;9(15):2975–2983. doi: 10.1021/bi00817a007. [DOI] [PubMed] [Google Scholar]
- Malik M. N., Martonosi A. The regulation of the rate of ATP hydrolysis by H-meromyosin. Arch Biochem Biophys. 1972 Sep;152(1):243–257. doi: 10.1016/0003-9861(72)90212-3. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Barry S. Affinity chromatography of lactate dehydrogenase Model studies demonstrating the potential of the technique in the mechanistic investigation as well as in the purification of multi-substrate enzymes. FEBS Lett. 1972 Apr 1;21(3):281–285. doi: 10.1016/0014-5793(72)80183-2. [DOI] [PubMed] [Google Scholar]
- Offer G., Baker H., Baker L. Interaction of monomeric and polymeric actin with myosin subfragment 1. J Mol Biol. 1972 May 28;66(3):435–444. doi: 10.1016/0022-2836(72)90425-1. [DOI] [PubMed] [Google Scholar]
- Perry S. V. The structure and interactions of myosin. Prog Biophys Mol Biol. 1967;17:325–381. doi: 10.1016/0079-6107(67)90010-7. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Harrington W. F. Cooperative role of two sulfhydryl groups in myosin adenosine triphosphatase. Biochemistry. 1974 May 7;13(10):2014–2022. doi: 10.1021/bi00707a003. [DOI] [PubMed] [Google Scholar]
- SEKINE T., BARNETT L. M., KIELLEY W. W. The active site of myosin adenosine triphosphatase. I. Localization of one of the sulfhydryl groups. J Biol Chem. 1962 Sep;237:2769–2772. [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G. Meromyosins, the subunits of myosin. Arch Biochem Biophys. 1953 Feb;42(2):305–320. doi: 10.1016/0003-9861(53)90360-9. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Gergely J. Electron spin resonance of myosin spin labeled at the S1 thiol groups during hydrolysis of adenosine triphosphate. Arch Biochem Biophys. 1973 Oct;158(2):853–863. doi: 10.1016/0003-9861(73)90581-x. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. The effects of actin on the electron spin resonance of spin-labeled myosin. Arch Biochem Biophys. 1973 Aug;157(2):588–596. doi: 10.1016/0003-9861(73)90678-4. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Syska H., Perry S. V., Trayer I. P. A new method of preparation of troponin I (inhibitory protein) using affinity chromatography. Evidence for three different forms of troponin I in striated muscle. FEBS Lett. 1974 Apr 1;40(2):253–257. doi: 10.1016/0014-5793(74)80238-3. [DOI] [PubMed] [Google Scholar]
- Tawada K., Oosawa F. Activation of H-meromyosin ATPase by polymers of actin and carboxymethylated actin. J Mol Biol. 1969 Sep 14;44(2):309–317. doi: 10.1016/0022-2836(69)90177-6. [DOI] [PubMed] [Google Scholar]
- Tawada K., Oosawa F. Effect of the H-meromyosin plus ATP system on F-actin. Biochim Biophys Acta. 1969 May;180(1):199–201. doi: 10.1016/0005-2728(69)90209-6. [DOI] [PubMed] [Google Scholar]
- Taylor E. W., Lymn R. W., Moll G. Myosin-product complex and its effect on the steady-state rate of nucleoside triphosphate hydrolysis. Biochemistry. 1970 Jul 21;9(15):2984–2991. doi: 10.1021/bi00817a008. [DOI] [PubMed] [Google Scholar]
- Tokiwa T. EPR spectral observations on the binding of ATP and F-actin to spin-labeled myosin. Biochem Biophys Res Commun. 1971 Jul 16;44(2):471–476. doi: 10.1016/0006-291x(71)90625-5. [DOI] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R. Affinity chromatography of nicotinamide nucleotide-dependent dehydrogenases on immobilized nucleotide derivatives. Biochem J. 1974 Sep;141(3):775–787. doi: 10.1042/bj1410775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R., Small D. P., Bottomley R. C. Preparation of adenosine nucleotide derivatives suitable for affinity chromatography. Biochem J. 1974 Jun;139(3):609–623. doi: 10.1042/bj1390609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson J. M., Perry S. V., Cole H. A., Trayer I. P. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972 Mar;127(1):215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Sekine T. Sulfhydryl groups involved in the active site of myosin A adenosine triphosphatase. I. Specific blocking of the SH group responsible for the inhibitory phase in "B phasic response" of the catalytic activity. J Biochem. 1966 Jan;59(1):24–33. doi: 10.1093/oxfordjournals.jbchem.a128254. [DOI] [PubMed] [Google Scholar]


