Abstract
There has been a marked increase in interest in high-temperature superconductors over the past few years, sparked by their potential to revolutionize multiple fields, including energy generation and transportation. A particularly promising avenue of exploration has emerged in the form of ternary superhydrides, compounds composed of hydrogen along with two other rare-earth elements. Our investigation focuses on the search for Y–Th–H ternary compounds; employing an evolutionary search methodology complemented by electron–phonon calculations reveals a stable superhydride, P6̅m2-YThH18, capable of exhibiting a critical temperature (Tc) as high as 222 K at 200 GPa along a few low-Tc novel hydrides. Our analysis explores the possibility of alloyed structure formation from the disordered condition of Th-doped YH9 and establishes that the P6̅m2-YThH18 is indeed a structurally ordered structure. This opens up an exciting avenue for research on multinary superhydrides, which could facilitate experimental synthesis and provides potential implications for high-temperature superconductivity research.
Introduction
Wigner and Huntington1theoretically predicted hydrogen to be metallic above 400 GPa and exhibit superconductivity, but such extreme pressures are experimentally unreachable even today. In 2004, Ashcroft2 argued that the incorporation of other metallic elements into the hydrogen system could significantly reduce the transition pressure. This realization prompted investigations into the possibility of high-temperature (high-Tc) superconductivity in metal hydrides, leading to the discovery of high-Tc superconductors, H3S (Tc ∼ 203 K at P = 150 GPa),3 YH10 (with Tc > 300 K at P ∼ 250 GPa),4 and LaH10 (Tc ∼ 280 K at P ∼ 200 GPa).4−7 Note that these discoveries were initially predicted using an evolutionary algorithm coupled with ab initio enthalpy calculations, followed by experimental confirmations.3,6−8 Recently, several binary hydrides have actually synthesized,6,7,9−12 which, in turn, have sparked extensive theoretical works into nearly all possible binary hydrides.13−15
Recently, a promising avenue has emerged in the field of hydride superconductors through the combination of binary hydrides to form ternary hydrides for their potential to allow for more prototypical superhydrides due to expanding combinatorial space in ternary composition.16 This development has led to the discovery of high-Tc ternary hydrides,17−34 some of which have the potential to exhibit higher Tc values at lower P compared to their binary counterparts. For example, Semenok et al.33 demonstrated experimentally that the unstable binary hydrides such as YH10 and LaH6 in their pure forms may be stabilized into combined ternary hydrides at a relatively lower P. Additionally, the synthesis of the La–Ce–H-based ternary hydride (La, Ce)H929 has demonstrated a substantial increase in Tc by 50–80 K, compared to binary CeH9. This enhancement was attributed to the doping effect of La within the Ce lattice, changing the electronic structure and Fermi surface topology and therefore increasing Tc. Similarly, it has been found experimentally and theoretically that the (La, Ce)H9,10 ternary hydrides are superior to their parent binaries.30 Currently, significant efforts are being directed toward discovering high-Tc superconductivity in hydrides at more moderate or ambient pressure conditions. In this vein, recent trends, reporting superconducting temperatures above 100 K at pressures below 100 GPa, suggest that ternary hydrides could offer a promising route to achieving this goal.20,27,35,36 Unlike the binary hydrides, however, an exhaustive exploration of ternary (or more) hydrogen-rich hydrides seem impossible because their exploration space gets expanded combinatorially. Although the expanded space increases the probability of finding novel high-Tc hydrides, it also becomes a bottleneck to screen potential thermodynamically stable and high-Tc hydrides even for ternary phases. Thus, the selection of two binaries for ternary candidates usually relies on heuristic strategies: (i) thermodynamically stable and potentially high-Tc binary compounds37 and (ii) their similarity in electronegativity and atomic size of metals in binary hydrides.38,39 In this context, we have explored new high-Tc superconductors within the Y–Th–H ternary hydrides employing the genetic algorithm combined with ab initio simulations. We discovered that a hydrogen-rich ternary P6̅m2-YThH18, exhibits a high Tc value of up to 222 K at 200 GPa, alongside two low-Tc hydrides, which can be interpreted as a a consequence of combining the recently synthesized P63/mmc-YH9 (∼243 at 201 K)11 and P63/mmc-ThH9 (146 K at 179 GPa)9 systems.
Even if the selection of the two binary hydrides successfully predict a high-Tc ternary hydride candidate, (iii) the third issue generally emerges for ternary (or more complex) hydrogen-rich hydrides, i.e., the structural degree of freedom of two (or more) different metal arrangements. It is widely recognized that elements sharing similar properties such as atomic radii, electronegativity, and electronic configuration typically exhibit a higher propensity for disordered substitutional alloy formation due to its higher configurational entropy.40−43 In this vein, recent works43−46 suggest that multiple predicted ternary phases could have more stable, disordered counterparts. This seems to hold even for our Y–Th–H systems because Y and Th atoms have similar physical characteristics (electronegativities of 1.3 and 1.22 on the Pauling scale, respectively, and identical atomic radii of 1.80 Å). This is generally important in multicomponent/high-entropy compounds at higher temperatures.47,48 Even for multicomponent compounds at higher temperatures, however, ordered structures overcome disordered ones owing to their overwhelming enthalpies arising from their interactions.49 The present study investigates the order–disorder-competition in the predicted high-Tc ternary phase (P6̅m2-YThH18), considering the configurational entropy in mixing the Y–H and Th–H building blocks.
The paper begins with computational details explaining our structure search, Tc evaluations, and structural modeling for investigating the order–disorder competition. The result section exhibits the outcomes of new phases predicted by the evolutionary algorithms, their convex-hull phase diagram analysis taking into account configurational entropy effects, superconducting properties, including the electron–phonon interaction. We then discuss how the method/results can provide a basis for predicting and investigating the superconducting property. Finally, we wrap up with the conclusion and acknowledgment sections.
Computational Details
This study aimed to investigate the existence of stable ternary hydrides with the stoichiometry (YHn)x(ThHn)y, where x and y are positive integers, and n = 4, 6, 9. Note that binary hydrides involving Yttrium (YH3, YH6, YH9)11,12,50 and Thorium (ThH4, ThH6, ThH10)9,51,52 have already demonstrated excellent high-Tc properties. This suggests a strong potential for the formation of stable ternary hydrides through the combination of Y and Th binary systems. Candidate structures for the (YHn)x(ThHn)y stoichiometry were explored using the variable-composition evolutionary algorithm implemented in the USPEX (Universal Structure Predictor: Evolutionary Xtallography)53,54 software; the USPEX generated 400 random structures for the given stoichiometry; the evolutionary algorithm was applied to 100 randomly selected structures from the 400 generated structures, and these structures were allowed to evolve with 40% heredity, 40% randomness, 10% soft mutation, and 10% transmutation in every subsequent generation. The search was set to terminate after 20 generations if no new viable candidate structure was detected.
During the structural search, electronic total energies at a given pressure were computed at the level of density functional theory (DFT) with the GGA-PBE exchange-correlation functional.55 Applied pressures were greater than 100 GPa up to 300 GPa, as both parent binaries are stabilized above 150 GPa. The calculations were conducted using the Vienna Ab-initio Simulation Package (VASP)56 with projector augmented wave (PAW)57 scheme. A plane-wave basis set cutoff energy of 600 eV was used, and the Brillouin zone was sampled with a k-point resolution of 2π × 0.03 Å–1. Full geometry optimization and enthalpy evaluation were performed for all candidate structures at the GGA-PBE level of theory, resulting in the convex-hull diagram used to obtain thermodynamically stable structures. The criteria for the convergence of the self-consistent field (SCF) and maximum force were set to 0.1 meV/atom. The structures were visualized using the VESTA 3 package.58 We performed first-principles phonon and electron–phonon coupling (EPC) calculations as implemented in the QUANTUM ESPRESSO (QE)59 package with a PAW pseudopotential57 at the GGA-PBE level. An energy cutoff of 90 and 700 Ry were employed for wave function and charge density, respectively. The q-point mesh in the Brillouin zone was set to 4 × 4 × 4, and the k-point mesh for the integral of the EPC constant and Tc was set to 16 × 16 × 16.
We applied both (1) the McMillan60 and (2) the Allen-Dynes (AD) modified McMillan formulas61 to evaluate Tc only for phases predicted as being stable thermodynamically and dynamically:
(1) The McMillan formula for the Tc value is given as
| 1 |
where ωD and μ* are respectively the Debye frequency and effective Coulomb pseudopotential that is taken as both 0.1 and 0.13 for hydrides. The electron–phonon coupling (EPC) constant (λ) is computed by
| 2 |
where the electron–phonon spectral function, α2F(ω), is given as
| 3 |
where N(εF) represents the density of states at the Fermi level, while γqν represents the phonon line width, ωqν is the phonon frequency for wave vector q and mode ν, and ℏ is the reduced Planck’s constant.
(2) The AD modified McMillan formulas is given as
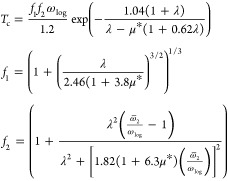 |
4 |
where f1 and f2 are λ/μ*/ωlog/ω̅2-dependent parameters; the logarithmic average frequency (ωlog) and the mean square frequency (ω̅2) are expressed in terms of phonon frequency (ω)
| 5 |
| 6 |
The present study focuses on the order–disorder competition which arises from the structural degree of freedom for atomic arrangements beyond the binary. To investigate this, a cost-effective structural modeling is critical. To construct the disordered solid solution structure of YxTh1–xH9, we employed the special quasi-random structures (SQS) method, as implemented in the sqsgen tool.62 This method optimizes the Warren-Cowley short-range-order (WC-SRO) parameters63,64 to ensure a random distribution of Y and Th atoms within the lattice, identifying an optimal disordered configuration. To accommodate the random occupation of Y and Th atoms across 16 lattice sites, we extended the standard unit cell of one of the parent structures (P63/mmc-YH9) to a 2 × 2 × 2 supercell, equivalent to 16 YxTh1–xH9 formula units. With increasing temperature (T), the configurational entropy of mixing (Sconf) can lower the Gibbs free energy (G) as described by G = H – TSconf. Equal molar ratios of atoms Y and Th in YThH18 configuration suggests the maximum configuration entropy in its disordered form, which can be understood from the following relation
| 7 |
where W represents the degree of disorder, kB is the Boltzmann constant, and R and N are the gas constant and number of elements (N = 2 for Y and Th elements) leading to a disordered phase, respectively.
Results
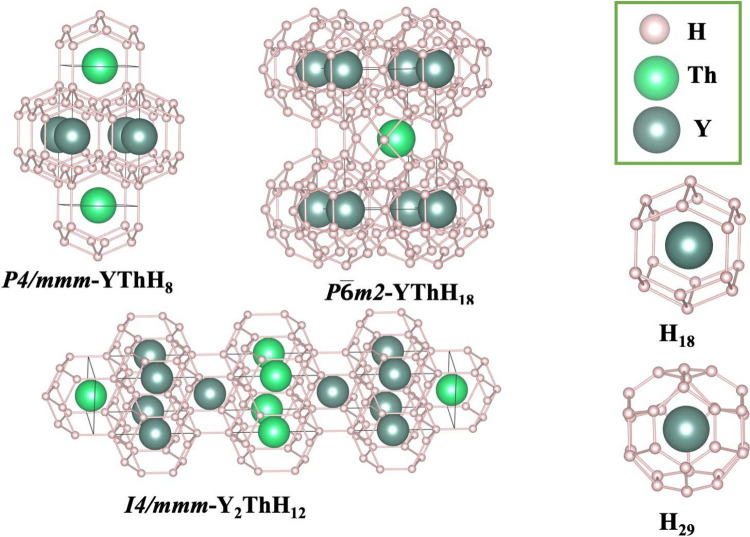
Our newly predicted stable structures for Y–Th–H ternary hydrides and their superconducting properties are given in Figure 2 and in Table 1, respectively. Our key findings reveal three stable structures at P = 200 GPa: (1) P4/mmm-YThH8, (2) I4/mmm-Y2ThH12, and (3) P6̅m2-YThH18. Among these, the P6̅m2-phase is a high-Tc hydride, exhibiting a transition temperature as high as 222 K at 200 GPa. While P4/mmm-YThH8 and I4/mmm-Y2ThH12 remain stable up to 300 GPa, they show low superconductivity (below 30 K). Therefore, the subsequent discussion will primarily focus on the promising high-TcP6̅m2-YThH18.
Figure 2.
Predicted stable hydrides. P4/mmm-YThH8 and I4/mmm-Y2ThH12 share H18 cages, while P6̅m2-YThH18 demonstrates H29 cages.
Table 1. Two Computed Tc Values at Certain Pressures for Newly Predicted Phases, along with Relevant Parameters (Electron–Phonon Coupling Constant, λ, Logarithmic Average Frequency, ωlog, and Root Mean Squared Phonon Frequency ω̅2)a.
| Tc(McM) | Tc(AD) | |||||
|---|---|---|---|---|---|---|
| System | P [GPa] | λ | ω̅2 | ωlog [K] | μ* (0.1–0.13) | μ* (0.1–0.13) |
| P6̅m2-YThH18 | 200 | 2.54 | 27.84 | 1041.5 | 173–164 | 222–205 |
| P4/mmm-YThH8 | 200 | 0.50 | 39.97 | 1351.4 | 16–10 | 17–10 |
| P4/mmm-YThH8 | 300 | 0.41 | 43.53 | 1317.4 | 6–3 | 6–3 |
| I4/mmm-Y2ThH12 | 200 | 0.60 | 37.42 | 1169.4 | 26–18 | 27–19 |
| I4/mmm-Y2ThH12 | 300 | 0.49 | 42.87 | 1356.8 | 16–9 | 16–10 |
McM and AD stand for McMillan and AD-modified McMillan formulas, respectively. For each formula, the listed Tc values, separated by a dash, correspond to μ* = 0.10–0.13.
Structure prediction and order–disorder competition
Y–H- and Th–H-based binary hydrides, YHx and ThHx (where x takes on values of 3, 4, 6, or 9), have already been investigated, some of which exhibit high Tc around P ∼ 200 GPa. Building on these compounds, we explored various combinations of binaries to form the ternary system Y–Th–H, specifically (YH4)x(ThH4)y, (YH6)x(ThH6)y, and (YH9)x(ThH9)y. The structural stability can be attributed to the dopant element having similar atomic radii and electronic properties, which indicates the stability of the structure against decomposition. Notably, the atomic radii and electronegativity of both elements (Y and Th) are closely matched, providing a strong motivation for our evolutionary search for Y–Th–H systems at pressures ranging from 100 to 300 GPa.
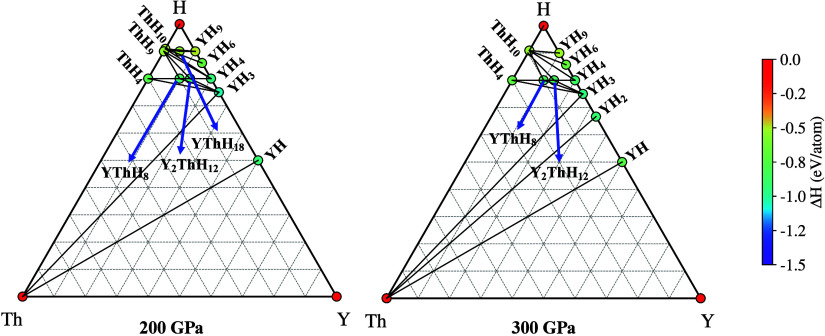
Once we generated novel structures using an evolutionary algorithm for the Y–Th–H chemical system, it was essential to assess their phase stability. Determining the phase stability of ternary hydride involves comparing its energy to that of competing phases. To achieve this, we looked into all the low-energy phases into which the Y–Th–H hydrides could potentially decompose, in addition to their binary (YHx, ThHy) and/or elemental constituents (Y, Th, H). To determine the most stable phases in the system, we employed ternary convex hull phase diagram analysis, the results of which are presented in Figure 1. Evidently, at 100 GPa, no stable phases were identified. However, at 200 GPa, the phase diagram analysis revealed three energetically stable structures: P6̅m2-YThH18, P4/mmm-Y2ThH12, and I4/mmm-YThH8. Lattice parameters of all the obtained hydride phases have been summarized in Supporting Information (Table S1). Upon increasing the pressure to 300 GPa, the P6̅m2-YThH18 phase disappeared from the convex hull diagram. The stable phases exhibit deep hull energies, exceeding −0.53 eV/atom relative to their elemental constituents. This signifies their thermodynamic stability; nevertheless, we can not exclude the possibility of these being disordered alloy structures or metastable phases.
Figure 1.
Ternary convex hull diagram of the Y–Th–H system at 200 and 300 GPa obtained using Pymatgen’s phase analysis module.65 Only the stable phases are shown. Black lines connecting the ternaries indicate the shortest synthesis pathways. Out of three stable compositions YThH8, Y2ThH12, and YThH18 was observed at 200 GPa; the YThH18 phase disappeared at 300 GPa.
The YThH18 composition, characterized
by a higher hydrogen
content composed of H29 cage structures, as shown in Figure 2, indicates its potential as a high-temperature superconductor
candidate. Moreover, it is important to consider that this predicted
stable structure may undergo phase decomposition with varying pressure.
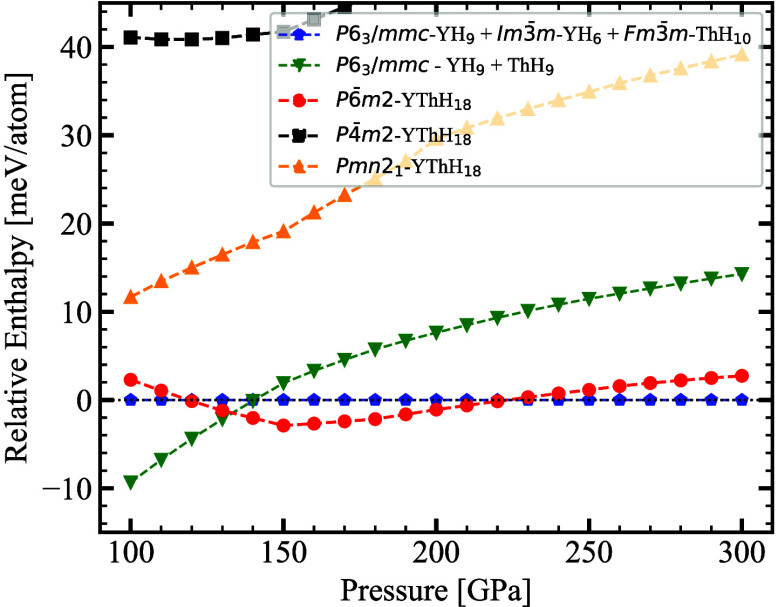
In this line, we analyzed its enthalpies with respect to highly competitive
ternary/binary phases identified in our convex hull analysis across
a pressure range from 100 to 300 GPa, as depicted in Figure 3. Our aim here is to identify
the pressure-dependent phase stability for the highest hydrogen-rich
compound against phase transition. There are two critical points corresponding
to a stability window ranging from 132 to 222 GPa, suggesting decomposition
of P6̅m2-YThH18 into binary hydrides: (1) at 132 GPa, P6̅m2-YThH18 → 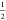 P63/mmc-YH9 +
P63/mmc-YH9 + 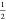 P63/mmc-ThH9, (2) at 222 GPa, P6̅m2-YThH18 →
P63/mmc-ThH9, (2) at 222 GPa, P6̅m2-YThH18 → 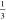 P63/mmc-Y2H18 +
P63/mmc-Y2H18 + 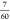 Im3̅m-YH6 +
Im3̅m-YH6 +  Fm3̅m-ThH10. We anticipate that this hydrogen-rich clathrate
hydride could exhibit higher critical temperatures (Tc) due to high-frequency phonon modes mediated by hydrogen
and the strong electron–phonon coupling.3,66,67 The YThH18 phase in the P6̅m2 symmetry exhibits a hydrogen-to-metal
ratio of 9:1, aligning with the expectations of being a promising
high-Tc candidate.
Fm3̅m-ThH10. We anticipate that this hydrogen-rich clathrate
hydride could exhibit higher critical temperatures (Tc) due to high-frequency phonon modes mediated by hydrogen
and the strong electron–phonon coupling.3,66,67 The YThH18 phase in the P6̅m2 symmetry exhibits a hydrogen-to-metal
ratio of 9:1, aligning with the expectations of being a promising
high-Tc candidate.
Figure 3.
Relative enthalpy of the P6̅m2-YThH18 phase compared to other competing phases. Labels in the figure denote the compounds contributing toward total enthalpy. This figure underscores the phase stability against decomposition under different pressure conditions.
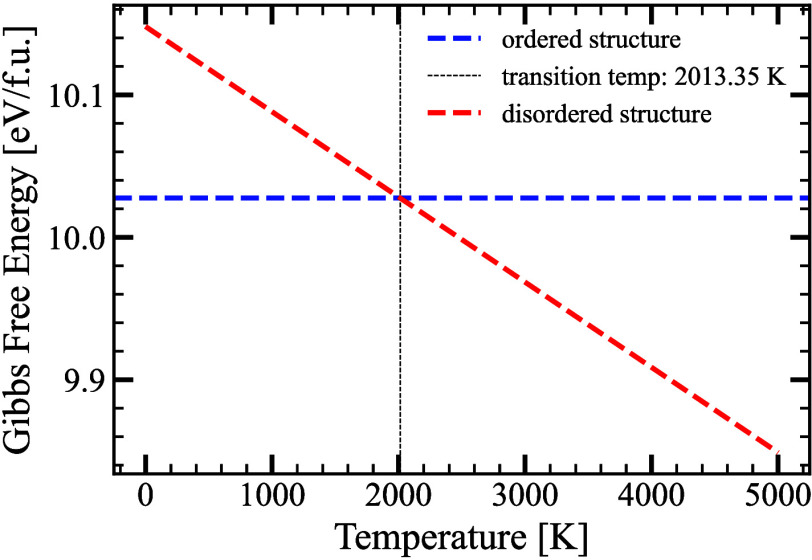
The predicted structure can be thought of as a derived structure resulting from one of its parent binaries, for example, Th-substitution on the site of Y in YH9 binary hydride. Given these observations, we analyzed the possibility that P6̅m2-YThH18 could be an alloy structure, potentially existing in a disordered form of Th-doped YH9. To investigate this phenomenon, we analyzed the stability of the ordered phase compared to the disordered structure. We carried out research on the relative stability between the disordered solid solution and the ordered structure in the case of YxTh1–xH9. To accomplish this, Gibbs free energy has been calculated in a range of temperatures to account for the increasing configuration entropy (Sconf). One can note that the Gibbs free energy follows a decreasing linear trend with temperature given in Figure 4. According to Sconf values computed from eq 7 for both ordered and disordered structures, our analysis reveals that the ordered form of the structure P6̅m2-YThH18 remains stable up to a temperature of 2013 K compared to the disordered structure as demonstrated in Figure 4.
Figure 4.
We depict the change in Gibbs free energy with configurational entropy of both the ordered and disordered structures as temperature increases. The vertical black-dotted line represents the transition temperature from the ordered to the disordered structure.
Dynamic stability and Superconductivity
Before delving into the prediction of superconductivity in a compound, it is important to analyze both its dynamic and thermodynamic stability. Thermodynamic stability pertains to the structural stability in the equilibrium configuration of atoms. At the same time, dynamical stability deals with the resilience of a crystal structure when subjected to small atomic vibrations or perturbations. To assess lattice stability under phonon vibrations, we conducted EPC calculations for all the stable hydrides, as depicted in the phonon dispersion relation in Figure 5 and Figure S2, at both 200 and 300 GPa. A consistent trend emerged across all hydrides, where phonon modes could be categorized into two distinct groups: low-frequency modes associated with Th and Y atoms and high-frequency modes composed mainly of H atoms. This observation aligns with the fact that Th and Y are significantly heavier atoms than H, resulting in phonon modes extending up to 8 THz, while H exhibits high-frequency modes ranging from 10 THz to 60 THz.
Figure 5.
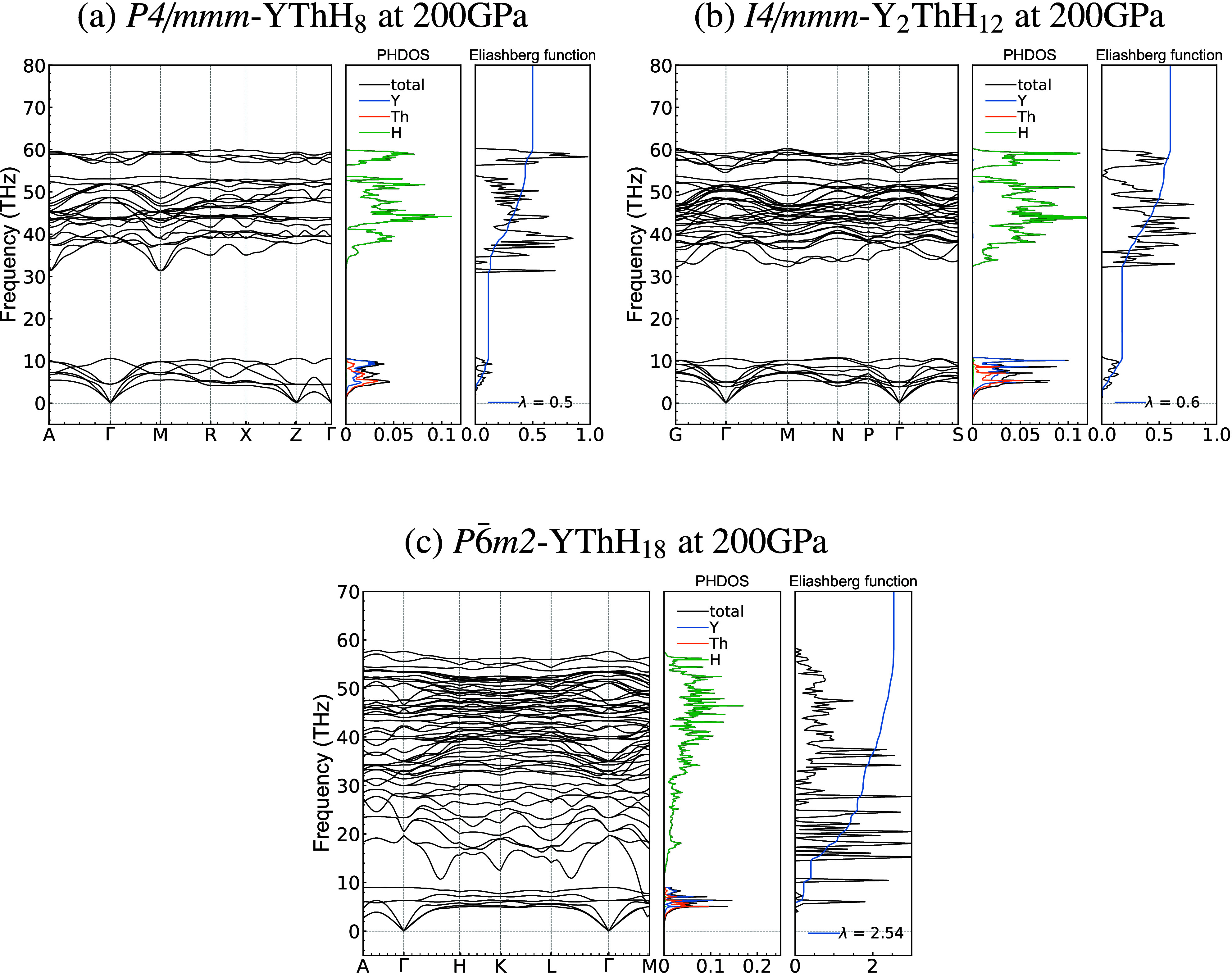
Phonon dispersion, projected phonon density of states (PHDOS), and Eliashberg spectral function α2F(ω) associated with the electron–phonon integral (λ(ω)) for all the stable compounds. No imaginary modes suggest that all the structures, in addition to their thermodynamic stability are dynamically stable.
Specifically, for P6̅m2-YThH18, the logarithmic frequency (ωlog) and the EPC parameter (λ) are 1041.48 K and 2.54, respectively (Figure 5 and Table 1). Notably, the λ value is relatively higher than the reported value for P63/mmc-ThH9, which was 1.73 at 150 GPa.9 Similarly, the λ value is quite high for the P63/mmc-YH9 case,5 reaching 4.42 at 150 GPa. It is essential to note that these λ values may decrease at higher pressures due to phonon hardening.31 The λ value of 2.54 for P6̅m2-YThH18, formed from the combination of the two binary hydrides (ThH9 and YH9), is reasonable when considering the pressure difference (150 GPa for binaries and 200 GPa for the ternary compound) and the synergistic effect resulting from the combination. The remaining predicted hydrides, specifically P4/mmm-YThH8 and I4/mmm-Y2ThH12, at both 200 and 300 GPa, exhibit relatively low λ values, peaking at 0.6 in most cases. The relatively higher λ value for the YThH18 structure, compared to other predicted Y–Th–H compositions can be primarily attributed to a significant contribution of H-modes. This is evident from the Eliashberg spectral function (α2F(ω)), as shown in Figure 5.
We computed the critical temperature (Tc) using both the McMillan formalism (1) and the AD-modified McMillan formula (4), and the results are summarized in Table 1. Typically, a Coulomb pseudopotential (μ*) ranging from 0.1 to 0.13 is selected for such calculations. As listed in Table 1 the calculation for Tc has been performed on both values. In light of these considerations, we can conclude that YThH8 and Y2ThH12 represent low-Tc hydrides, with typical Tc values of 16 and 26 K at 200 GPa, decreasing by 10 K as the pressure is raised to 300 GPa. In contrast, P6̅m2-YThH18 demonstrates a significantly high Tc of 222 K when evaluated using the AD formalism, but this value reduces to 173 K when calculated using McMillan’s formula. This observation positions YThH18 among the ternary superhydrides capable of achieving Tc values exceeding 200 K at pressures not exceeding 200 GPa.
Discussion
The reliability of our genetic algorithm, which is based on the USPEX structure search method to predict structures for the Y–Th–H ternary composition can be assessed by comparing it to recent experimental synthesis efforts guided by structure predictions for Y–H12 and Th–H9 binary hydrides. This study assesses phase stability by examining their potential decomposition into various low-order compositions, in addition to evaluating large hull energies and a wide range of pressure stability. These factors collectively increase the likelihood of guiding successful experimental synthesis. It is important to note that the approximations made in predicting critical temperatures (Tc) using the BCS theory-based Eliashberg models (both AD and McMillan formulations) arise from the harmonic approximation. Moreover, a prior prediction51 using the harmonic approximation with the AD formalism showed good agreement with the synthesis results.9
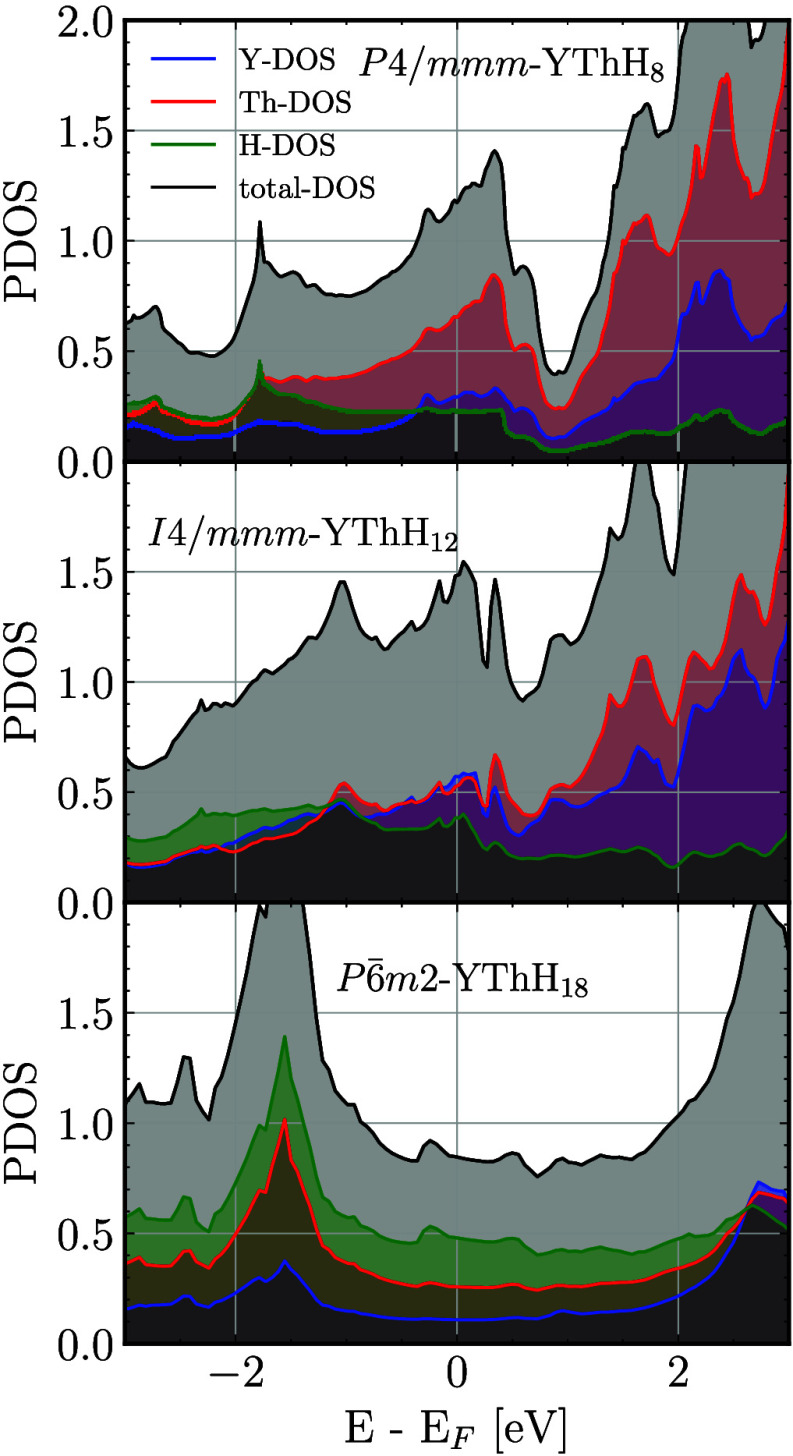
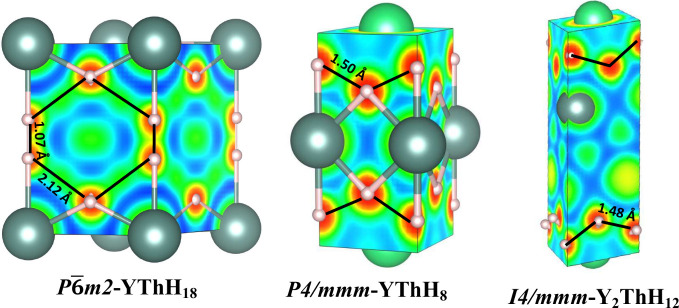
To gain further insights into the high-Tc compounds, it is crucial to quantify the density of states (DOS) derived from hydrogen (H) near the Fermi level, which is a highly sought-after key characteristic of high-Tc hydride compounds.5 As illustrated in Figure 6 and Figure 5 (also in Figure S1), it is evident that YThH18 exhibits the highest H-derived DOS among the compositions considered. This structure also dominates the highest relative DOS attributed to the H element, whereas the other two low-Tc hydrides exhibit a lower H contribution. Apart from the H-derived DOS, the relative hydrogen content in hydride compounds, strongly correlates with superconductivity. For example, in their analysis of over 500 compounds, Wrona et al.68 found that a lower ratio of the mass of metal atoms to the mass of all hydrogen atoms is a strong predictor of high-Tc compounds. Additionally, our analysis of bonding information reveals that the H–H bond lengths exceed 1.06 Å (for reference, a H2 molecule has a bond length of approximately 0.74 Å), which is larger than the H–H distance of 0.98 Å in the high-pressure monatomic phase of metallic hydrogen.69 This suggests the dissociation of H–H molecular bonds. The electron localization function (ELF) values correspond to the lowest H–H bond midpoint exhibiting the highest value of 0.75, indicating that the predicted hydrides imply the presence of weak covalent bonds. It can be seen from Figure 7 that the ELF reduces with increasing bond separation (H–H bonding). Nevertheless, a significant amount of ELF remains at interstitial sites, which, according to chemical template theory,70 helps explain the stability of hydride compounds. This stabilization favors the formation of an H-sublattice over H2 units in the presence of metallic sublattices. Further, Bader charge analysis shows that each Y and Th atom loses 1.48 and 1.5 electrons to H atoms, resulting in an average of 0.33 electrons to every H–H bond in the YThH18 phase. Additionally, rare earth (RE) elements Y and Th can exhibit oxidation states of +1, +2, and +3 and +1, + 2, + 3, and +4, respectively, which can readily allow for electron donation to the H-sublattice. The electrons acquired by the H–H bonds lead to increased H-derived DOS, which are located in the antibonding σ*-orbitals since the bonding σ-orbitals, which have lower energies, are already occupied.71,72 These properties collectively help stabilize the structure and lend an explanation as to why our predicted phase (P6̅m2-YThH18) can allow for the existence of such a high Tc superconducting property.
Figure 6.
H-derived DOS comparison with different hydrides. The superconducting transition temperature Tc agrees with the observed trend in H-dos.
Figure 7.
Electron localization function (ELF) presented in all the predicted structures. The lower ELF between H–H bonds indicates a weak covalent bonding. An isosurface value of 0.5 has been used in these plots.
In experiments, the use of Y–Th alloy as a precursor for the synthesis of Y–Th–H hydrides under high-temperature and high-pressure conditions can facilitate the formation of disordered alloyed ternary hydrides by readily overcoming the energy barrier posed by the enthalpy of formation.45 A recent investigation into the La–Th–H-based ternary hydrides by Song et al.46 demonstrated the possibility of disordered alloy structure formation. This study also suggests that the ordered compositions at ambient conditions gradually stabilize into disordered phases with increasing temperature due to an increase in the configurational entropy of mixing.43 Along the same line of reasoning, our study involves the calculation of configurational entropy to predict the transition temperature for stable ordered ternary hydride to disordered alloy composition. The results indicate that the P6̅m2-YThH18 remains an ordered structure up to a high temperature of over 2000 K. In addition, considering previous studies, where Y-based hydrides33 generally yield higher Tc than those of La-based,46 further highlights the significance of this research. In the end, we propose that the experimental synthesis of P6̅m2-YThH18 can be realized through a usual approach utilizing a diamond anvil cell to produce pressurized hydrides. An alloy of rare-earth metals (Y, Th in the present study) forming the predicted ternary hydrides with NH3BH3 as a hydrogen source can be used as precursors. Samples prepared from these can then be loaded into the diamond anvil cell, applying the required pressure, followed by the application of high temperatures up to several hundred degrees Celsius or higher using resistive heating or laser heating. This allows the starting sample to undergo chemical reactions and structural transformations leading to the formation of ternary hydrides. Such synthesis method has been successfully utilized for several recently discovered superconducting hydrides.26,29,30,33
Conclusion
Our work explores Y–Th–H-based ternary hydrides, a promising avenue for high-temperature superconductivity. By exploiting the chemical similarities of Yttrium and Thorium, and the fact that Y–H and Th–H-based parent binaries have already been synthesized, we identified a novel YThH18 superhydride in the P6̅m2 phase using a combined evolutionary algorithm and electron–phonon calculations. This material exhibits an impressive critical temperature (Tc) of 222 K at 200 GPa, exceeding many known binary hydride superconductors.68 Additionally, analysis suggests the ordered structure of P6̅m2-YThH18 remains stable up to around 2000 K. These findings pave the way for future experimental efforts to synthesize and explore these novel high-temperature superconductors.
Acknowledgments
The computations in the present study were performed using the facilities of the Research Center for Advanced Computing Infrastructure (RCACI) at JAIST. A.G. is thankful for the financial support from the MEXT scholarship provided by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. P.S. is grateful for financial support from Grant-in-Aid for JSPS Research Fellow (Grant No. 22J10527). R.M. is grateful for financial support from MEXT-KAKENHI (JP22H05146, JP21K03400), from the Air Force Office of Scientific Research (AFOSR-AOARD/FA2386-17-1-4049; FA2386-19-1-4015), and from JSPS Bilateral Joint Projects (JPJSBP120197714). K.H. is grateful for financial support from MEXT-KAKENHI, Japan (JP19K05029, JP21K03400, JP22H02170, and JP23H04623), and the Air Force Office of Scientific Research, United States (Award Numbers: FA2386-22-1-4065).
Data Availability Statement
The data supporting the findings of this study are available at Figshare with the following https://doi.org/10.6084/m9.figshare.27020479.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c07199.
Crystallographic information on the predicted crystals (Table S1), electronic band structure and density of state plots (Figure S2), phonon dispersion, projected phonon density of states (PHDOS), and Eliashberg spectral function (Figure S2), and project workflow (PDF)
Author Present Address
⊥ Materials Science and Technology Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831-2008, United States
Author Present Address
# Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, Miyagi 980-8577, Japan
The authors declare no competing financial interest.
Supplementary Material
References
- Wigner E.; Huntington H. B. On the Possibility of a Metallic Modification of Hydrogen. J. Chem. Phys. 1935, 3, 764–770. 10.1063/1.1749590. [DOI] [Google Scholar]
- Ashcroft N. W. Hydrogen Dominant Metallic Alloys: High Temperature Superconductors. Phys. Rev. Lett. 2004, 92, 187002. 10.1103/PhysRevLett.92.187002. [DOI] [PubMed] [Google Scholar]
- Drozdov A. P.; Eremets M. I.; Troyan I. A.; Ksenofontov V.; Shylin S. I. Conventional superconductivity at 203 K at high pressures in the sulfur hydride system. Nature 2015, 525, 73–76. 10.1038/nature14964. [DOI] [PubMed] [Google Scholar]
- Liu H.; Naumov I. I.; Hoffmann R.; Ashcroft N. W.; Hemley R. J. Potential high-Tc superconducting lanthanum and yttrium hydrides at high pressure. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 6990–6995. 10.1073/pnas.1704505114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F.; Sun Y.; Pickard C. J.; Needs R. J.; Wu Q.; Ma Y. Hydrogen Clathrate Structures in Rare Earth Hydrides at High Pressures: Possible Route to Room-Temperature Superconductivity. Phys. Rev. Lett. 2017, 119, 107001. 10.1103/PhysRevLett.119.107001. [DOI] [PubMed] [Google Scholar]
- Drozdov A. P.; Kong P. P.; Minkov V. S.; Besedin S. P.; Kuzovnikov M. A.; Mozaffari S.; Balicas L.; Balakirev F. F.; Graf D. E.; Prakapenka V. B.; Greenberg E.; Knyazev D. A.; Tkacz M.; Eremets M. I. Superconductivity at 250K in lanthanum hydride under high pressures. Nature 2019, 569, 528–531. 10.1038/s41586-019-1201-8. [DOI] [PubMed] [Google Scholar]
- Somayazulu M.; Ahart M.; Mishra A. K.; Geballe Z. M.; Baldini M.; Meng Y.; Struzhkin V. V.; Hemley R. J. Evidence for Superconductivity above 260K in Lanthanum Superhydride at Megabar Pressures. Phys. Rev. Lett. 2019, 122, 027001. 10.1103/PhysRevLett.122.027001. [DOI] [PubMed] [Google Scholar]
- Snider E.; Dasenbrock-Gammon N.; McBride R.; Wang X.; Meyers N.; Lawler K. V.; Zurek E.; Salamat A.; Dias R. P. Synthesis of Yttrium Superhydride Superconductor with a Transition Temperature up to 262 K by Catalytic Hydrogenation at High Pressures. Phys. Rev. Lett. 2021, 126, 117003. 10.1103/PhysRevLett.126.117003. [DOI] [PubMed] [Google Scholar]
- Semenok D. V.; Kvashnin A. G.; Ivanova A. G.; Svitlyk V.; Fominski V. Y.; Sadakov A. V.; Sobolevskiy O. A.; Pudalov V. M.; Troyan I. A.; Oganov A. R. Superconductivity at 161K in thorium hydride ThH10: Synthesis and properties. Mater. Today 2020, 33, 36–44. 10.1016/j.mattod.2019.10.005. [DOI] [Google Scholar]
- Chen W.; Semenok D.; Huang X.; Shu H.; Li X.; Duan D.; Cui T.; Oganov A. High-Temperature Superconducting Phases in Cerium Superhydride with a Tc up to 115K below a Pressure of 1Megabar. Phys. Rev. Lett. 2021, 127, 117001. 10.1103/PhysRevLett.127.117001. [DOI] [PubMed] [Google Scholar]
- Kong P.; Minkov V. S.; Kuzovnikov M. A.; Drozdov A. P.; Besedin S. P.; Mozaffari S.; Balicas L.; Balakirev F. F.; Prakapenka V. B.; Chariton S.; Knyazev D. A.; Greenberg E.; Eremets M. I. Superconductivity up to 243K in the yttrium-hydrogen system under high pressure. Nat. Commun. 2021, 12, 5075. 10.1038/s41467-021-25372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyan I. A.; Semenok D. V.; Kvashnin A. G.; Sadakov A. V.; Sobolevskiy O. A.; Pudalov V. M.; Ivanova A. G.; Prakapenka V. B.; Greenberg E.; Gavriliuk A. G.; Lyubutin I. S.; Struzhkin V. V.; Bergara A.; Errea I.; Bianco R.; Calandra M.; Mauri F.; Monacelli L.; Akashi R.; Oganov A. R. Anomalous High-Temperature Superconductivity in YH6. Adv. Mater. 2021, 33, 2006832. 10.1002/adma.202006832. [DOI] [PubMed] [Google Scholar]
- Semenok D. V.; Kruglov I. A.; Savkin I. A.; Kvashnin A. G.; Oganov A. R. On Distribution of Superconductivity in Metal Hydrides. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100808 10.1016/j.cossms.2020.100808. [DOI] [Google Scholar]
- Flores-Livas J. A.; Boeri L.; Sanna A.; Profeta G.; Arita R.; Eremets M. A perspective on conventional high-temperature superconductors at high pressure: Methods and materials. Phys. Rep. 2020, 856, 1–78. 10.1016/j.physrep.2020.02.003. [DOI] [Google Scholar]
- Gao G.; Wang L.; Li M.; Zhang J.; Howie R. T.; Gregoryanz E.; Struzhkin V. V.; Wang L.; Tse J. S. Superconducting binary hydrides: Theoretical predictions and experimental progresses. Materials Today Physics 2021, 21, 100546 10.1016/j.mtphys.2021.100546. [DOI] [Google Scholar]
- Flores-Livas J. A.; Boeri L.; Sanna A.; Profeta G.; Arita R.; Eremets M. A perspective on conventional high-temperature superconductors at high pressure: Methods and materials. Phys. Rep. 2020, 856, 1–78. 10.1016/j.physrep.2020.02.003. [DOI] [Google Scholar]
- Liu Z.; Hou W.; Botana J.; Sun Y.; Sun Y.; Yi W.; Chen Y. Unexpected Metastable High-Temperature Superconducting Hydride LaCaH12 with an Irregular Cage Structure at High Pressure. J. Phys. Chem. C 2023, 127, 23870–23878. 10.1021/acs.jpcc.3c05654. [DOI] [Google Scholar]
- Chen S.; Wang Y.; Bai F.; Wu X.; Wu X.; Pakhomova A.; Guo J.; Huang X.; Cui T. Superior Superconducting Properties Realized in Quaternary La-Y-Ce Hydrides at Moderate Pressures. J. Am. Chem. Soc. 2024, 146, 14105–14113. 10.1021/jacs.4c02586. [DOI] [PubMed] [Google Scholar]
- Song X.; Hao X.; Wei X.; He X.-L.; Liu H.; Ma L.; Liu G.; Wang H.; Niu J.; Wang S.; Qi Y.; Liu Z.; Hu W.; Xu B.; Wang L.; Gao G.; Tian Y. Superconductivity above 105 K in Nonclathrate Ternary Lanthanum Borohydride below Megabar Pressure. J. Am. Chem. Soc. 2024, 146, 13797–13804. 10.1021/jacs.3c14205. [DOI] [PubMed] [Google Scholar]
- Wei X.; Hao X.; Bergara A.; Zurek E.; Liang X.; Wang L.; Song X.; Li P.; Wang L.; Gao G.; Tian Y. Designing ternary superconducting hydrides with A15-type structure at moderate pressures. Materials Today Physics 2023, 34, 101086 10.1016/j.mtphys.2023.101086. [DOI] [Google Scholar]
- Gao K.; Cui W.; Shi J.; Durajski A. P.; Hao J.; Botti S.; Marques M. A. L.; Li Y. Prediction of high-Tc superconductivity in ternary actinium beryllium hydrides at low pressure. Phys. Rev. B 2024, 109, 014501. 10.1103/PhysRevB.109.014501. [DOI] [Google Scholar]
- Sun W.; Chen B.; Li X.; Peng F.; Hermann A.; Lu C. Ternary Na-P-H superconductor under high pressure. Phys. Rev. B 2023, 107, 214511. 10.1103/PhysRevB.107.214511. [DOI] [Google Scholar]
- Shutov G. M.; Semenok D. V.; Kruglov I. A.; Oganov A. R. Ternary superconducting hydrides in the La-Mg-H system. Materials Today Physics 2024, 40, 101300 10.1016/j.mtphys.2023.101300. [DOI] [Google Scholar]
- Durajski A. P.; Szcześniak R. First-Principles Estimation of Low-Pressure Superconductivity in KC2H8 Ternary Hydride. physica status solidi (RRL)Rapid Research Letters 2023, 17, 2300043. 10.1002/pssr.202300043. [DOI] [Google Scholar]
- Qin K. S.; Song P.; Hongo K.; Maezono R. First-Principles Investigation of Stability and Superconductivity in Ternary Yttrium-Praseodymium Hydrides under High Pressure. J. Phys. Chem. C 2023, 127, 21242–21249. 10.1021/acs.jpcc.3c02968. [DOI] [Google Scholar]
- Song Y.; Bi J.; Nakamoto Y.; Shimizu K.; Liu H.; Zou B.; Liu G.; Wang H.; Ma Y. Stoichiometric Ternary Superhydride LaBeH8 as a New Template for High-Temperature Superconductivity at 110K under 80 GPa. Phys. Rev. Lett. 2023, 130, 266001. 10.1103/PhysRevLett.130.266001. [DOI] [PubMed] [Google Scholar]
- Di Cataldo S.; Heil C.; von der Linden W.; Boeri L. La BH 8: Towards high-T c low-pressure superconductivity in ternary superhydrides.. Phys. Rev. B 2021, 104, L020511 10.1103/PhysRevB.104.L020511. [DOI] [Google Scholar]
- Semenok D. V.; Troyan I. A.; Sadakov A. V.; Zhou D.; Galasso M.; Kvashnin A. G.; Kruglov I. A.; Bykov A. A.; Terent’ev K. Y.; Cherepahin A. V.; et al. Effect of paramagnetic impurities on superconductivity in polyhydrides: s-wave order parameter in Nd-doped LaH10. 2022. 10.48550/arXiv.2203.06500. [DOI] [Google Scholar]
- Bi J.; Nakamoto Y.; Zhang P.; Shimizu K.; Zou B.; Liu H.; Zhou M.; Liu G.; Wang H.; Ma Y. Giant enhancement of superconducting critical temperature in substitutional alloy (La, Ce)H9. Nat. Commun. 2022, 13, 5952. 10.1038/s41467-022-33743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Huang X.; Semenok D. V.; Chen S.; Zhou D.; Zhang K.; Oganov A. R.; Cui T. Enhancement of superconducting properties in the La-Ce-H system at moderate pressures. Nat. Commun. 2023, 14, 2660. 10.1038/s41467-023-38254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; Bergara A.; Wang L.; Wen B.; Zhao Z.; Zhou X.-F.; He J.; Gao G.; Tian Y. Potential high-T c superconductivity in CaYH 12 under pressure. Phys. Rev. B 2019, 99, 100505 10.1103/PhysRevB.99.100505. [DOI] [Google Scholar]
- Xie H.; Duan D.; Shao Z.; Song H.; Wang Y.; Xiao X.; Li D.; Tian F.; Liu B.; Cui T. High-temperature superconductivity in ternary clathrate YCaH12 under high pressures. J. Phys.: Condens. Matter 2019, 31, 245404 10.1088/1361-648X/ab09b4. [DOI] [PubMed] [Google Scholar]
- Semenok D. V.; Troyan I. A.; Ivanova A. G.; Kvashnin A. G.; Kruglov I. A.; Hanfland M.; Sadakov A. V.; Sobolevskiy O. A.; Pervakov K. S.; Lyubutin I. S.; Glazyrin K. V.; Giordano N.; Karimov D. N.; Vasiliev A. L.; Akashi R.; Pudalov V. M.; Oganov A. R. Superconductivity at 253K in lanthanum-yttrium ternary hydrides. Mater. Today 2021, 48, 18–28. 10.1016/j.mattod.2021.03.025. [DOI] [Google Scholar]
- Song P.; Hou Z.; de Castro P. B.; Nakano K.; Hongo K.; Takano Y.; Maezono R.. High-Tc ternary metal hydrides, YKH12 and LaKH12, discovered by machine learning. 2021. 10.48550/arXiv.2103.00193. [DOI]
- Vocaturo R.; Tresca C.; Ghiringhelli G.; Profeta G. Prediction of ambient-pressure superconductivity in ternary hydride PdCuHx. J. Appl. Phys. 2022, 131, 033903. 10.1063/5.0076728. [DOI] [Google Scholar]
- Sun Y.; Sun S.; Zhong X.; Liu H. Prediction for high superconducting ternary hydrides below megabar pressure. J. Phys.: Condens. Matter 2022, 34, 505404 10.1088/1361-648X/ac9bba. [DOI] [PubMed] [Google Scholar]
- Bi J.; Nakamoto Y.; Shimizu K.; Zhou M.; Wang H.; Liu G.; Ma Y.. Efficient route to achieve superconductivity improvement via substitutional La-Ce alloy superhydride at high pressure. 2022. 10.48550/arXiv.2204.04623. [DOI]
- Rahm M.; Cammi R.; Ashcroft N. W.; Hoffmann R. Squeezing All Elements in the Periodic Table: Electron Configuration and Electronegativity of the Atoms under Compression. J. Am. Chem. Soc. 2019, 141, 10253–10271. 10.1021/jacs.9b02634. [DOI] [PubMed] [Google Scholar]
- George E. P.; Raabe D.; Ritchie R. O. High-entropy alloys. Nature Reviews Materials 2019, 4, 515–534. 10.1038/s41578-019-0121-4. [DOI] [Google Scholar]
- Inoue A.; Ohtera K.; Masumoto T. New Amorphous Al-Y, Al-La and Al-Ce Alloys Prepared by Melt Spinning. Jpn. J. Appl. Phys. 1988, 27, L736. 10.1143/JJAP.27.L736. [DOI] [Google Scholar]
- Solozhenko V. L.; Kurakevych O. O.; Andrault D.; Le Godec Y.; Mezouar M. Ultimate Metastable Solubility of Boron in Diamond: Synthesis of Superhard Diamond-like BC5. Phys. Rev. Lett. 2009, 102, 015506. 10.1103/PhysRevLett.102.015506. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Liu H.; Li Q.; Gao B.; Wang Y.; Li H.; Chen C.; Ma Y. Superhard in BC3 Cubic Diamond Structure. Phys. Rev. Lett. 2015, 114, 015502. 10.1103/PhysRevLett.114.015502. [DOI] [PubMed] [Google Scholar]
- Bi J.; Nakamoto Y.; Zhang P.; Wang Y.; Ma L.; Wang Y.; Zou B.; Shimizu K.; Liu H.; Zhou M.; Wang H.; Liu G.; Ma Y. Stabilization of superconductive La-Y alloy superhydride with Tc above 90K at megabar pressure. Materials Today Physics 2022, 28, 100840 10.1016/j.mtphys.2022.100840. [DOI] [Google Scholar]
- Wang T.; Flores-Livas J. A.; Nomoto T.; Ma Y.; Koretsune T.; Arita R. Optimal alloying in hydrides: Reaching room-temperature superconductivity in LaH10. Phys. Rev. B 2022, 105, 174516. 10.1103/PhysRevB.105.174516. [DOI] [Google Scholar]
- George E. P.; Raabe D.; Ritchie R. O. High-entropy alloys. Nature Reviews Materials 2019, 4, 515–534. 10.1038/s41578-019-0121-4. [DOI] [Google Scholar]
- Song P.; Durajski A. P.; Hou Z.; Ghaffar A.; Dahule R.; Szczȩśniak R.; Hongo K.; Maezono R. (La,Th)H10: Potential High-Tc (242 K) Superconductors Stabilized Thermodynamically below 200 GPa. J. Phys. Chem. C 2024, 128, 2656–2665. 10.1021/acs.jpcc.3c07213. [DOI] [Google Scholar]
- Nix F. C.; Shockley W. Order-Disorder Transformations in Alloys. Rev. Mod. Phys. 1938, 10, 1–71. 10.1103/RevModPhys.10.1. [DOI] [Google Scholar]
- Zawadzki P.; Zakutayev A.; Lany S. Entropy-Driven Clustering in Tetrahedrally Bonded Multinary Materials. Physical Review Applied 2015, 3, 034007. 10.1103/PhysRevApplied.3.034007. [DOI] [Google Scholar]
- Mizuseki H.; Sahara R.; Hongo K. Order-disorder competition in equiatomic 3d-transition-metal quaternary alloys: phase stability and electronic structure. Science and Technology of Advanced Materials: Methods 2023, 3, 2153632 10.1080/27660400.2022.2153632. [DOI] [Google Scholar]
- Heil C.; Di Cataldo S.; Bachelet G. B.; Boeri L. Superconductivity in sodalite-like yttrium hydride clathrates. Phys. Rev. B 2019, 99, 220502 10.1103/PhysRevB.99.220502. [DOI] [Google Scholar]
- Kvashnin A. G.; Semenok D. V.; Kruglov I. A.; Wrona I. A.; Oganov A. R. High-temperature superconductivity in a Th-H system under pressure conditions. ACS Appl. Mater. Interfaces 2018, 10, 43809–43816. 10.1021/acsami.8b17100. [DOI] [PubMed] [Google Scholar]
- Yao S.; Wang C.; Jeon H.; Liu L.; Bok J. M.; Bang Y.; Jia Y.; Cho J.-H.. Origin of the large differences in high-pressure stability and superconductivity between ThH9 and ThH18. 2023. 10.48550/arXiv.2302.06956. [DOI]
- Glass C. W.; Oganov A. R.; Hansen N. USPEX—Evolutionary crystal structure prediction. Comput. Phys. Commun. 2006, 175, 713–720. 10.1016/j.cpc.2006.07.020. [DOI] [Google Scholar]
- Oganov A. R.; Glass C. W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704. 10.1063/1.2210932. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- Blöchl P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- Momma K.; Izumi F. VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. 10.1107/S0021889811038970. [DOI] [Google Scholar]
- Giannozzi P.; Baroni S.; Bonini N.; Calandra M.; Car R.; Cavazzoni C.; Ceresoli D.; Chiarotti G. L.; Cococcioni M.; Dabo I.; Corso A. D.; de Gironcoli S.; Fabris S.; Fratesi G.; Gebauer R.; Gerstmann U.; Gougoussis C.; Kokalj A.; Lazzeri M.; Martin-Samos L.; Marzari N.; Mauri F.; Mazzarello R.; Paolini S.; Pasquarello A.; Paulatto L.; Sbraccia C.; Scandolo S.; Sclauzero G.; Seitsonen A. P.; Smogunov A.; Umari P.; Wentzcovitch R. M. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter 2009, 21, 395502 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- McMillan W. L. Transition Temperature of Strong-Coupled Superconductors. Phys. Rev. 1968, 167, 331–344. 10.1103/PhysRev.167.331. [DOI] [Google Scholar]
- Allen P. B.; Dynes R. Transition temperature of strong-coupled superconductors reanalyzed. Phys. Rev. B 1975, 12, 905. 10.1103/PhysRevB.12.905. [DOI] [Google Scholar]
- Gehringer D.; Friák M.; Holec D. Models of configurationally-complex alloys made simple. Comput. Phys. Commun. 2023, 286, 108664 10.1016/j.cpc.2023.108664. [DOI] [Google Scholar]
- Cowley J. An approximate theory of order in alloys. Phys. Rev. 1950, 77, 669. 10.1103/PhysRev.77.669. [DOI] [Google Scholar]
- Cowley J. Short-range order and long-range order parameters. Phys. Rev. 1965, 138, A1384 10.1103/PhysRev.138.A1384. [DOI] [Google Scholar]
- Ong S. P.; Wang L.; Kang B.; Ceder G. Li-Fe-P-O2 Phase Diagram from First Principles Calculations. Chem. Mater. 2008, 20, 1798–1807. 10.1021/cm702327g. [DOI] [Google Scholar]
- Mao W. L.; Mao H.-k. Hydrogen storage in molecular compounds. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 708–710. 10.1073/pnas.0307449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D.; Liu Y.; Tian F.; Li D.; Huang X.; Zhao Z.; Yu H.; Liu B.; Tian W.; Cui T. Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity. Sci. Rep. 2014, 4, 6968. 10.1038/srep06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrona I. A.; Niegodajew P.; Durajski A. P. A recipe for an effective selection of promising candidates for high-temperature superconductors among binary hydrides. Materials Today Physics 2024, 46, 101499 10.1016/j.mtphys.2024.101499. [DOI] [Google Scholar]
- McMahon J. M.; Ceperley D. M. Ground-State Structures of Atomic Metallic Hydrogen. Phys. Rev. Lett. 2011, 106, 165302. 10.1103/PhysRevLett.106.165302. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Miao M. Chemical templates that assemble the metal superhydrides. Chem. 2023, 9, 443–459. 10.1016/j.chempr.2022.10.015. [DOI] [Google Scholar]
- Wang H.; Tse J. S.; Tanaka K.; Iitaka T.; Ma Y. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 6463–6466. 10.1073/pnas.1118168109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Wang Y.; Lv J.; Ma Y. Materials discovery at high pressures. Nature Reviews Materials 2017, 2, 17005. 10.1038/natrevmats.2017.5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available at Figshare with the following https://doi.org/10.6084/m9.figshare.27020479.