Abstract
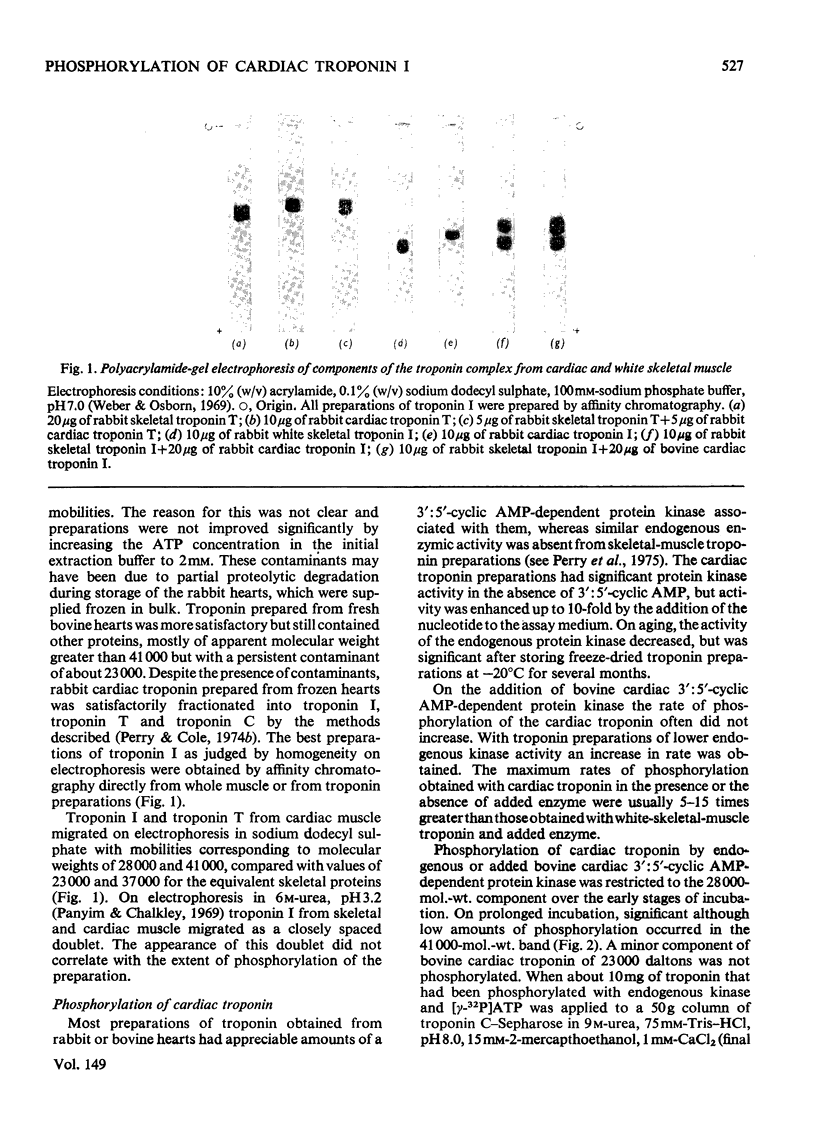
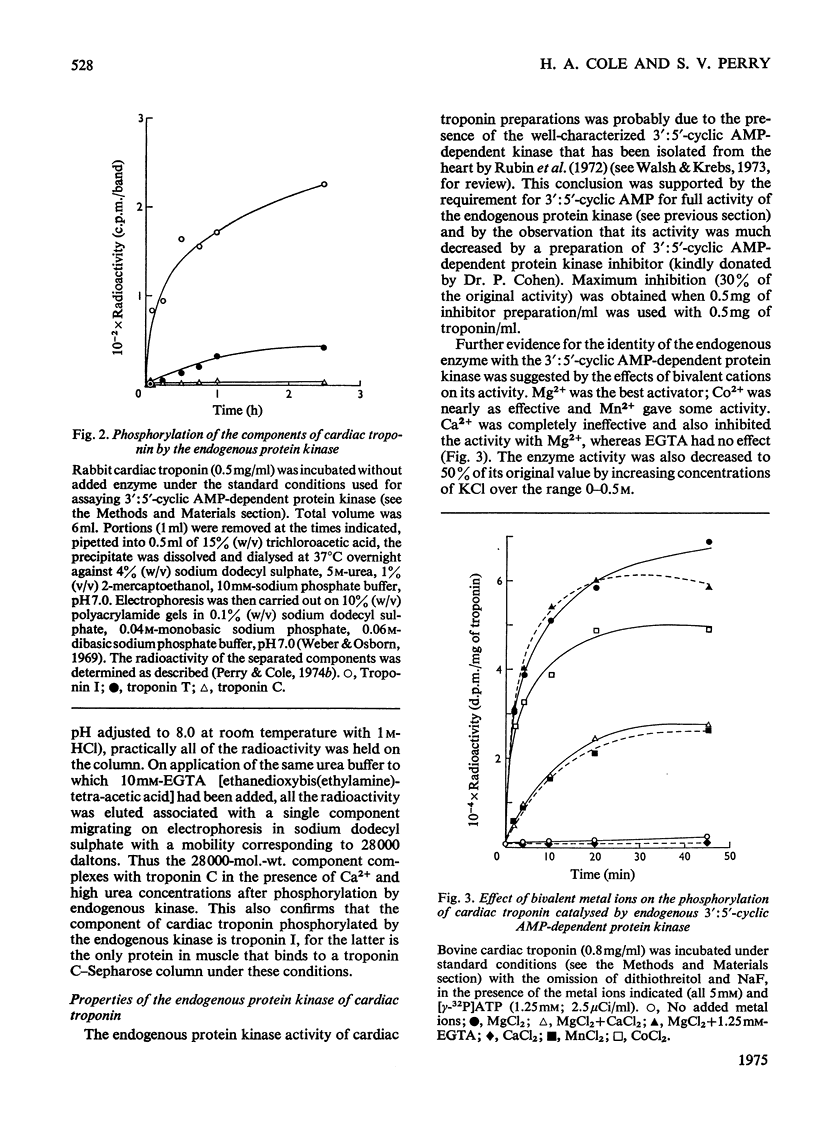
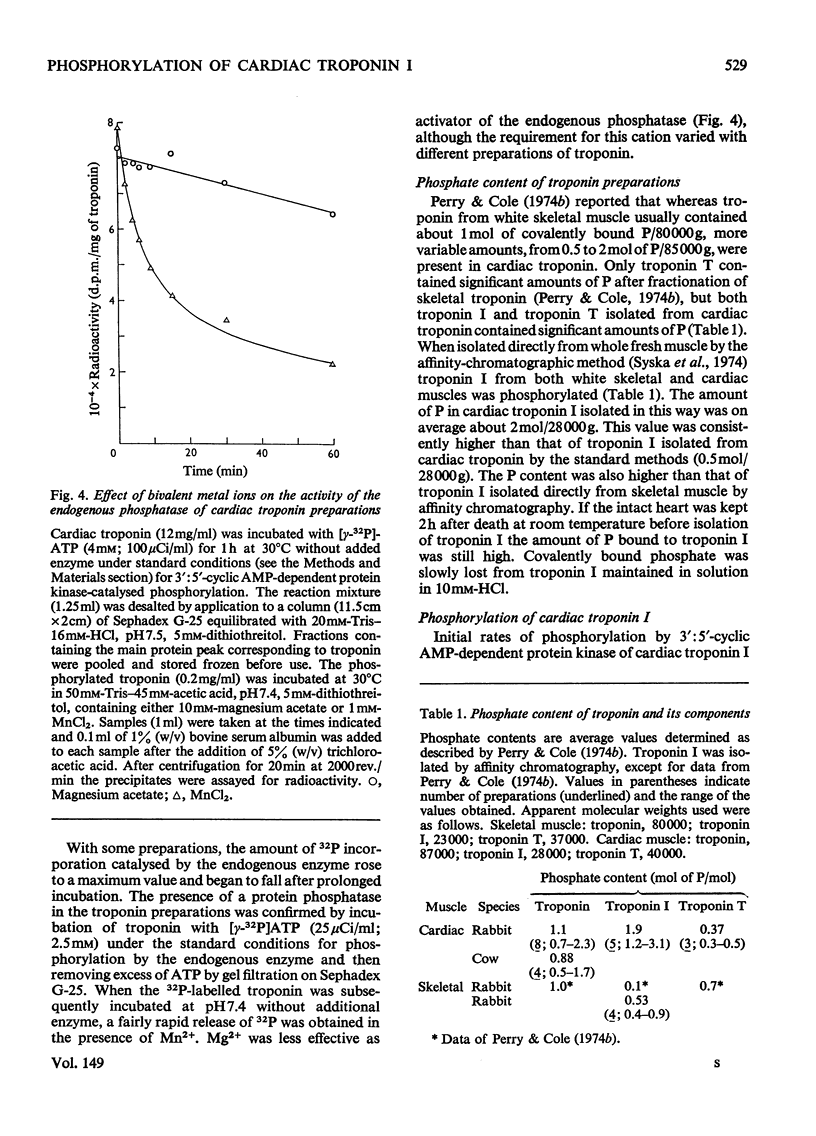
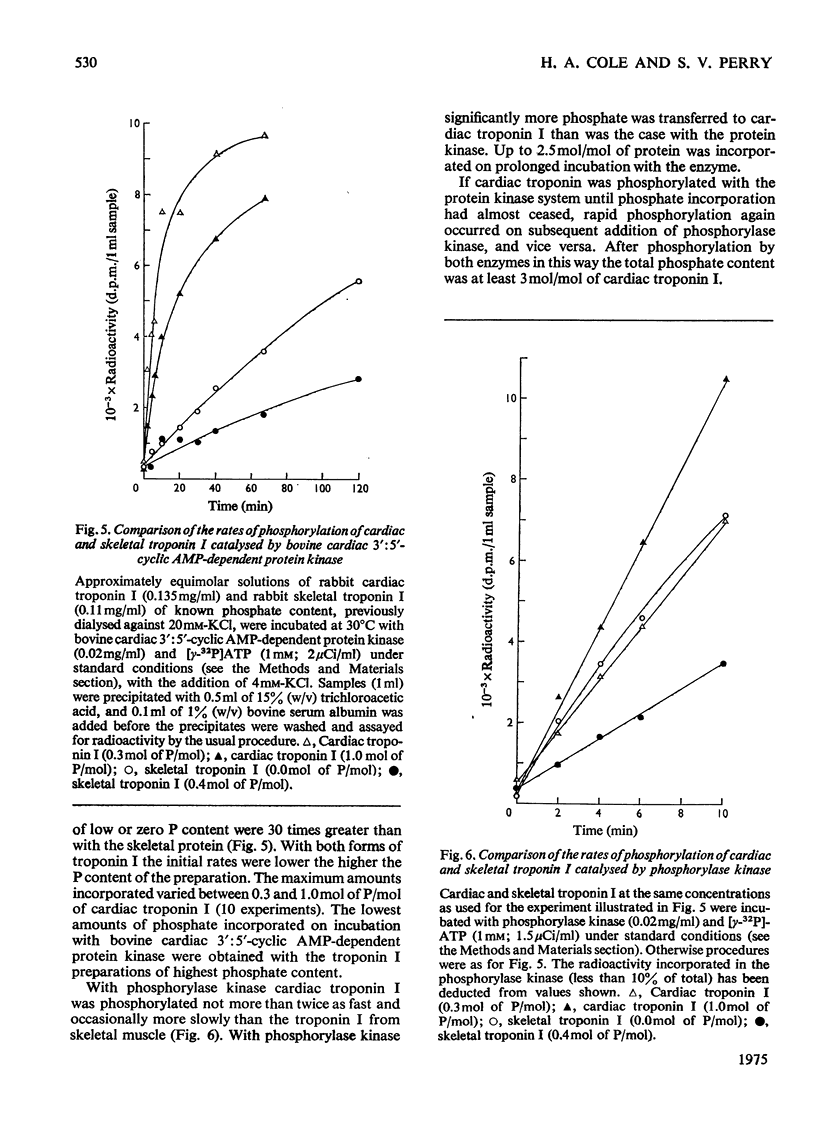
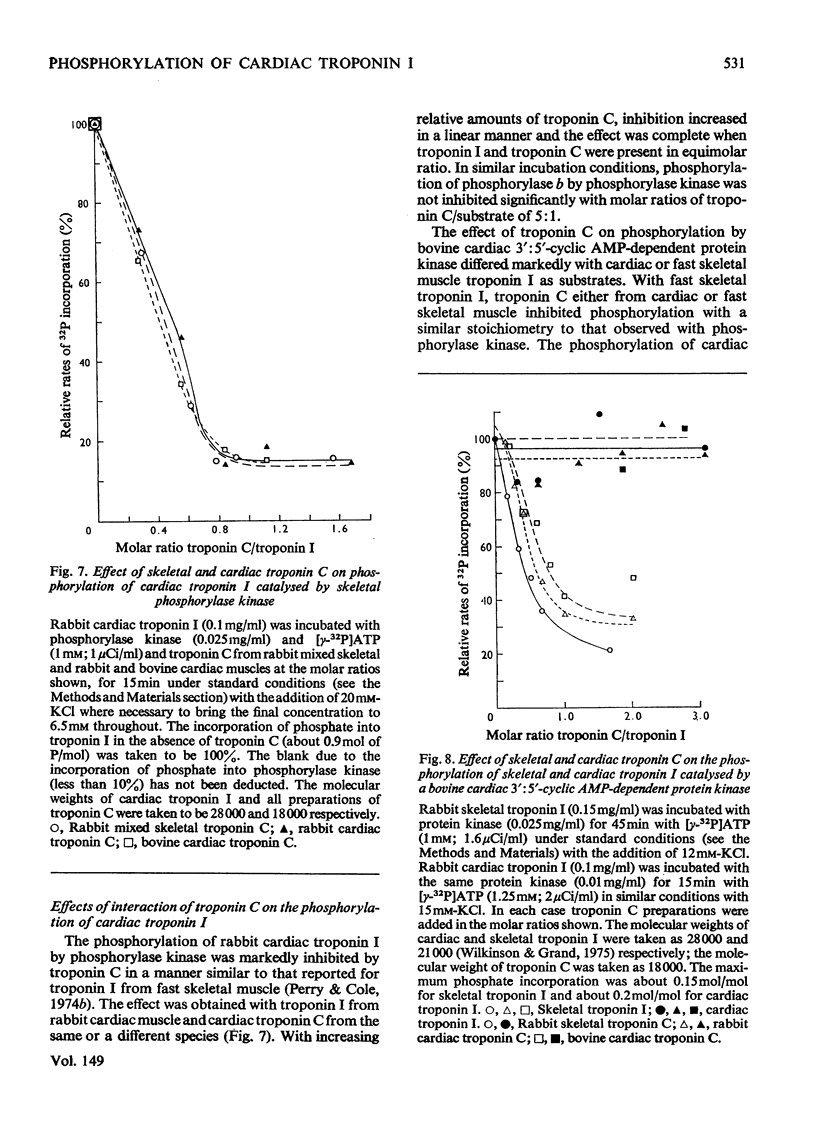
1. Troponin I isolated from fresh cardiac muscle by affinity chromatography contains about 1.9 mol of covalently bound phosphate/mol. Similar preparations of white-skeletal-muscle troponin I contain about 0.5 mol of phosphate/mol. 2. A 3':5'-cyclic AMP-dependent protein kinase and a protein phosphatase are associated with troponin isolated from cardiac muscle. 3. Bovine cardiac 3':5'-cyclic AMP-dependent protein kinase catalyses the phosphorylation of cardiac troponin I 30 times faster than white-skeletal-muscle troponin I. 4. Troponin I is the only component of cardiac troponin phosphorylated at a significant rate by the endogenous or a bovine cardiac 3':5'-cyclic AMP-dependent protein kinase. 5. Phosphorylase kinase catalyses the phosphorylation of cardiac troponin I at similar or slightly faster rates than white-skeletal-muscle troponin I. 6. Troponin C inhibits the phosphorylation of cardiac and skeletal troponin I catalysed by phosphorylase kinase and the phosphorylation of white skeletal troponin I catalysed by 3':5'-cyclic AMP-dependent protein kinase; the phosphorylation of cardiac troponin I catalysed by the latter enzyme is not inhibited.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. Correlation between contraction and phosphorylation of the inhibitory subunit of troponin in perfused rat heart. FEBS Lett. 1975 Jan 15;50(1):57–60. doi: 10.1016/0014-5793(75)81040-4. [DOI] [PubMed] [Google Scholar]
- Huang T. S., Bylund D. B., Stull J. T., Krebs E. G. The amino acid sequences of the phosphorylated sites in troponin-I from rabbit skeletal muscle. FEBS Lett. 1974 Jun 15;42(3):249–252. doi: 10.1016/0014-5793(74)80738-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moir A. J., Wilkinson J. M., Perry S. V. The phosphorylation sites of troponin I from white skeletal muscle of the rabbit. FEBS Lett. 1974 Jun 15;42(3):253–256. doi: 10.1016/0014-5793(74)80739-8. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of the "37000 component" of the troponin complex (troponin-t). Biochem J. 1973 Feb;131(2):425–428. doi: 10.1042/bj1310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratje E., Heilmeyer L. M.G. Phosphorylation of rabbit muscle troponin and actin by a 3', 5'-c-AMP-dependent protein kinase. FEBS Lett. 1972 Oct 15;27(1):89–93. doi: 10.1016/0014-5793(72)80416-2. [DOI] [PubMed] [Google Scholar]
- Reddy Y. S., Ballard D., Giri N. Y., Schwartz A. Phosphorylation of cardiac native tropomyosin and troponin: inhibitory effect of actomyosin and possible presence of endogenous myofibrillar-located cyclic-AMP-dependent protein kinase. J Mol Cell Cardiol. 1973 Oct;5(5):461–471. doi: 10.1016/0022-2828(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Stull J. T., Brostrom C. O., Krebs E. G. Phosphorylation of the inhibitor component of troponin by phosphorylase kinase. J Biol Chem. 1972 Aug 25;247(16):5272–5274. [PubMed] [Google Scholar]
- Syska H., Perry S. V., Trayer I. P. A new method of preparation of troponin I (inhibitory protein) using affinity chromatography. Evidence for three different forms of troponin I in striated muscle. FEBS Lett. 1974 Apr 1;40(2):253–257. doi: 10.1016/0014-5793(74)80238-3. [DOI] [PubMed] [Google Scholar]
- Tsukui R., Ebashi S. Cardiac troponin. J Biochem. 1973 May;73(5):1119–1121. doi: 10.1093/oxfordjournals.jbchem.a130168. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



