Abstract
Introduction
Recurrent respiratory papillomatosis (RRP) is a chronic disease caused by human papillomavirus (HPV), characterized by recurrent papillomas in the respiratory tract. Presenting as either juvenile-onset RRP (JoRRP) or adult-onset RRP (AoRRP), the severity of the disease is subjective and unpredictable. Lack of curative therapies necessitates disease management involving repeated surgical removal of lesions. The review aimed to assess the clinical, humanistic and economic burden associated with RRP.
Methods
Systematic literature reviews of Embase®, MEDLINE® and Cochrane databases were conducted for epidemiology, clinical, humanistic, and economic burden, from database inception to November 30, 2022. Conference abstracts were also searched (2019–2022). Key inclusion criteria consisted of juveniles or adults with RRP/laryngeal papillomatosis, with no restriction on study country, interventions, or comparators. Outcomes of interest included incidence, prevalence, risk factors, symptomatic presentation, HPV genotype, cost burden, resource use and health related quality of life (HRQoL).
Results
In JoRRP, the incidence rate ranged from 0.2–2.1 per 100,000 and the prevalence rate ranged from 0.8–4.3 per 100,000. Incidence and prevalence of AoRRP were 0.2–3.9 and 0.4–8.4 per 100,000, respectively. Limited studies reported the subsequent impact of introducing national prophylactic HPV immunisation programs on JoRRP epidemiology, but where available, they were associated with significantly reduced incidence rates. Symptomatic presentations were diverse, with voice impact and breathing difficulties commonly reported. More aggressive disease was linked to earlier age of onset and HPV11 genotype. Healthcare utilisation was largely driven by surgical interventions, due to lack of curative treatments. Cost burden was substantial, with JoRRP associated with triple the costs of AoRRP in the US. Patients with JoRRP and AoRRP experienced considerable HRQoL impairment, particularly relating to voice disorder.
Conclusion
Extensive clinical, humanistic and economic disease burden was reported for both JoRRP and AoRRP, as it is a chronic condition, with propensity to recur and spread. Feasibility of improving HPV prophylactic vaccination coverage against HPV6/HPV11 should be explored to reduce incidence, alongside efforts to improve treatment of JoRRP and AoRRP patients. Despite the existing literature, RRP remains a poorly understood disease, and future research on risk factors and medical options are needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-03057-w.
Keywords: Recurrent respiratory papillomatosis (RRP), JoRRP, AoRRP, Human papillomavirus (HPV), Clinical burden, Epidemiology, Treatment patterns, Economic burden, Humanistic burden, Health related quality of life (HRQoL)
Background
Recurrent Respiratory Papillomatosis (RRP) is a chronic, and poorly understood condition occurring in the respiratory tract. RRP usually presents with non-specific symptoms of airway involvement, including chronic cough, hoarseness, wheezing, voice change, stridor, and, chronic dyspnea [1]. The disease is caused by the growth of papillomas due to human papillomavirus (HPV), a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. It presents as a benign neoplasm that may arise in children, and adults, associated with a characteristic bimodal age distribution; however, a recent study identified three peaks for disease onset, at ages 7, 35, and 64 [2, 3]. HPV6 and HPV11 are among the most common HPV genotypes associated with RRP [4, 5]. Among adults, increased sexual activity with multiple partners is an important risk factor for HPV infection, this association has not been proven in AoRRP, whilst in juveniles, there is considerable evidence to suggest that RRP results from vertical HPV transmission from mother to child during the pregnancy, and child birth [1].
Based on the age at which RRP presents, the disease is classified into juvenile onset RRP (JoRRP) or adult onset RRP (AoRRP), with JoRRP typically representing the more aggressive form of the disease, with increased likelihood of recurrence [1]. No treatments are indicated for AoRRP or JoRRP, with disease management currently limited to surgical removal of papillomas, although some adjuvant treatment options have shown promise [6]. Intralesional administration of cidofovir (a cytosine nucleotide analogue which inhibits viral replication) has been shown to prolong the interval between surgeries, bevacizumab (a vascular endothelial growth factor-A inhibitor) may help reduce lesion size in patients with aggressive JoRRP including pulmonary involvement and pembrolizumab (programmed cell death protein 1 inhibitor) has shown encouraging results in a recent Phase II study [6–12]. Meanwhile, peri-treatment prophylactic HPV quadrivalent and nonavalent vaccination has also demonstrated potential to reduce the number of surgeries and increase intersurgical interval [13–15]. Due to the similarities in disease management, AoRRP and JoRRP are often presented together. However, given the inherent heterogeneity between the patient populations associated with AoRRP, and JoRRP, and the associated variations in epidemiology, symptomatic burden and healthcare resource utilization, the two sub-types may be considered distinctive diseases [16]. As such, it is important to assess the current disease landscape for these distinct patient populations.
Although reviews have been published which describe the therapeutic strategies among patients with RRP, this study aimed to summarize those areas which have been of lesser focus among the published literature to date [17–19]. That is, to summarize the available evidence for JoRRP, and AoRRP, for epidemiology, clinical burden (specifically including treatment complications, risk factors, and symptomatic presentations), humanistic burden (i.e. health related quality of life), and economic burden. This study also aims to provide greater awareness, and understanding of this uncommon, chronic, and underrecognized HPV-associated disease.
Methodology
Data sources
Systematic literature reviews were conducted for epidemiology, clinical burden, humanistic burden, and economic burden in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Excerpta Medica Database (Embase®), Medical Literature Analysis and Retrieval System Online (MEDLINE®), MEDLINE® In-Process, Cochrane Central Register of Controlled Trials (CENTRAL) and Cochrane Database of Systematic Reviews (CDSR). Databases were searched from database inception to November 30, 2022 (Table 1).
Table 1.
Databases searched for the literature review and the search platform
| Data sources | Platform | Timeframe |
|---|---|---|
| Embase® | Embase.com; https://www.embase.com/#advancedSearch/resultspage | Database inception to November 30, 2022 |
| MEDLINE® | ||
| MEDLINE® In-process | PubMed; https://pubmed.ncbi.nlm.nih.gov/advanced/ | |
| CENTRAL | Cochrane library; https://www.cochranelibrary.com/advanced-search/search-manager | |
| CDSR | ||
| Conferences |
• International Society for Pharmacoeconomics and Outcomes Research (ISPOR) [All Regions] • European Research Organization on Genital Infection and Neoplasia (EUROGIN) • International Papillomavirus Conference (IPVC) |
2019 to 2022 |
CDSR Cochrane Database of Systematic Reviews, CENTRAL Cochrane Controlled Register of Trials MEDLINE: Medical Literature Analysis and Retrieval System Online
Additional searches of conferences were also conducted to capture studies that may not yet have been published in full-text articles, or to supplement the results of previously published studies. Abstracts were manually searched from 2019 to 2022 (Table 1). Bibliographic searching of review publications was also conducted.
Search strategy
A systematic search was designed for each of the electronic databases searched. Search terms used included keywords and medical subject headings (MeSH terms) based on predefined inclusion criteria using a Population, Intervention, Comparator, Outcomes, Timeframe and Study design (PICOTS) framework (Supplementary Appendix S1, Table 2).
Table 2.
Eligibility criteria for study inclusion
| PICOTS | Inclusion Criteria |
|---|---|
| Population | Juveniles and adults with RRP/laryngeal papillomatosis (stratification by age groups) |
| Interventions | No restriction |
| Comparisons | No restriction |
| Outcomes |
Epidemiology burden: Incidence Prevalence Mortality/survival Proportion of RRP progressing to cancer Tumor progression Note: HPV genotype was also assessed to identify distinct types of HPV Clinical burden: Treatment complications Risk factors Symptoms Humanistic burden: All patient-reported outcomes such as voice impact or HRQoL instruments and utility values Any disease specific and general PRO instruments Utility Economic review (cost and resource use and economic evaluations): Intervention and comparator details Evaluation details Cost-analysis Cost-effectiveness analysis Resource use |
| Study design |
Epidemiology burden: Cohort studies including historical cohort studies and nested case–control studies Cross-sectional studies Registry/database studies Clinical and humanistic burden: Randomized controlled trials Non-randomized controlled trials Single arm trials Cross-sectional and longitudinal database studies Registry studies Pragmatic clinical trials Cohort studies /longitudinal studies (retrospective/prospective) Case–control studies Analysis of hospital records/database All studies reporting utility values Note: For clinical burden, clinical trials were deprioritized Economic burden: Cost studies/surveys/analyses Database studies collecting cost data (e.g., claims databases and hospital records) Cost resource use studies/surveys Economic evaluations Economic modelling studies Observational cost studies |
| Timeframe |
Database inception till date (November 2022) Note: For clinical burden last ten years data was prioritized (2012–2022) |
| Regions | Global |
| Other | No language restriction was used in the searches. Studies with title and abstract in English and full text in non-English were evaluated on the basis of title and abstract |
PICOTS Population, intervention, comparisons, outcomes, time, study design
Data extraction and validation
Data extraction and validation was conducted using a two-step process. First screening (using study titles and abstracts) and second screening (based upon the full text of articles) was undertaken by two reviewers, with any discrepancies resolved by a third, independent reviewer. Data extraction was again conducted by two independent reviewers, followed by a quality check by the independent reviewer.
Quality rating was conducted using the Newcastle–Ottawa Scale for cohort studies and case–control studies, and adapted Newcastle–Ottawa Scale for cross-sectional studies. The maximum score for the Newcastle–Ottawa Scale was 9. Majority of the studies were graded as medium quality.
Results
Overall, 1,886 studies were identified across the four reviews. Upon the completion of the two-step screening process, 139 studies were identified for inclusion in total (epidemiology n = 56, clinical burden n = 75, humanistic burden n = 18, economic burden n = 23) (Supplementary Appendix S2).
Epidemiology
Upon the completion of the screening procedure, 56 studies were included. Multiple reports of the same studies were linked, so that each study is represented as a whole, rather than each of the related publications considered separately. Among the evidence included, 39 studies evaluated patients with JoRRP, and 23 studies evaluated patients with AoRRP (Supplementary Appendix S2). Ten studies evaluated overall RRP patients, where age of onset was unclear (not presented).
Epidemiology of JoRRP
Eighteen studies provided data on incidence and prevalence across the globe. Studies evaluating the impact of prophylactic HPV vaccination on incidence of RRP were only identified for the US, and Australia while studies retrieved from other countries were conducted in the pre-vaccination era (Table 3).
Table 3.
Incidence and prevalence among patients with JoRRP and AoRRP
| Study | Country | Year | Age-range (years) | Incidence per 100,000 children | Prevalence per 100,000 children |
|---|---|---|---|---|---|
| JoRRP | |||||
| Armstrong 2000 | US | 1996 | 0–18 | 0.1–2.1 | 1.0–4.0 |
| Derkay 1995 | US | 1993 | < 14 | 0.1 | 4.3 |
| Meites 2021 | US | 2004–2005 | < 18 | 2.0 | - |
| 2012–2013 | < 18 | 0.5a | - | ||
| Marsico 2014 | US | 2006 | 0–17 |
0.5 (privately insured) 1.0 (publicly insured) |
1.5 (privately insured) 2.9 (publicly insured) |
| Cristensen 1984 | Denmark | Pre-1961 | 0–15 | 0.2 | - |
| Denmark | After 1961 | 0–15 | 0.7 | - | |
| Bomholt 1988 | Denmark | 1980–1983 | 0–14 | 0.6 | 0.8 |
| Lindeberg 1990 | Denmark | 1974–1993 | 0–14 | 0.4 | - |
| Omland 2012 | Norway | 1987–2009 | 0–17 | 0.2 | - |
| Campisi 2010 | Canada | 1994–2007 | 0–14 | 0.2 | 1.1 |
| Novakovic 2010 | Australia | 1998–2008 | 5–9 | - | 1.2–1.8 |
| Novakovic 2016 | Australia | 2000–2013 | < 15 | - | 0.8 |
| Australia | 2000–2013 | 5–9 | - | 1.1 | |
| Novakovic 2018 | Australia | 2012 | < 15 | 0.1† | - |
| Australia | 2016 | < 15 | 0.02† | - | |
| Novakovic 2016 | Australia | 2012 | - | 7 casesa | - |
| Australia | 2013 | - | 3 casesa | - | |
| Australia | 2014 | - | 2 casesa | - | |
| Australia | 2015 | - | 1 casea | - | |
| Teutsch 2022 | Australia | 2021 | < 15 | 0.1a | - |
| Zurynski 2018 | Australia | 2012 | < 16 | 0.2a | - |
| Australia | 2016 | < 16 | 0.02a | - | |
| Seedat 2018 | Lesotho | 2011–2013 | 0–14 | 0.5 | 1.0 |
| South Africa | 2011–2013 | 0–14 | 1.3 | 3.9 | |
| Oh 2021 | Korea | 2002–2014 | 0–12 | 0.3 | - |
| AoRRP | |||||
| Derkay 1995 | US | 1993–1994 | 18 + | 1.8 | - |
| Bomholt 1988 | Denmark | 1980–1983 | 18 + | 0.8 | 2.3 |
| Lindeberg 1990 | Denmark | 1974–1993 | 18 + | 3.9 | - |
| Omland 2012 | Norway | 1987–2009 | 18 + | 0.5 | - |
| Blackwell 2015 | Scotland | 2003–2014 | 18 + | - | 8.4 |
| Ndour 2020 | South Africa | 2009–2018 | 18 + | 3.1 | - |
| Seedat 2018 | South Africa | 2011–2013 | 18 + | 0.2 | 0.4 |
aIndicates those incidence values reporting data from the post-vaccination era
Pre-vaccination era
During the pre-vaccine era (up to 2006), a small number of studies (n = 3) aimed to assess burden of JoRRP in the US. In 1995, Derkay et al. reported incidence in the US of 4.3 per 100,000 among children < 14 years; calculated by extrapolating the results from a survey of board-certified otolaryngologists and members of two different medical associations comprising physicians specialized in treating diseases of the aerodigestive tract to the US population [20]. Incidence and prevalence estimates were also reported for JoRRP in Atlanta and Seattle (US) by identifying children < 18 years treated for JoRRP at a practicing otolaryngologist (1996) [21]. Incidence rates ranged from 0.4–1.1 per 100,000 persons, with prevalence of 1.7–2.6 in 100,000 persons. In addition, analysis of health insurance claims data from 2006 estimated that JoRRP incidence was 0.5 per 100,000 privately insured, and 1.0 per 100,000 publicly insured children aged < 18 years [22].
Outside of the US, robust data were reported for Scandinavian countries (Denmark and Norway), and Australia, but limited for the rest of the world (Canada, Africa, Korea: n = 4). Between 1980 and 1983, seven new cases of JoRRP presented out of an at-risk population of 300,000 children aged 0–14 years, indicating an incidence of 0.6 per 100,000 children in Denmark [23]. The incidence of JoRRP was calculated among Danish patients that lived in Fyn or Jutland at first presentation between 1965 and 1984. The observed incidence of JoRRP among children aged < 15 years was 0.5 per 100,000 [24]. Elsewhere, Omland et al. conducted a study to estimate the incidence of juvenile, and adult RRP in two Norwegian regions from 1987–2009 [25]. The overall incidence of JoRRP was 0.2 per 100,000 children per year. The median age at diagnosis was four years with a 3:1 male preponderance. The analysis did not detect a statistically significant change in incidence over the study period [25].
In Canada, a multicenter survey included 243 children < 15 years of age (1994–2007) estimated a mean incidence rate of 0.2 per 100,000 [26]. An earlier study in Denmark made comparable observations, estimating JoRRP incidence of 0.4 per 100,000 children [24]. Estimates of incidence and prevalence for JoRRP, between 2011–2015 for Free State province in South Africa among patients aged < 15 years were 1.3 per 100,000 per year, and 3.9 per 100,000, respectively [27]. In Korea, the incidence of JoRRP was estimated to be 0.3 per 100,000 person years during 2002–2014 [28]. The median age at diagnosis was four years (mean, 4.3) [28]. In Australia (2000–2013), the estimated national prevalence rate of RRP was 0.8 per 100,000 children aged < 15 years, peaking within the age range of 5–9 years (1.1 per 100,000) [29].
Post-vaccination era
Assessment of trends in JoRRP cases before, and after HPV vaccine introduction in the US found that JoRRP incidence was significantly lower among children born in the years following HPV quadrivalent vaccine introduction in 2006 [30]. Numbers of JoRRP cases, and incidences declined significantly, with an incidence rate ratio (IRR) of 0.2 when comparing births in 2012–2013 to those in 2004–2005, which authors concluded was indicative of the potential impact of HPV vaccination [30].
Similar declines in JoRRP were observed in Australia following the introduction of HPV quadrivalent vaccination in 2007. Novakovic et al. first reported the decreased incidence of RRP from 0.2 in 2012 to 0.02 in 2016 per 100,000 children in Australia, with subsequent analyses also observing significant decline since, with no reported new cases in 2022 [31–33].
HPV genotype distribution among JoRRP
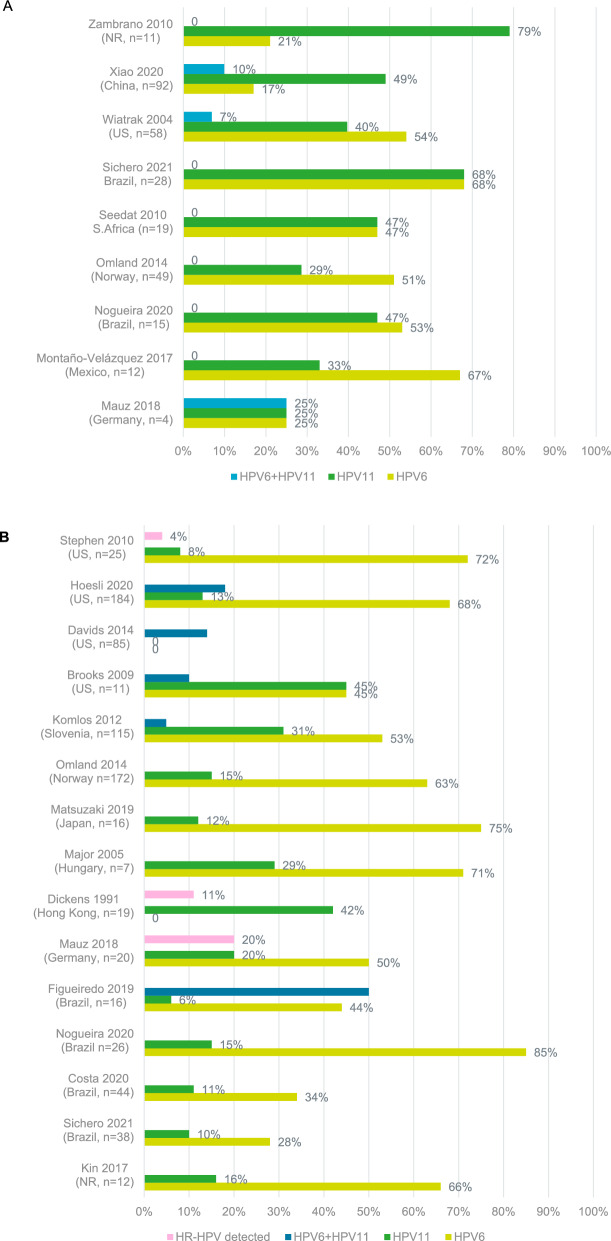
Twenty-one studies were identified which reported HPV status among JoRRP patients. HPV6 and HPV11 were found in more than 95% of JoRRP cases. The proportion of patients found to be positive for HPV6 ranged from 17–68%, whilst HPV11 was identified in 25–79% of patients. Co-infection with both HPV6 and HPV11 was observed in 7–25% of patients [34–42] (Fig. 1A).
Fig. 1.
Prevalence of HPV genotypes among patients with JoRRP (A) and AoRRP (B). HPV human papilloma virus, HR-HPV high risk human papilloma virus genotype, n number of patients included in sample, NR not reported, US United States. HP6 + HPV11 indicates co-infection with both genotypes
Epidemiology of AoRRP
Among the 23 studies identified in the epidemiology review for AoRRP, only seven studies provided data on incidence and prevalence across the globe and 16 studies reported HPV status among patients with AoRRP (Table 3). No study reported data on incidence in AoRRP pre- and post- HPV vaccination era.
Two studies reported AoRRP incidence in Denmark, and no recent data were identified. In the Copenhagen region (1980–1983) estimated incidence was 0.8 per 100,000 adults while the prevalence was 2.3 per 100,000 adults [43]. Meanwhile, Lindeberg et al. estimated that the incidence of RRP in Denmark was 0.4 per 100,000 in adults (1964–1984) [24]. In Norway, a population-based study reported incidence rates of RRP in adults to be 0.5 per 100,000 (1987–2009) [25]. Elsewhere in Europe, the prevalence of RRP in Scotland was reported to be 8.4 per 100,000 (2003–2014) [44].
Two studies provided epidemiology data for AoRRP in Africa. In South Africa, the incidence and prevalence of AoRRP were 0.2 per 100,000 per year, and 0.38 per 100,000, respectively (2011–2015) [27]. In Senegal, annual incidence rates were reported for adult laryngeal papillomatosis from 2009–2018, with an estimate of 3.1 cases of laryngeal papillomatosis per year [45].
HPV genotype distribution among AoRRP
Sixteen studies investigating HPV status of AoRRP patients were identified, with HPV6 and HPV11 representing the most common genotypes identified. The proportion of patients found to be positive for HPV6 ranged from 24–85%, whilst HPV11 was identified in 6–45% of patients [34, 36, 37, 39, 46–57] (Fig. 1B).
Summary of epidemiology findings
The majority of the studies presented data for patients with JoRRP. Among the studies that evaluated the impact of prophylactic HPV vaccination against genotypes HPV6 and HPV11 on incidence rates, a decline in the incidence of JoRRP was reported following the introduction of HPV quadrivalent vaccination. HPV6 and HPV11 were the most commonly identified genotypes among patients with JoRRP, accounting for more than 95% of JoRRP cases. Limited epidemiological evidence was available for AoRRP, however, HPV6 and HPV11 were also the most commonly identified genotypes among patients with AoRRP.
Clinical burden
Due to the volume of evidence identified in the clinical burden review (N = 1,033), the most recent data was prioritized to include only those studies from 2012 onwards. Upon the completion of the screening process, 75 studies were identified (n = 24 for AoRRP, and n = 48 for JoRRP) (PRISMA flow included in Supplementary Appendix S2).
The sample size for studies evaluating JoRRP was varied and ranged from 4–721 patients while for AoRRP it ranged from 8–174 patients. Median age of onset reported for JoRRP ranged from 1.6–3.5 years, and median age of onset for AoRRP was 34 years. Across the studies, balanced gender distribution was generally observed; 66% of studies which provided gender distribution had male population that was greater than 50%. In terms of ethnicity, only 14 studies reported these data: non-Hispanic White, White, or Caucasian were the most frequently reported. A detailed summary of the study details, along with population characteristics is included in Supplementary Appendix 3. The literature search identified more studies for JoRRP providing data on disease symptoms compared with AoRRP studies (n = 21, and n = 11, respectively).
Disease manifestations
The clinical manifestation of RRP is heterogenous; making it difficult to understand the natural history of disease. This was reflected in the symptomatic presentations reported among the studies, with a wide range of symptoms reported among patients with JoRRP or AoRRP. This was particularly evident among studies which reported the manifestations of JoRRP, due to the age variations among the children included.
Impaired voice quality is one of the hallmark symptoms in both JoRRP, and AoRRP, as reflected in the symptoms reported in both groups. Due to upper airway involvement, the other most common disease presentation in RRP was related to breathing difficulty. Dyspnea and upper airway obstruction were also reported among both AoRRP, and JoRRP patients, whilst stridor, shortness of breath, respiratory difficulty, and respiratory distress were reported among JoRRP patients only. The less common presentations included mouth breathing, noisy breath, snoring, weight loss, and abnormal cry in JoRRP patients. Dysphonia and dysplasia were also frequently reported (Table 4).
Table 4.
Symptomatic presentations among patients with JoRRP and AoRRP
| Symptom | Proportion of patients, range |
|---|---|
| JoRRP | |
| Cough [64–67] | 4–89% |
| Dysphagia [65, 66, 68, 69] | 1–16% |
| Weak/abnormal crying [58, 70] | 20–24% |
| Difficulty speaking [67] | 46–56% |
| Difficulty breathing [66] | 29%* |
| Difficulty swallowing [58] | 8%* |
| Dysphonia [50, 69, 70] | 80–100% |
| Dysplasia [69, 71] | 10–21% |
| Dyspnea [50, 67–70] | 44–96% |
| Severe dyspnea [72, 73] | 67–69% |
| Failure to thrive [64] | 26%* |
| Horseness [58, 64–66, 74–78] | 33–100% |
| Raspy voice [67] | 63–97% |
| Respiratory difficulty [47, 58, 65] | 27–66% |
| Respiratory disorder [74] | 50%* |
| Respiratory distress [75, 78] | 29–60% |
| Severe airway obstruction [65, 66] | 6–7% |
| Shortness of breath [75] | 40%* |
| Snoring [66, 75] | 1–40% |
| Stridor [47, 58, 64–67, 75] | 5–100% |
| Throat pain [67] | 13–22% |
| Upper airway obstruction [64, 79] | 42–100% |
| Voice impairment [58, 80–82] | 57–100% |
| Noisy breath [75] | 13%* |
| AoRRP | |
| Dysphagia [68, 69] | 14–38% |
| Dysphonia [68, 69] | 63–100% |
| Dysplasia [48, 59, 69, 83] | 19–50% |
| Dyspnea [68, 69] | 13–20% |
| Upper airway obstruction [79] | 70%* |
| Voice impairment [84–87] | 100%* |
*Study does not report a range
Risk factors and factors impacting severity
The factors generally associated with more aggressive or severe RRP were observed to be frequency of repeated surgeries, higher Derkay score (0–75, used to quantify RRP severity by grading on extent of anatomic involvement across 25 respiratory sites; with 0 = no lesion, 1 = surface lesion, 2 = raised lesion, and 3 = bulky lesion) history of tracheostomy, or distal spread (involvement of glottic, subglottic region, trachea or lungs) across the evidence [50, 58–61]. Younger age at diagnosis has also been found to be associated with more aggressive disease, indicative of the more burdensome nature of JoRRP compared with AoRRP. Patients with JoRRP were observed to have more instances of distal spread. Glottic involvement, supraglottic involvement, lower respiratory tract spread, along with pulmonary lesions has been frequently reported among patients with JoRRP.
AoRRP may be associated with an increased number of lifetime sexual partners; however, further research is required to determine which sexual practices are specifically associated with AoRRP [62]. Additionally, laryngo-pharyngeal reflux was also identified as a risk factor in AoRRP, although no study has yet identified any reduction in disease recurrence or severity with treatment of laryngo-pharyngeal reflux [63]. Children with JoRRP were commonly firstborn and delivered vaginally to young mothers; most of the mothers reported no HPV vaccination prior to delivery [58].
Across the identified evidence, many patients with JoRRP had received tracheostomy, and demonstrated greater severity of the disease. Omland et al. confirmed that patients with JoRRP are more severely affected by distal spread of the disease compared with AoRRP (p = 0.03) and that the disease was more aggressive in juveniles than adults (p < 0.001); with aggressive disease defined as distal spread, tracheostomy, four surgical operations annually or more than 10 surgeries in total. Along with the distal spread, a significantly higher frequency of operations was observed in JoRRP patients during first three years from onset (p < 0.001). The number of tracheostomies were also significantly higher in JoRRP patients (p < 0.001) [61].
HPV genotyping also appears to be an important consideration in JoRRP. Patients with JoRRP positive for HPV11 infection have more aggressive disease than those with reported HPV6 [36]. A cross-sectional study conducted among young Mexican patients with RRP concluded that children infected by HPV11 were more impaired by the disease, as they experienced tracheobronchial involvement, and more frequent surgical excisions (p = 0.03) [35]. Elsewhere, it was reported that the number of procedures per child with JoRRP was 20, and 25 for HPV6 and HPV11, respectively. HPV11 also appears more frequently in children than in adults [88]. One study reported that among JoRRP patients, the mean disease severity score with HPV11 infection was significantly higher than that with HPV6 infection (p = 0.013) [75]. Elsewhere, Montano-Velasquez et al. reported that when compared to patients with JoRRP, those testing positive for HPV6 received a significantly higher number of surgical excisions compared with those with HPV11 (median 12 vs 3; p = 0.03) [35]. Notably, HPV6 detection was significantly higher in AoRRP cases (p = 0.03), whereas HPV11 was more prevalent in JoRRP cases (p = 0.02), with prevalence increasing with JoRRP severity [47].
An association of multiple HPV coinfections with increasing disease severity was also highlighted. Derkay’s score (assessing anatomical severity, ranging from 0–75) at the diagnosis increased with the increasing number of co-infections, reaching 21 for ≥ 3 HPV co-infections vs. 9 for patients with mono-infection (p = 0.018) [89]. In addition to the maximum number of surgical procedures per year (p = 0.012), the average number of surgical procedures/year (p = 0.022), and the total number of surgeries also increased with increasing number of HPV co-infections. Patients with HPV6/HPV11 co-infection showed the worse clinical course [89]. HPV typing can be used as a potential tool for predicting the aggressiveness of disease.
It was also reported that a younger age at presentation of the disease is a key predictor for worse outcome [36, 61, 90]. The younger the patient at diagnosis, the higher the number of surgeries required, and the worse the Derkay score is at each surgery [36, 71]. Younger age at diagnosis and infection with HPV11 were both significantly associated with more severe disease among children with JoRRP [58]. It was also found that children diagnosed at an earlier age are more likely to present with airway symptoms and require more frequent surgeries to control the disease [81]. However, gender was not found to be a predictor of disease aggressiveness in JoRRP or AoRRP [71].
One study reported that based on histopathology, AoRRP patients experienced significantly more dysplastic features on compared to the JoRRP population (50% vs. 10%, respectively; p < 0.001), and 4.5% of AoRRP patients had directly developed malignant degeneration [69]. Moreover, pulmonary involvement, which has been linked to malignant degeneration, was identified in 8–9% of patients with JoRRP [91, 92]. Elsewhere, it was also demonstrated that smoking increased the chance of malignant transformation in RRP patients and led to a more severe clinical course, requiring additional surgical interventions. Smokers who developed carcinoma had significantly more debulking during their disease course than those not developing carcinoma (6.6 vs. 2.0, p = 0.019) [93].
Treatment burden
As there is no cure for RRP, surgical intervention is required to manage the disease, to ensure airway patency, and improve voice quality [81]. Some individuals may require surgery every few weeks while others may only require surgery twice a year or only a few times during their life as the recurrence of papilloma is unpredictable [81]. Surgical techniques used to treat individuals with RRP include “cold” excision, micro debridement, various pulsed dye lasers, or carbon dioxide lasers. More frequent or repeated surgical procedures in a short span of time complicates patients’ day-to-day life [38, 40–42, 46, 48–55]. Among the few studies that compared AoRRP and JoRRP, it was observed that multiple surgical excisions were documented more significantly in the JoRRP group than the AoRRP group [61, 71, 79]. A summary of the burden incurred by repeated surgeries has also been included in the economic burden review, from a healthcare resource utilization perspective.
In JoRRP patients, HPV11 genotype and increased number of distal spread locations were linked to increased surgical excisions [35, 41, 58, 94]. Young age was observed to be the most important determinant of disease severity, when looking at frequency of surgery [61, 81, 90, 95]. Children presenting with airway symptoms were younger at presentation (median 2.1 years, range 0.4–8.8 years) than those presenting with voice symptoms (median 6.7 years, range 1.0–15.1 years). It was reported that the number of surgeries per year, over the course of the disease, were 1.2 times higher in patients with airway symptoms at time of diagnosis, compared to patients with voice symptoms at time of diagnosis [81]. The evidence also demonstrated a statistically significant decrease in surgery rate with increasing age, and time from initial diagnosis [81].
Few adjuvant therapies are being investigated to manage the treatment burden, by increasing the time interval between surgeries, or by improving the Derkay score [82, 96, 97]. Further complications after surgical procedures contribute to the additional overall burden incurred. Post-operative complications usually include laryngeal sequelae, vocal cord adhesion, laryngotracheal stenosis, lower airway dissemination, surgical scarring, and lost voice, among others. [61, 70, 72, 73, 98].
Summary of clinical burden findings
The clinical manifestation was heterogenous, as reflected by the wide range of symptoms reported among patients with JoRRP or AoRRP. Impaired voice quality was identified as a hallmark symptom, and was reported in patients with both JoRRP and AoRRP. Patients with JoRRP were observed to have more aggressive disease, greater instances of distal spread, and more surgical excisions compared to patients with AoRRP. Furthermore, it was also reported that a younger age at presentation of the disease represents a key predictor for worse outcome. Patients with JoRRP who tested positive for HPV11 were found to have more aggressive disease than those with HPV6.
Humanistic burden
In the humanistic burden review, 18 studies were identified in total. Of those, seven studies evaluated patients with JoRRP, and 12 studies evaluated patients with AoRRP; one study reported on both JoRRP and AoRRP (PRISMA flow included in Supplementary Appendix S2). A summary of study characteristics is included in Supplementary Appendix S4. Due to the absence of a validated disease-specific tool for measuring health related quality of life (HRQoL) in RRP, generic scales were used to evaluate the humanistic burden among patients with RRP.
Humanistic burden in JoRRP
Pediatric Voice-Related Quality-of-Life (PVRQoL) is a measure of voice-related quality of life (scoring from 0–100, with higher scores indicating higher HRQoL) that has been validated in the pediatric population, consisting of 10 items that can be used for proxy-rating or self-rating in competent older children. Rogers et al. identified a marked impact on voice-related quality of life in children with RRP [82]. A total score of 52.5 was recorded at baseline which was improved to 70 (p = 0.02) post-treatment with bevacizumab [82].
The general HRQoL of pediatric RRP population was studied employing the Pediatric Quality of Life Inventory (PedsQL) in two studies. Lindman et al. demonstrated that compared with healthy controls, children with RRP aged 5–18 years self-reported a significantly worse HRQoL as measured by the PedsQL (p < 0.05) [99]. There were lower mean PedsQL Total Scores, Psychosocial Health scores, School Functioning scores, and Social Functioning scores self-reported by children with RRP. The parent reports also reflected lower quality of life for children with RRP compared with healthy children. Elsewhere, it was also demonstrated that children with RRP and their parents perceive a poor quality of life, compared with the healthy children. Additionally, it was found that the infection by HPV11 might increase the impact of the disease on quality of life in children [35].
The effect of the voice disorder on daily life was studied with a Voice Handicap Index (VHI) questionnaire in a retrospective study conducted by Ilmarinen et al. in adult patients with a history of JoRRP. A score from 0–30 represents ‘‘minimal handicap’’, a score from 31–60 ‘‘moderate handicap’’, and a score from 61–120 ‘‘serious handicap” [100]. It was found that the patients’ mean total score (21.1) was higher than that of controls (13.6), but no statistically significant differences occurred between RRP patients, and controls in any of the VHI subscales. According to the values of VHI, five patients (28%) had a moderate handicap, while all others had minimal handicap [100].
So et al. reported quality of life outcomes of an international cohort of patients with JoRRP derived from the Recurrent Respiratory Papillomatosis Foundation (RRPF)—Coordination of Rare Diseases at Sanford registry from a quality of life questionnaire designed by patients, and their family members of the RRPF [67]. Voice quality was a significant source of concern for patients in the study cohort, with many patients avoiding social engagement due to voice issues (p = 0.05). The vast majority of patients with JoRRP (90.6%) reported experiencing taunting or bullying from peers leading to social anxiety and worsened HRQoL, by impacting day-to-day activities [67].
Two studies provided data on health state utility values (HSUV). Ilmarinen et al. conducted a retrospective study to evaluate the HSUV in JoRRP by using 15D questionnaire (utility score scale ranging from 0 to 1 across 15 different dimensions of health). The results of the study found that a mean score of 0.9 was comparable to that reported for controls (1.0) [100].
Chadha et al. conducted a cross-sectional study to evaluate the utility burden of RRP in children by using four tools: the Health Utilities Index version three (HUI-3), PVRQoL, the impact of Family scale (IFS), and a visual analogue scale (VAS). The study estimated that the health utility of children with RRP was impaired and established an undesirable patient health state [101].
Humanistic burden in AoRRP
Voice handicap index (VHI; n = 3 studies), voice related quality of life (VRQoL) (n = 2), 36-Item Short Form Health Survey (SF-36; n = 2), 12-Item Short Form Health Survey (SF-12; n = 1), RAND-36 (n = 1), and a self-reported impact on quality-of-life questionnaire (n = 2) were among the generic scales used to measure humanistic burden in AoRRP. One study also reported patient reported outcomes in terms of impact of RRP on social interactions, and personal feelings [102].
Awad et al. identified a marked impact on voice-related quality of life in adults with RRP [84]. The initial VHI mean score was 47.0 (SD 24.7), which improved to 23.4 (SD 25.7) (p = 0.01) after surgical treatment with coblation [84]. Another study also reported significant improvements in VHI scores post-surgical ablation of papillomas (p < 0.001) [85]. HRQoL in AoRRP patients was also measured using VHI-10. Scores for the VHI-10 range from 0–40, with higher scores indicating a greater voice-related handicap [103]. A score greater than 11 is considered abnormal, and scores ranging from 0–10 are considered normal, or nearly normal. The mean VHI-10 score for patients with AoRRP included in the study fell within the abnormal category (17.2) [103].
Two studies evaluated HRQoL in AoRRP using SF-36 compared with a general population. Patients with AoRRP scored lower than those of the general population, specifically in domains for role limitation, energy/vitality, and pain [104]. Elsewhere, AoRRP patients were reported to be equivalent to controls in every category on the SF-36 except for physical functioning, and emotional problems [62]. Further, the patients were arbitrarily assigned to two groups; a ‘mild group” with lesion score (0–2), and a “marked group” with lesion score (3–5). These two groups were then scored separately on the various domains of the SF-36 and compared with the general population, with marked disease patients indicating lower SF-36 scores than those of the general population in all domains [62].
Two studies reported HRQoL in AoRRP using VRQoL; a questionnaire based on 10 core items, with responses scored on a scale from 1–5 (excellent to very bad). Both studies reported the impact of treatment on HRQoL in AoRRP. Zeitels et al. conducted a prospective study to evaluate the HRQoL in AoRRP using VRQoL, on which 0 indicates the lowest voice-related quality of life, and 100 indicates the highest voice-related quality of life. Total score at baseline was 42.9, which significantly improved to 83.3 post-treatment with bevacizumab and laser treatment of RRP (p < 0.001) [105]. Suter et al. reported initial VRQoL mean score among patients to be 38, which significantly improved to 21.1 after treatment with adjuvant therapy, based on the immunomodulatory effects of pegylated interferon α−2a and GM-CSF (p < 0.001) [87]. A phase II trial also reported that treatment with pembrolizumab resulted in improved social interactions, and decreased work-related absences in patients with AoRRP [102]. Meanwhile, in another study which used SF-12 to determine the impact of cidofovir treatment in patients with AoRRP, no significant improvement in HRQoL was reported after receiving treatment [106].
Using the same methods as for patients with JoRRP, So et al. also reported HRQoL outcomes in patients with AoRRP. The results of the study demonstrated high mental and fiscal burden, and worsening HRQoL among patients with AoRRP. Voice quality was a significant source of concern for patients in the study cohort, with many patients (46%) avoiding social engagement due to voice issues. The vast majority (78%) of patients with AoRRP experienced limitations in their career options due to RRP, and 18% reported experiencing job loss due to RRP-related work absence [67].
Summary of humanistic burden findings
Across the range of patient reported outcome (PRO) measures used to assess HRQoL, considerable impairment was observed in patients with JoRRP or AoRRP compared with healthy controls across a number of domains, including aspects relating to voice quality, and overall HRQoL.
Economic burden of JoRRP and AoRRP
In total, 23 studies were identified in the economic burden review (PRISMA flow included in Supplementary Appendix S2). A summary of the study details is also available in Supplementary Appendix 5.
Of the 12 studies identified which included economic data for patients with RRP, five studies provided data on cost burden (US, n = 4; UK, n = 1). In the US, JoRRP was associated with substantial economic burden. Based on data from the National Registry for RRP, and the Maryland Health Services Cost Review Commission, annual medical costs associated with treatment of JoRRP were estimated at 42–67 million USD in the year 1994 [107]. Furthermore, another survey in 1993–1994 found that among US pediatric patients 16,597 (95% CI 6938–26,255) surgical procedures were performed for JoRRP over the time period, at a cost of approximately 109 million USD [20]. By comparison, among AoRRP patients, 9,284 surgical procedures were performed at a cost of about 42 million USD [20].
In 2012, an estimated overall annual direct medical cost of 8.0 billion USD (10.8 billion USD in 2023, adjusted for inflation) was reported relating to the prevention and treatment of HPV-associated disease. Of this total, $0.2 billion (2.1%) was associated with RRP, with JoRRP accountable for a larger proportion of this figure compared to AoRRP (1.5% vs. 0.6%, respectively). That is, an annual cost of treatment for JoRRP of 123 million USD (2010 cost year) [108]. Using commercial insurance claims data from 2011–2014, and Texas Medicaid from 2008–2012, patients with JoRRP were compared to a matched control population to determine the cost differences incurred between the two groups in the first two years following diagnosis of JoRRP. In commercially insured patients, the mean first two years’ cost difference between cases and controls was $58,733. In the Texas Medicaid population, the mean first two years’ cost difference between cases and controls was $76,115 [109]. For AoRRP, the analysis demonstrated that costs were higher compared toto controls during the first two months following diagnosis, then decreased significantly, and remained similar to costs for controls for the remaining 22 months of follow-up [109].
In a tertiary health care setting in the UK, a total of 18 JoRRP cases were identified in the preceding six years (2005 onwards), from proven histological reports. Comprehensive clinical coding was carried out for 14 patients to reflect accurately their entire clinical management journey within the hospital, with costs calculated using standard National Health Service tariffs. The summative cost of JoRRP over the time period was reported to be £225,000 among the 14 patients [110]. Notably, these data should be used with caution as the study was published as a conference abstract with limited information.
Eight studies provided data on resource use (US, n = 6; Brazil, n = 1, and Norway, n = 1) (Table 5 and Table 6). However, the primary objectives of the identified studies were not to evaluate the health care resource utilization in JoRRP, but the studies did include data on surgical procedures, the standard treatment in RRP. In the US, the annual mean number of surgeries per child with JoRRP ranged from 4.4–5.1 [107, 111, 112]. Although not all studies were consistent, as another study reported a median of six lifetime surgeries for JoRRP [58]. Furthermore, the number of lifetime papilloma operations was also reported, which indicated that whilst 1–5 was the most common response (25%), many patients received considerably more surgeries (6–10: 21%, 11–20: 21%, 21–40: 16%, 41–100: 10%, > 100: 7%) [113]. So et al. reported that 84% of patients required 0–2 healthcare related visits per month, and 16% required 3–5 visits per month [67].
Table 5.
Summary of studies assessing cost burden and healthcare resource utilisation in patients with JoRRP
| Study | Country | Year of data collection | Parameter | Value [cost year] |
|---|---|---|---|---|
| Studies presenting cost data in patients with JoRRP | ||||
| Bishai 2000 | US | 1994 | Annual medical costs | $42–67 m USD [NR] |
| Estimated cost per case | $29,946 USD [NR] | |||
| Chesson 2012 | US | 2004–2007 | Annual medical costs | $123 m USD [2010] |
| Cost per case | Mean (range): 150,000 (72,000–387,000) USD [2010] | |||
| Derkay 1995 | US | 1993–1994 | Total cost of surgical procedures | $109 m USD [NR] |
| Per surgical procedure | $6,593 USD [NR | |||
| Narasimhan 2012 | UK | 2005–2011 | Summative treatment cost among 14 patients | £225,000 [NR] |
| Tam 2020 | US | 2011–2014 and 2008–2012 | Medical cost in the first 2 years after diagnosis for patients with newly diagnosed JoRRP |
Approx. $76,000 USD [2015] (Medicaid patients) |
|
Approx. $59,000 USD [2015] (privately insured) | ||||
| Studies presenting resource data in patients with JoRRP | ||||
| Armstrong 1999 | US | 1997–1998 | Annual surgical treatments | Mean (range): 4.4 (0.2–19.3) |
| Bishai 2000 | US | 1994 |
Number of office visits (per surgery) |
Mean (range): 3 (2.4–2.6) |
| Derkay 1995 | US | 1993–1994 | Lifetime papilloma operations |
11–20 operations: 21% 21–40 operations: 16% 41–100 operations: 10% > 100 operations: 7% |
| Reeves 2003 | US | 1996–2002 | Annual surgical treatments | Mean (range): 5.1 (0.4–21.5) |
| Amiling 2021 | US | 2015–2020 | Lifetime surgeries | Median (IQR): 6 (3–13) |
| So 2022 | US | NR | RRP-related healthcare visit |
0–2 visits: 84% 3–5 visits: 16% |
| Nogueira 2021 | Brazil | 2008–2015 | Number of surgeries | Mean (SD): 7.0 (5.71) |
| Omland 2014 | Norway | 1987–2009 | Annual surgical treatment |
Median (IQR): 1.2 (0.5–3.4) Mean (SD): 2.0 (2.1) |
AoRRP adult-onset recurrent respiratory papillomatosis, IQR Interquartile range, JoRRP juvenile onset recurrent respiratory papillomatosis, NR not reported, RRP Recurrent respiratory papillomatosis, SD standard deviation, USD US dollar
Table 6.
Summary of studies assessing cost burden and healthcare resource utilisation in patients with AoRRP
| Study | Country | Year | Parameter | Value [cost year] |
|---|---|---|---|---|
| Studies presenting cost data in patients with AoRRP | ||||
| Harrison 2016 | UK | 2013–2014 | Total cost | £107,478 [2014] |
| Operating theatre cost | £92,828 [2014] | |||
| Chesson 2012 | US | 2010 | Annual medical costs | Baseline (range): $48 m (2–236 m) USD [2010] |
| Derkay 1995 | US | 1993–1994 | Surgical procedures | $42 m USD [NR] |
| Tam 2020 | US | 2011–2014 and 2008–2012 | Medical cost in the first 2 years after diagnosis for patients with newly diagnosed AoRRP |
Approx. $5,000 USD [2015] (Medicaid patients) |
|
Approx. $11,000 USD [2015] (privately insured) | ||||
| Miller 2017 | UK | 2005–2012 | Operating theatre cost | Mean: $12,382 USD [NR] |
| Office procedure cost | Mean: $3,413 [NR] | |||
| Studies presenting resource data in patients with AoRRP | ||||
| Miller 2017 | UK | 2005–2012 |
Number of surgeries In-office laser procedures per patient: |
Mean (SD): 13.5 (19.1) Mean (SD): 4.2 (17.1) |
| Derkay 1995 | US | 1993–1994 | Lifetime papilloma operations |
11–20 operations: 15% 21–40 operations: 5% 41–100 operations: 10% > 100 operations: 9% |
| Nogueira 2020 | Brazil | 2008–2015 | Number of surgeries |
HPV6 mean (SD): 3.4 (2.0) HPV11 mean (SD): 4.5 (3.5) |
| Omland 2014 | Norway | 1987–2009 | Annual surgical treatment per year | Median: 1.2 |
| Annual surgical treatment during first three years | Median: 2.3 | |||
| Studies evaluating the impact of HPV vaccination on resource utilization in patients with AoRRP | ||||
| Kin 2017 | Germany | NR | Incidence rate of surgeries |
Pre-vaccination: 47.4/1000 patient months Post-vaccination: 6.7/1000 patient months p < 0.0001 |
| Matsuzaki 2020 | Japan | 2012–2018 | Number of surgeries |
Mean before combination therapy: 3.3 Mean after combination therapy: 1.6 |
| Yiu 2019 | US | 2012–2017 | Number of surgeries |
Pre-vaccination: 2.7 Post-vaccination: 0.8 p = 0.014 |
AoRRP adult-onset recurrent respiratory papillomatosis, HPV Human papillomavirus, IQR Interquartile range, JoRRP juvenile onset recurrent respiratory papillomatosis, RRP Recurrent respiratory papillomatosis, NR not reported, USD US dollar
Among AoRRP patients in the US, the mean number of in-office laser procedures per patient lifetime was reported as 4.2, with 5.4 months mean interval between procedures (N = 17; although 10 patients underwent only 1 office procedure). The mean number of operating room procedures was 13.5, and the mean interval between procedures was 14.3 months. The difference in cost between the office procedure (mean: $3,413.00) and the operating room procedure (mean: $12,382.59) was almost $9,000, but these savings were offset by the fact that the office procedures needed to be performed three times as often [114]. A prospective study conducted in Brazil observed that patients with AoRRP needed on average 3.6 surgeries to control the disease [36]. Another study conducted in Norway reported an average of 2.4 surgeries per year were required [37].
A retrospective registry-based study suggested a large impact of RRP on employment-related measures posing a fiscal burden on the patients in the US. About 37% of participants reported missing > 10 workdays per month due to RRP related complications and/or treatment [67]. Most patients (78%) admitted feeling limited in their career options due to RRP, while 24% of patients lost a job due to absences related to AoRRP [67].
Whilst not included as the primary objective of the economic burden review, several studies which evaluated HPV vaccination in patients with AoRRP, and its impact on healthcare resource utilization were also captured [14, 53, 56]. The results of a retrospective study conducted in the US showed an increase in time between procedures, and a decrease in number of procedures needed per year in patients with RRP [14]. Meanwhile, in Germany a seven-fold reduction in incidence rate of surgeries from the post-vaccine period was reported, compared to the pre-vaccine period (p < 0.0001) [53]. Whilst in Japan, a decrease in the number of surgeries required was reported post-vaccination (mean: 3.3 vs 1.6, respectively) [56].
Summary of economic burden findings
The economic burden was reported to be considerable, particularly among patients with JoRRP. Healthcare resource utilization was driven by the need for surgical interventions. The mean annual number of surgeries among patients with JoRRP ranged from 2.0–5.1, however one study reported that 7% of patients underwent over 100 lifetime papilloma surgeries (Table 5). Evidence also indicated that the number of surgeries decreased post-HPV vaccination in patients with AoRRP (Table 6).
Discussion
This review provided a detailed summary of the currently available published evidence on the disease landscape for RRP, characterizing the epidemiology, clinical burden, humanistic burden and economic burden in this underrecognized, and life-threatening disease. Particular attention was given to ensure that the nuances between JoRRP and AoRRP were captured, reflective of the different mode of transmission, and distinct patient populations associated with each disease subtype.
RRP is a lesser-known disease due to HPV, and disease is classified into two distinct sub-types based on age at diagnosis: commonly with JoRRP (< 18 years old) and AoRRP (> 18 years old). As has been previously reported, RRP is associated with a characteristic bimodal age distribution, which was validated by the median age of onset reported in this study (4 years and 34 years). However, elsewhere, a recent study identified a third peak in disease onset at 64 years, which represents an important aspect for future research (2, 3). When considering the epidemiological findings presented in this study, it is important to note the lack of specific International Classification of Diseases (ICD) codes regarding JoRRP or AoRRP, which may contribute towards the underestimation of the disease, and account for some of the heterogeneity observed among the studies included. However, the introduction of an RRP-specific ICD-11 code in 2024 represents a positive step, and may help facilitate improved accuracy of reporting going forward [115].
The incidence and prevalence of RRP among low-to-middle income countries are likely underestimated when compared to high income countries. Patients in low-to-middle income countries may have reduced access to healthcare, including ear, nose, and throat (ENT) services which, in turn, might result in fewer cases being diagnosed and recorded. In some cases, patients may also succumb to upper airway obstruction prior to presentation [4]. Unfortunately, the healthcare limitations associated with RRP in low-to-middle income countries also extend to both its prevention and management. For instance, a 2023 study reported notable limitations of HPV vaccination programs in Africa. National vaccination programs were only present in 9/19 countries surveyed, and of those, crucially, only four programs used a vaccine which protected against HPV6 and HPV11, highlighting the need for an updated approach to HPV vaccination in the region [116]. Furthermore, whilst surgical techniques to manage RRP have evolved from the use of non-powered laryngeal instruments to less invasive techniques, including lasers, and microdebriders, such advancements may be less readily available in low-to-middle countries which in turn impacts the effectiveness of treatment received [117–121].
Patients with RRP usually present with symptoms of airway involvement, including chronic cough, hoarseness, voice change, and breathing difficulties. However, due to the physiological differences associated with disease onset at different ages, considerable heterogeneity is observed between JoRRP and AoRRP, as well as between different patients with each respective condition. Due to the unspecific clinical presentations (particularly in JoRRP), the disease can mimic other common laryngeal and respiratory diseases such as laryngitis, asthma, bronchitis, and croup, which can negatively impact the accuracy of diagnosis leading to inadequate initial treatment [58, 64, 68, 76]. Importantly, delayed diagnosis is associated with a greater number of surgical procedures needed to control disease. Patients who underwent surgical excision within a year of symptom onset required fewer surgical procedures than those patients with delays of more than one year (p = 0.015) [78]. Notably, Derkay et al. reported that 7% of patients with JoRRP, and 9% of patients with AoRRP had undergone over 100 papilloma-related surgeries in their lifetime [20]. As such, improving awareness of the condition to help provide timely, and accurate diagnoses represents an opportunity to alleviate the clinical, humanistic, and economic burden associated with JoRRP and AoRRP.
Patients with JoRRP, and AoRRP, experienced considerably impaired HRQoL, mainly due to the severity and unpredictability of the disease. JoRRP, and AoRRP are devastating diseases for the patients, and for the parents of children suffering from JoRRP. Across both populations, assessment of the impact of voice disorder was among the most assessed domain, with significant impairment identified in RRP compared to healthy controls in several studies. There remains an unmet need for additional studies to measure the extent of HRQoL impairment across other domains, to effectively capture the full extent of the humanistic burden, particularly considering the wide array of symptoms reported in this review. As highlighted by San Giorgio et al., habituation to chronic diseases, such as RRP, can also lead to the negative impact on HRQoL reported by patients to be diminished due to response shift [122, 123]. Therefore, the burden experienced by patients with RRP may be underrepresented among the studies included in this review. Furthermore, humanistic burden was exclusively measured using generic PRO measures, as no RRP-specific PRO measure currently exists. The development of an appropriate disease-specific measure may prove challenging due to the heterogeneous symptomatic presentations, but efforts to improve the specificity of HRQoL assessment are to be encouraged. Notably, in addition to voice disorder, one study reported that infection with HPV11 may be associated with increased impact of RRP on HRQoL in children with JoRRP [35]. Although not included in the scope of this review, it is important to consider the substantial impact of JoRRP on patient’s families, and caregivers, due to the patients inability to use their voice, and the constant interruptions to daily life cause by repeated surgical interventions [124].
HPV infection is intrinsically linked to the development of both AoRRP and JoRRP. In contrast to other more common HPV-related pathologies, HPV genotypes with a high-risk of cancer were not frequent among those most associated with RRP. Across all the studies HPV6, HPV11, or co-infection with both HPV6 and HPV11 were the most frequently identified. Patients with HPV11 were found to have more aggressive disease, were more likely to require surgical intervention, and patients with HPV11 also present at a significantly younger age than those with HPV6 disease [40, 61, 125, 126]. HPV genotyping has therefore been suggested as a possible means of predicting disease aggressiveness, and its consideration during HPV-screening among at risk individuals is pertinent and should be encouraged [126]. In the classification of HPV-associated cancers, HPV6 and HPV11 are both classified as “low-risk” which may further contribute to the unrecognition of the severity of the disease. Importantly, HPV6 and HPV11 genotypes are not among those currently included in the available bivalent HPV vaccines. In Australia and the US, the introduction of vaccination programs including HPV6 and HPV11 were found to help contribute to a significant reduction in the incidence of JoRRP [30–33]. Improved primary prevention through vaccination is essential, and the effective rollout of HPV vaccination programs will not only help to reduce incidence rates, but also alleviate the substantial clinical, humanistic and economic burden associated with RRP. Encouragingly, quadrivalent and nonavalent HPV vaccines have an updated label within European Union and in the UK on the prevention of JoRRP by vaccinating girls and women of childbearing potential [127, 128]. The potential impact of vaccination has also been highlighted in studies among small cohorts of patients with RRP, providing preliminary evidence that it may be associated with a reduction of autoinoculation, through increased antibody responses to specific HPV genotypes, and a reduced incidence of new lesions necessitating surgical removal [129].
The management of JoRRP and AoRRP remains challenging since there no current conclusive treatment protocol exists, and the focus is primarily on maintaining airway patency, and voice quality [130, 131]. In 2020, The International Pediatric Otolaryngology Group (IPOG) published consensus recommendations to improve care provision and outcomes among patients with JoRRP [132]. The recommendations layout diagnostic considerations, surgical management, systemic adjuvant therapies, postoperative management, surveillance, and voice evaluation. Patients often require multiple surgeries with short intervals, and occasionally adjuvant therapy when surgery alone is unsuccessful. As such, JoRRP and AoRRP are associated with high-cost burden, and resource utilization. In the US for example, the annual mean number of surgeries per child with JoRRP ranged from 4.4–5.1, and 15% of patients required ≥ 3 healthcare related visits per month [67, 107, 111, 112]. For patients with AoRRP in the US, the mean number of lifetime operating room procedures was 13.5, and the mean interval between procedures was 14.3 months [114]. Whilst high rates of surgical interventions were generally reported, variation in severity of the disease was also reflected in resource utilization. Notably, JoRRP ($123 million, USD) was accountable for approximately three times the cost burden of AoRRP in 2010, although this was considerably lower than the costs associated chronic diseases such as cancer ($125 billion in 2010), and diabetes ($245 billion in 2012) [133, 134]. The high costs associated with RRP could be avoided by effective prophylactic measures, and efforts to improve prevention efforts should be encouraged. Limited evidence was available outside of the US, and up to date studies evaluating economic burden, and resource utilization are required. In addition to the economic costs incurred, repeated surgery affects the structure of the vocal folds, causing scars, incomplete vocal fold closure, and poor mucosal wave, which may ultimately reduce vocal quality [135].
Whilst adjuvant therapies have been explored as an addition to the treatment options available for patients with JoRRP or AoRRP, as yet, none appear to provide patients with a cure [136]. Preliminary data suggest that cidofivir, bevacizumab, and pembrolizumab may be beneficial therapeutic options in some patients [6–12]. However, their administration is restricted to off-label use, since none of these treatments are currently indicated for the treatment of RRP. It is important to note that costs associated with such off-label treatments can be considerable, and since the evidence captured in this review largely predate their use, the economic burden described herein likely represents an underestimation of the costs associated with disease management. Peri-treatment prophylactic HPV quadrivalent and nonavalent vaccination is also under investigation and has been shown to be effective in reducing recurrence among patients undergoing surgical procedures [13–15]. Although not included as the primary objective of the economic burden review, three studies were identified which reported the impact of peri-treatment HPV vaccination on healthcare resource utilization associated with AoRRP. Among the studies, HPV vaccination among patients with AoRRP was associated with increased intersurgical intervals, and fewer surgical procedures per year [14, 53, 56]. The results of these studies are consistent with the findings of a number of recent systematic literature reviews and meta-analyses in this area, which indicated that vaccination, in combination with surgical interventions, may be an effective approach to help reduce the clinical burden (and therefore the associated healthcare resource utilization) incurred through the surgical management of RRP [13, 137–139].
This study provides a detailed, and exhaustive overview of the current disease landscape, providing a global view of the current status quo, whilst capturing the nuances between patients with AoRRP and JoRRP. To date, a limited number of systematic reviews have been conducted to evaluate the clinical, humanistic, and economic burden in patients with RRP and as such, this study provides a valuable summary of the current literature for this underrecognized and poorly understood disease. Whilst the lack of comparable studies limits our ability to contextualize the findings of this review within existing literature, this review helps fill the current evidence gap in the literature. A limitation of this systematic review is that the studies included in the economic burden were often outdated (> 10 years), which may underrepresent the economic burden associated with RRP. Additional studies which provide timely estimates of the economic burden in this setting will be important. There was also limited evidence available evaluating the impact of HPV vaccination compared to the pre-vaccination era.
Conclusion
Extensive clinical, humanistic and economic burden is associated with both JoRRP and AoRRP, particularly given the chronic nature of the condition and its tendency to recur and spread throughout the respiratory tract. The findings of this review suggest that JoRRP is more burdensome than AoRRP and that HPV11 genotype is particularly associated with more aggressive disease. The repeated surgical interventions required to manage the disease contribute to the high clinical and cost burden associated with the condition, as well as negatively impacting the HRQoL of patients. In the absence of curative treatment, preventive measures should be considered, as the rollout of HPV vaccination programs against HPV6 and HPV11 has been shown to reduce the number of new cases of JoRRP. Further studies should be performed regarding the impact on AoRRP cases. Efforts to improve the coverage of primary prevention strategies represents a key area of opportunity, and should be encouraged, alongside further research to find an effective approach to adjuvant therapy to reduce the number of surgical procedures. Despite the existing literature, RRP remains a poorly understood disease, and future research evaluating risk factors, treatment/disease management, and prevention options are needed.
Supplementary Information
Acknowledgements
The authors thank Adam Hall of Parexel, UK, for providing editorial and writing support for the development of the manuscript, which was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The authors would also like to thank Elizabeth Russel of Merck & Co., Inc., Rahway, NJ, USA for contributions in the scoping of this study.
Abbreviations
- AoRRP
Adult-onset recurrent respiratory papillomatosis
- CA
Conference abstract
- CDSR
Cochrane database of systematic reviews
- CI
Confidence interval
- DNA
Deoxyribonucleic acid
- ENT
Ear, nose, and throat
- EUROGIN
European research organization on genital infection and neoplasia
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- HPV
Human papillomavirus
- HR-HPV
High risk HPV
- HRQoL
Health related quality of life
- HSUV
Health state utility values
- HUI-3
Health utilities index version three
- ICD
International classification of diseases
- IFS
Impact on family life scale
- IPOG
International pediatric otolaryngology group
- IPVC
International papillomavirus conference
- IQR
Interquartile range
- IRR
Incidence rate ratio
- ISPOR
International society for pharmacoeconomics and outcomes research
- JA
Journal article
- JoRRP
Juvenile-onset recurrent respiratory papillomatosis
- MSD
Merck, sharp & dohme
- NICE
National institute for health and care excellence
- NR
Not reported
- PedsQL
Pediatric quality of life inventory
- PICOTS
Population, intervention, comparisons, outcomes, time, study design
- PRISMA
Preferred reporting items for systematic reviews and meta-analysis
- PRO
Patient reported outcome
- PVRQoL
Pediatric voice-related quality of life
- RAND-36
RAND corporation 36-item health-related quality-of-life survey instrument
- RCT
Randomized controlled trial
- RRP
Recurrent respiratory papillomatosis
- RRPF
Recurrent respiratory papillomatosis foundation
- SD
Standard deviation
- SF-36
36-Item short form health survey
- UK
United Kingdom
- US
United States
- USD
United States dollars
- VAS
Visual analogue scale
- VHI
Voice handicap index
- VRQoL
Voice related quality of life
Author contributions
Study concept and design: OO, EM Acquisition of data: MV, RP Analysis and interpretation of data: MV, RP, OO, EM, KE Drafting of the manuscript: MV, RP, OO, EM, KE Critical revision of the manuscript for important intellectual content: EM, OO, KE. Acquisition of data: MV, RP. Analysis and interpretation of data: MV, RP, OO, EM, KE. Drafting of the manuscript: MV, RP, OO, EM, KE. Critical revision of the manuscript for important intellectual content: EM, OO, KE.
Funding
Funding for the development of this manuscript has been provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
OO and KE are employees of MSD (UK) Limited, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock in Merck & Co., Inc., Rahway, NJ, USA. EM is an employee of Merck Sharp & Dohme. MV and RP and employees of Parexel International.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fortes HR, Escuissato DL, Araujo Neto CA, Zanetti G, Hochhegger B, et al. Recurrent respiratory papillomatosis: a state-of-the-art review. Respir Med. 2017;126(116):116–21. [DOI] [PubMed] [Google Scholar]
- 2.San Giorgi MR, van den Heuvel ER, Tjon Pian Gi RE, Brunings JW, Chirila M, Friedrich G, et al. Age of onset of recurrent respiratory papillomatosis: a distribution analysis. Clin Otolaryngol. 2016;41(5):448–53. [DOI] [PubMed] [Google Scholar]
- 3.Stamataki SNT, Korres S, Felekis D, Tzangaroulakis A, Ferekidis E. Juvenile recurrent respiratory papillomatosis: still a mystery disease with difficult management. Head Neck. 2007;29(2):155–62. [DOI] [PubMed] [Google Scholar]
- 4.Seedat RY. Juvenile-onset recurrent respiratory papillomatosis diagnosis and management - a developing country review. Pediatric Health Med Ther. 2020;11:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez GI, Jaramillo R, Cuello G, Quintero K, Baena A, O’Byrne A, et al. Human papillomavirus genotype detection in recurrent respiratory papillomatosis (RRP) in Colombia. Head Neck. 2013;35(2):229–34. [DOI] [PubMed] [Google Scholar]
- 6.Lepine C, Leboulanger N, Badoual C. Juvenile onset recurrent respiratory papillomatosis: what do we know in 2024? Tumour Virus Res. 2024. 10.1016/j.tvr.2024.200281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derkay CS, Wikner EE, Pransky S, Best SR, Zur K, Sidell DR, et al. Systemic use of bevacizumab for recurrent respiratory papillomatosis: who, what, where, when, and why? Laryngoscope. 2023;133(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Xu J, Liu M, Zhu D, Xiao Y. 155P Systemic bevacizumab in pediatric patients with aggressive recurrent respiratory papillomatosis. ESMO Open. 2024. 10.1016/j.esmoop.2024.102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pai S, Muniappan A, Park J, Friedman A, Campbell N, Krantz S, et al. 992O Pembrolizumab for HPV-associated recurrent respiratory papillomatosis. Ann Oncol. 2024;35:S676. [Google Scholar]
- 10.So RJ, Hidalgo Lopez JC, Ballestas SA, Klein AM, Steuer C, Shin DM, et al. Efficacy of systemic bevacizumab for recurrent respiratory papillomatosis with pulmonary involvement. Laryngoscope. 2024;134(2):577–81. [DOI] [PubMed] [Google Scholar]
- 11.Tjon Pian Gi R, Ilmarinen T, Van Den Heuvel E, Aaltonen L, Andersen J, Brunings J, et al. Safety of intralesional cidofovir in patients with recurrent respiratory papillomatosis: an international retrospective study on 635 RRP patients. Euro Archi Oto-Rhino-Laryngol. 2013;270:1679–87. [DOI] [PubMed] [Google Scholar]
- 12.Tran MN, Galt L, Bashirzadeh F. Recurrent respiratory papillomatosis: the role of cidofovir. Respirol Case Rep. 2018;6(8): e00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg T, Philipsen BB, Mehlum CS, Dyrvig A-K, Wehberg S, Chirilǎ M, et al. Therapeutic use of the human papillomavirus vaccine on recurrent respiratory papillomatosis: a systematic review and meta-analysis. J Infect Dis. 2019;219(7):1016–25. [DOI] [PubMed] [Google Scholar]
- 14.Yiu Y, Fayson S, Smith H, Matrka L. Implementation of routine HPV vaccination in the management of recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2019;128(4):309–15. [DOI] [PubMed] [Google Scholar]
- 15.Papaioannou V, Lux A, Voigt-Zimmermann S, Arens C. Treatment outcomes of recurrent respiratory papillomatosis: retrospective analysis of juvenile and adult cases. HNO. 2018;66(Suppl 1):7–15. [DOI] [PubMed] [Google Scholar]
- 16.Welschmeyer A, Berke GS. An updated review of the epidemiological factors associated with recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol. 2021;6(2):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertino G, Pedretti F, Mauramati S, Filauro M, Vallin A, Mora F, et al. Recurrent laryngeal papillomatosis: multimodal therapeutic strategies Literature review and multicentre retrospective study. Acta Otorhinolaryngol Ital. 2023. 10.14639/0392-100X-suppl.1-43-2023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman E, Reuschenbach M, Kaminski A, Ronnebaum S. Human papillomavirus vaccine impact and effectiveness in six high-risk populations: a systematic literature review. Vaccines. 2022. 10.3390/vaccines10091543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivancic R, Iqbal H, deSilva B, Pan Q, Matrka L. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol. 2018;3(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derkay CS. Task force on recurrent respiratory papillomas: a preliminary report. Arch Otolaryngol Head Neck Surg. 1995;121(12):1386–91. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong LRPEJD, Reichert M, Phillips DL, Nisenbaum R, Todd NW, Jacobs IN, Inglis AF, Manning SC, Reeves WC. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis. 2000;31(1):107–9. [DOI] [PubMed] [Google Scholar]
- 22.Marsico M, Mehta V, Chastek B, Liaw KL, Derkay C. Estimating the incidence and prevalence of juvenile-onset recurrent respiratory papillomatosis in publicly and privately insured claims databases in the United States. Sex Transm Dis. 2014;41(5):300–5. [DOI] [PubMed] [Google Scholar]
- 23.Cristensen P-HJK, Grontved A. Juvenile papillomatosis of the larynx A 45 years’ follow up from the county of Funen Denmark. Acta Otolaryngol. 1984;98:37–9. [Google Scholar]
- 24.Lindeberg H, Elbrond O. Laryngeal papillomas: the epidemiology in a Danish subpopulation 1965–1984. Clin Otolaryngol Allied Sci. 1990;15(2):125–31. [DOI] [PubMed] [Google Scholar]
- 25.Omland T, Akre H, Vardal M, Brondbo K. Epidemiological aspects of recurrent respiratory papillomatosis: a population-based study. Laryngoscope. 2012;122(7):1595–9. [DOI] [PubMed] [Google Scholar]
- 26.Campisi P, Hawkes M, Simpson K. Canadian juvenile onset recurrent respiratory papillomatosis working G The epidemiology of juvenile onset recurrent respiratory papillomatosis derived from a population level national database. Laryngoscope. 2010;120(6):1233–45. [DOI] [PubMed] [Google Scholar]
- 27.Seedat RY, Schall R. Age of diagnosis, incidence and prevalence of recurrent respiratory papillomatosis-a South African perspective. Clin Otolaryngol. 2018;43(2):533–7. [DOI] [PubMed] [Google Scholar]
- 28.Oh JK, Choi HY, Han M, Jung YS, Lee SJ, Ki M. Estimated incidence of juvenile-onset recurrent respiratory papillomatosis in Korea. Epidemiol Health. 2021;43: e2021019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novakovic D, Cheng AT, Baguley K, Walker P, Harrison H, Soma M, et al. Juvenile recurrent respiratory papillomatosis: 10-year audit and Australian prevalence estimates. Laryngoscope. 2016;126(12):2827–32. [DOI] [PubMed] [Google Scholar]
- 30.Meites E, Stone L, Amiling R, Singh V, Unger ER, Derkay CS, et al. Significant declines in juvenile-onset recurrent respiratory papillomatosis following human papillomavirus (HPV) vaccine introduction in the United States. Clin Infect Dis. 2021;73(5):885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novakovic DBJ, Garland S, Chen A, Zurynski Y, Tabrizi S. An australian national prospective study of the incidence of juvenile onset recurrent respiratory papillomatosis. Intern Med J. 2016;46:23–4. [Google Scholar]
- 32.Zurynski YBJML, Novakovic D, Cheng ATL, Booy R, Berkowitz R, Garland SM, Elliott E, Black R. Reduced rates of juvenile onset recurrent respiratory papillomatosis in Australia after implementation of a national HPV vaccination program. Arch Disease Childhood. 2018;103:203. [DOI] [PubMed] [Google Scholar]
- 33.Teutsch SM, Nunez CA, Morris A, Eslick GD, Berkhout A, Novakovic D, et al. Australian paediatric surveillance unit (APSU) annual surveillance report 2021. Commun Dis Intell. 2018;2022:46. [DOI] [PubMed] [Google Scholar]
- 34.Mauz PS, Schafer FA, Iftner T, Gonser P. HPV vaccination as preventive approach for recurrent respiratory papillomatosis - a 22-year retrospective clinical analysis. BMC Infect Dis. 2018;18(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montano-Velazquez BB, Nolasco-Renero J, Parada-Banuelos JE, Garcia-Vazquez F, Flores-Medina S, Garcia-Romero CS, et al. Quality of life of young patients with recurrent respiratory papillomatosis. J Laryngol Otol. 2017;131(5):425–8. [DOI] [PubMed] [Google Scholar]
- 36.Nogueira RL, Bonfim CM, Aragon DC, Damico TA, Miura CS, Passos IM, Nogueira ML, Rahal P, Valera FCP. HPV genotype is a prognosticator for recurrence of respiratory papillomatosis in children Clinical. Otolaryngology. 2020;46(1):181–8. [DOI] [PubMed] [Google Scholar]
- 37.Omland T, Lie KA, Akre H, Sandlie LE, Jebsen P, Sandvik L, et al. Recurrent respiratory papillomatosis: HPV genotypes and risk of high-grade laryngeal neoplasia. PLoS ONE. 2014;9(6): e99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seedat RY, Thukane M, Jansen AC, Rossouw I, Goedhals D, Burt FJ. HPV types causing juvenile recurrent laryngeal papillomatosis in South Africa. Int J Pediatr Otorhinolaryngol. 2010;74(3):255–9. [DOI] [PubMed] [Google Scholar]
- 39.Sichero L, Ferreira S, Lopez RVM, Mello BP, Costa V, El-Achkar VNR, et al. Prevalence of human papillomavirus 6 and 11 variants in recurrent respiratory papillomatosis. J Med Virol. 2021;93(6):3835–40. [DOI] [PubMed] [Google Scholar]
- 40.Wiatrak BJWDW, Broker TR, Lewis L. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114:1–23. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Y, Zhang X, Ma L, Wang J. Long-term outcomes of juvenile-onset recurrent respiratory papillomatosis. Clin Otolaryngol. 2021;46(1):161–7. [DOI] [PubMed] [Google Scholar]
- 42.Zambrano HAC, Vigliotti V, Lee SH, Zambrano E. Identification of human papillomavirus (HPV) genotypes in pediatric laryngeal papillomatosis (PLP). Lab Invest. 2010;90(2):296. [Google Scholar]
- 43.Bomholt A. Laryngeal papillomas with adult onset: an epidemiological Study from the copenhagen Region. Acta Otolaryngol. 1988;106:140–4. [DOI] [PubMed] [Google Scholar]
- 44.Blackwell NBA, MacDougall G. Adult onset recurrent respiratory papillomatosis: an evolving pattern. Int J Surge. 2015. 10.1016/j.ijsu.2015.07.255. [Google Scholar]
- 45.Ndour N, Maiga S, Houra A, Deguenonvo REA, Ndiaye C, Pilor N, et al. Laryngeal papillomatosis in adults: assessment for ten years at the ENT department of the national university hospital of fann (Dakar, Senegal). Int J Otolaryngol. 2020;2020:2782396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooks EG, Evans MF, Adamson CS, Peng Z, Rajendran V, Laucirica R, et al. In situ hybridization signal patterns in recurrent laryngeal squamous papillomas indicate that HPV integration occurs at an early stage. Head Neck Pathol. 2012;6(1):32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costa V, El-Achkar VN, de Barros PP, Leon JE, Ribeiro-Silva A, Carlos R, et al. Role of epstein-barr virus in the severity of recurrent respiratory papillomatosis. Laryngoscope. 2020;130(11):E611–8. [DOI] [PubMed] [Google Scholar]
- 48.Davids TMS, Wise JC, Johns MM, Klein A. Laryngeal papillomatosis associated dysplasia in the adult population: an update on prevalence and HPV subtyping. Ann Otol Rhinol Laryngol. 2014;123(6):402–8. [DOI] [PubMed] [Google Scholar]
- 49.Dickens P, Srivastava G, Loke SL, Larkin S. Human papillomavirus 6, 11, and 16 in laryngeal papillomas. J Pathol. 1991;165(3):243–6. [DOI] [PubMed] [Google Scholar]
- 50.Figueiredo C, et al. Prevalence and clinical implications of low-risk human papillomavirus among patients with recurrent respiratory papillomatosis in Rio de Janeiro. Brazil Auris Nasus Larynx. 2019;46:570–5. [DOI] [PubMed] [Google Scholar]
- 51.Hoesli R, Wingo ML, Richardson BE, Bastian RW. Identification of 11 different HPV subtypes in adult patients with recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg. 2020;163(4):785–90. [DOI] [PubMed] [Google Scholar]
- 52.Ilboudo M, Zohoncon TM, Traore IMA, Traore EMA, Kande A, Obiri-Yeboah D, Djigma FW, Gyebre YMC, Simpore J. Implication of low risk human papillomaviruses, HPV6 and HPV11 in laryngeal papillomatosis in Burkina Faso. Am J Otolaryngol. 2019;40(3):368–71. [DOI] [PubMed] [Google Scholar]
- 53.Kin Cho Goon PSL-U, Sudhoff H. Recurrent respiratory papillomatosis (RRP)–time for a reckoning? Laryngoscope Investigative Otolaryngol. 2017;2(4):184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komlos KF, Kocjan BJ, Kosorok P, Luzar B, Meglic L, Potocnik M, et al. Tumor-specific and gender-specific pre-vaccination distribution of human papillomavirus types 6 and 11 in anogenital warts and laryngeal papillomas: a study on 574 tissue specimens. J Med Virol. 2012;84(8):1233–41. [DOI] [PubMed] [Google Scholar]
- 55.Major T, Szarka K, Sziklai I, Gergely L, Czegledy J. The characteristics of human papillomavirus DNA in head and neck cancers and papillomas. J Clin Pathol. 2005;58(1):51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuzaki HMK, Hirai R, Suzuki H, Asai R, Oshima T. Multi-year effect of human papillomavirus vaccination on recurrent respiratory papillomatosis. Laryngoscope. 2020;130(2):442–7. [DOI] [PubMed] [Google Scholar]
- 57.Stephen J, Chen KM, Shah V, et al. Consistent DNA hypermethylation patterns in laryngeal papillomas. Int J Head Neck Surg. 2010;1(2):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amiling R, Meites E, Querec TD, Stone L, Singh V, Unger ER, et al. Juvenile-onset recurrent respiratory papillomatosis in the United States, epidemiology and HPV types-2015-2020. J Pediatric Infect Dis Soc. 2021;10(7):774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gluvajić DŠ-BM, Jerin A, Janša R, Hočevar-Boltežar I. The impact of laryngopharyngeal reflux on occurrence and clinical course of recurrent respiratory papillomatosis. Laryngoscope. 2022;132:619–25. [DOI] [PubMed] [Google Scholar]
- 60.Rodier C, Lapointe A, Coutlee F, Mayrand MH, Dal Soglio D, Roger M, et al. Juvenile respiratory papillomatosis: risk factors for severity. J Med Virol. 2013;85(8):1447–58. [DOI] [PubMed] [Google Scholar]
- 61.Omland T, Akre H, Lie KA, Jebsen P, Sandvik L, Brondbo K. Risk factors for aggressive recurrent respiratory papillomatosis in adults and juveniles. PLoS ONE. 2014;9(11): e113584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruiz R, Achlatis S, Verma A, Born H, Kapadia F, Fang Y, et al. Risk factors for adult-onset recurrent respiratory papillomatosis. Laryngoscope. 2014;124(10):2338–44. [DOI] [PubMed] [Google Scholar]
- 63.Formánek MJD, Komínek P, Matoušek P, Zeleník K. Laryngopharyngeal reflux and herpes simplex virus type 2 are possible risk factors for adult-onset recurrent respiratory papillomatosis (prospective case–control study). Clin Otolaryngol. 2017;42:597–601. [DOI] [PubMed] [Google Scholar]
- 64.Adebola S, Dunmade AD. Impediments to clinical diagnosis and management of juvenile-onset recurrent respiratory papillomatosis in Ilorin Nigeria. Open J Pediatrics. 2013;3:127–32. [Google Scholar]
- 65.Labedz G, Scatolini ML, Ruvinsky S, Rodriguez HA. Factors related to extralaryngeal spread in juvenile recurrent respiratory papillomatosis. Laryngoscope. 2021;131(7):1652–6. [DOI] [PubMed] [Google Scholar]
- 66.Scatolini MLCA, Pérez CG, Rodríguez HA. Laryngeal reconstruction in children with recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2018;15:120–4. [DOI] [PubMed] [Google Scholar]
- 67.So RJ, McClellan K, Best SR. Recurrent respiratory papillomatosis: quality of life data from an international patient registry. Laryngoscope. 2022. 10.1002/lary.30401. [DOI] [PubMed] [Google Scholar]
- 68.Carvalho A, Brito DS, Cunha APA, Monteiro PM, Ferreira MB, Assis D, et al. Recurrent respiratory papillomatosis: clinical characteristics and viral genotyping in a Brazilian population. Rev Inst Med Trop Sao Paulo. 2021;63: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hock K, Kennedy A, Howell R, Friedman A, de Alarcon A, Khosla S. Surgery and adjuvant therapy improve derkay scores in adult and pediatric respiratory papillomatosis. Laryngoscope. 2022;132(12):2420–6. [DOI] [PubMed] [Google Scholar]
- 70.Hermann JSPP, Weckx LLM, Fujita R, Avelino M, Pignatari SSN. Laryngeal sequelae of recurrent respiratory papillomatosis surgery in children. Rev Assoc Med Bras. 2012;58(2):204–8. [PubMed] [Google Scholar]
- 71.Khan M, Naidu TK. Risk factors associated with severe recurrent respiratory papillomatosis. S Afr J Infect Dis. 2019;34(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niu Z, Xiao Y, Ma L, Qu X, Wang Y, Zhou S, et al. Tracheotomy as a predictor of remission and demise for juvenile-onset recurrent respiratory papillomatosis. Acta Otolaryngol. 2022;142(1):84–8. [DOI] [PubMed] [Google Scholar]
- 73.Niu Z, Xiao Y, Ma L, Qu X, Zhou S, Wang Y, et al. The duration of tracheostomy dependence in patients with juvenile-onset recurrent respiratory papillomatosis. Acta Otolaryngol. 2022;142(7–8):610–5. [DOI] [PubMed] [Google Scholar]
- 74.Eftekhaar N, Niya M, Izadi F, et al. Human papillomavirus (HPV) genotype distribution in patients with recurrent respiratory papillomatosis (RRP) in Iran. Asian Pac J Cancer Prev. 2017;18:1973–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Intakorn P, Sonsuwan N. Human papillomatosis genotyping and severity in patients with recurrent respiratory papillomatosis. J Med Assoc Thai. 2014;97:136–41. [PubMed] [Google Scholar]
- 76.Martha VKS. Laryngeal papillomatosis: Treatment, trends and results among children in a developing country. Clin Otolaryngol. 2012;37:122–3. [Google Scholar]
- 77.Matinhira N, Soko ND, Bandason T, Jenson RG, Dzongodza T, von Buchwald C, et al. Human papillomavirus types causing recurrent respiratory papillomatosis in Zimbabwe. Int J Pediatr Otorhinolaryngol. 2019;116:147–52. [DOI] [PubMed] [Google Scholar]
- 78.Silva L, Goncalves CP, Fernandes AM, Damrose EJ, Costa HO. Laryngeal papillomatosis in children: the impact of late recognition over evolution. J Med Virol. 2015;87(8):1413–7. [DOI] [PubMed] [Google Scholar]
- 79.Orji FT, Okorafor IA, Akpeh JO. Experience with recurrent respiratory papillomatosis in a developing country: impact of tracheostomy. World J Surg. 2013;37(2):339–43. [DOI] [PubMed] [Google Scholar]
- 80.Delgado Malave BCAL, Bastidas Y, Arrieta N. Voice evaluation in juvenile respiratory papillomatosis. Otolaryngol Head Neck Surgery (United States). 2017;157:P232–3. [Google Scholar]
- 81.Mudd P, Wikner E, Rana MS, Zalzal G. Presenting symptom as a predictor of clinical course in juvenile onset recurrent respiratory papillomatosis. Laryngoscope. 2021;131(7):1670–5. [DOI] [PubMed] [Google Scholar]
- 82.Rogers DJOS, Maurer R, Hartnick CJ. Use of adjuvant intralesional bevacizumab for aggressive respiratory papillomatosis in children. JAMA Otolaryngol Head Neck Surgery. 2013;139(5):496–501. [DOI] [PubMed] [Google Scholar]
- 83.Lee C-JACT, Merati AL. Prevalence of diabetes mellitus and its impact on disease severity in adult recurrent respiratory papillomatosis. Otolaryngol Head Neck Surgery (United States). 2013;149(4):603–7. [DOI] [PubMed] [Google Scholar]
- 84.Awad R, Shamil E, Aymat-Torrente A, Gibbins N, Harris S. Management of laryngeal papillomatosis using coblation: another option of surgical intervention. Eur Arch Otorhinolaryngol. 2019;276(3):793–800. [DOI] [PubMed] [Google Scholar]
- 85.Chung TK, Hu A, Sardesai MG, Wilcox H, Jiang L, Meyer TK. Evaluating the effect of recurrent respiratory papillomatosis on work productivity. Ann Otol Rhinol Laryngol. 2022;131(7):709–14. [DOI] [PubMed] [Google Scholar]
- 86.Hung W-CLW-C, Fang K-M, Cheng P-W, Wang C-T. Longitudinal voice outcomes following serial potassium Titanyl phosphate laser procedures for recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2021;130(4):363–9. [DOI] [PubMed] [Google Scholar]
- 87.Suter-Montano T, Montano E, Martinez C, Plascencia T, Sepulveda MT, Rodriguez M. Adult recurrent respirator papillomatosis: a new therapeutic approach with pegylated interferon alpha 2a (Peg-IFNalpha-2a) and GM-CSF. Otolaryngol Head Neck Surg. 2013;148(2):253–60. [DOI] [PubMed] [Google Scholar]
- 88.Gruber M, Mills N, Blair D, Van Der Meer G, Mahadevan M. Safety of paediatric day-stay laryngeal surgery for recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2016;82:116–9. [DOI] [PubMed] [Google Scholar]
- 89.Bertinazzi M, Gheit T, Polesel J, McKay-Chopin S, Cutrone C, Sari M, et al. Clinical implications of alpha, beta, and gamma HPV infection in juvenile onset recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol. 2022;279(1):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buchinsky FJ, Valentino WL, Ruszkay N, Powell E, Derkay CS, Seedat RY, et al. Age at diagnosis, but not HPV type, is strongly associated with clinical course in recurrent respiratory papillomatosis. PLoS ONE. 2019;14(6): e0216697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lepine C, Voron T, Berrebi D, Mandavit M, Nervo M, Outh-Gauer S, et al. Juvenile-onset recurrent respiratory papillomatosis aggressiveness in situ study of the level of transcription of HPV E6 and E7. Cancers. 2020. 10.3390/cancers12102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Q, Li Y, Ma L, Xiao Y, Wang H, Ding Y, et al. Long-term outcomes of juvenile onset recurrent respiratory papillomatosis with pulmonary involvement. Laryngoscope. 2021;131(7):2277–83. [DOI] [PubMed] [Google Scholar]
- 93.Freeman TAD, Erickson E, Kim B, DeSilva B, Matrka L. Smoking and carcinoma trends in recurrent respiratory papillomatosis patients. Otolaryngol Head Neck Surg. 2021;165:P121–2. [DOI] [PubMed] [Google Scholar]
- 94.Moreddu E, Lambert E, Kacmarynski D, Nicollas R, Triglia JM, Smith RJ. Risk factors for severity of juvenile-onset recurrent respiratory papillomatosis at first endoscopy. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136(1):25–8. [DOI] [PubMed] [Google Scholar]
- 95.Qu X, Xiao Y, Ma L, Wang J. Distribution characteristics of juvenile-onset recurrent respiratory papillomatosis at first-time surgery. Ear Nose Throat J. 2021. 10.1177/01455613211049845. [DOI] [PubMed] [Google Scholar]
- 96.Ruiz R, Balamuth N, Javia LR, Zur KB. Systemic bevacizumab treatment for recurrent respiratory papillomatosis: long-term follow-up. Laryngoscope. 2022;132(10):2071–5. [DOI] [PubMed] [Google Scholar]
- 97.Meacham K, Thompson JW. Comparison of cidofovir and the measles, mumps, and rubella vaccine in the treatment of recurrent respiratory papillomatosis. Ear Nose Throat J. 2017;96(2):69–74. [DOI] [PubMed] [Google Scholar]
- 98.Shen M. XY, Ma L., Wang J. 2021 Analysis of related factors of postoperative complications of Juvenile-onset recurrent respiratory papillomatosis. Lin chuang er bi yan hou tou jing wai ke za zhi. Journal of clinical otorhinolaryngology head and neck surgery 35(4):312–5. [DOI] [PMC free article] [PubMed]
- 99.Lindman JPGMD, Morlier R, Wiatrak BJ. Voice quality of prepubescent children with quiescent recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2004;68(5):529–36. [DOI] [PubMed] [Google Scholar]
- 100.Ilmarinen T, Nissila H, Rihkanen H, Roine RP, Pietarinen-Runtti P, Pitkaranta A, et al. Clinical features, health-related quality of life, and adult voice in juvenile-onset recurrent respiratory papillomatosis. Laryngoscope. 2011;121(4):846–51. [DOI] [PubMed] [Google Scholar]
- 101.Chadha NK, Allegro J, Barton M, Hawkes M, Harlock H, Campisi P. The quality of life and health utility burden of recurrent respiratory papillomatosis in children. Otolaryngol Head Neck Surg. 2010;143(5):685–90. [DOI] [PubMed] [Google Scholar]
- 102.Pai SIFR, Muniappan A, Friedman AD, Park JC, Campbell NP, Krantz SB, Song PC, Nocon C, Carroll TL, Faquin WC, Shin K-Y, O’Neill AM, Wirth LJ. Patient-reported outcomes (PROs) from a phase II trial of pembrolizumab for HPVassociated papilloma patients with laryngeal, tracheal and/or pulmonary involvement. J Clin Oncol. 2021. 10.1200/JCO.2021.39.15_suppl.6080.34871101 [Google Scholar]
- 103.Kupfer RAÇTE, Barry JO, Allen CT, Merati AL. Anatomic derkay score is associated with voice handicap in laryngeal papillomatosis in adults. Otolaryngology Head Neck Surgery (United States). 2016;154(4):689–92. [DOI] [PubMed] [Google Scholar]
- 104.Hill DS, Akhtar S, Corroll A, Croft CB. Quality of life issues in recurrent respiratory papillomatosis. Clin Otolaryngol Allied Sci. 2000;25(2):153–60. [DOI] [PubMed] [Google Scholar]
- 105.Zeitels SM, Barbu AM, Landau-Zemer T, Lopez-Guerra G, Burns JA, Friedman AD, et al. Local injection of bevacizumab (Avastin) and angiolytic KTP laser treatment of recurrent respiratory papillomatosis of the vocal folds: a prospective study. Ann Otol Rhinol Laryngol. 2011;120(10):627–34. [DOI] [PubMed] [Google Scholar]
- 106.McMurray JS, Connor N, Ford CN. Cidofovir efficacy in recurrent respiratory papillomatosis: a randomized, double-blind, placebo-controlled study. Ann Otol Rhinol Laryngol. 2008;117(7):477–83. [DOI] [PubMed] [Google Scholar]
- 107.Bishai DKH, Shah K. The cost of juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2000;126(8):935–9. [DOI] [PubMed] [Google Scholar]
- 108.Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30(42):6016–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tam S, Wu CF, Peng HL, Dahlstrom KR, Sturgis EM, Lairson DR. Cost of treating recurrent respiratory papillomavirus in commercially insured and medicaid patients. Laryngoscope. 2020;130(5):1186–94. [DOI] [PubMed] [Google Scholar]
- 110.Narasimhan GKA, Davies K, Donne A. Paediatric recurrent respiratory papillomatosis-at what cost. Clin Otolaryngol. 2012;37:179. [Google Scholar]
- 111.Armstrong LR, Derkay CS, Reeves WC. Initial results from the national registry for juvenile-onset recurrent respiratory papillomatosis RRP Task Force. Arch Otolaryngol Head Neck Surg. 1999;125(7):743–8. [DOI] [PubMed] [Google Scholar]
- 112.Reeves WCRSS, Swanson KI, Derkay CS, Marcus A, Unger ER. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2003;129(9):976–82. [DOI] [PubMed] [Google Scholar]
- 113.Derkay CS, Bluher AE. Update on recurrent respiratory papillomatosis. Otolaryngol Clin North Am. 2019;52(4):669–79. [DOI] [PubMed] [Google Scholar]
- 114.Miller AJ, Gardner GM. In-office vs. operating room procedures for recurrent respiratory papillomatosis. Ear Nose Throat J. 2017;96(4–5):24–8. [PubMed] [Google Scholar]
- 115.WHO. WHO-FIC Foundation, RRP ICD-11. Available at: https://icd.who.int/browse/2024-01/foundation/en#151039887. 2024.
- 116.McGuire JK, Kabagenyi F, Peer S. Human papillomavirus vaccination in Africa: an airway perspective. Int J Pediatr Otorhinolaryngol. 2023;165: 111423. [DOI] [PubMed] [Google Scholar]
- 117.Dedo HH, Yu KC. CO(2) laser treatment in 244 patients with respiratory papillomas. Laryngoscope. 2001;111(9):1639–44. [DOI] [PubMed] [Google Scholar]
- 118.El-Bitar MA, Zalzal GH. Powered instrumentation in the treatment of recurrent respiratory papillomatosis: an alternative to the carbon dioxide laser. Arch Otolaryngol Head Neck Surg. 2002;128(4):425–8. [DOI] [PubMed] [Google Scholar]
- 119.Pasquale K, Wiatrak B, Woolley A, Lewis L. Microdebrider versus CO2 laser removal of recurrent respiratory papillomas: a prospective analysis. Laryngoscope. 2003;113(1):139–43. [DOI] [PubMed] [Google Scholar]
- 120.Patel N, Rowe M, Tunkel D. Treatment of recurrent respiratory papillomatosis in children with the microdebrider. Ann Otol Rhinol Laryngol. 2003;112(1):7–10. [DOI] [PubMed] [Google Scholar]
- 121.Schraff S, Derkay CS, Burke B, Lawson L. American society of Pediatric otolaryngology members’ experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg. 2004;130(9):1039–42. [DOI] [PubMed] [Google Scholar]
- 122.San Giorgi MRMAL-M, Rihkanen H, Tjon Pian Gi REA, van der Laan BFAM, Hoekstra-Weebers JEHM, Dikkers FG. Quality of life of patients with recurrent respiratory papillomatosis. Laryngoscope. 2017;127(8):1826–31. [DOI] [PubMed] [Google Scholar]
- 123.Schwartz CE, Bode R, Repucci N, Becker J, Sprangers MA, Fayers PM. The clinical significance of adaptation to changing health: a meta-analysis of response shift. Qual Life Res. 2006;15(9):1533–50. [DOI] [PubMed] [Google Scholar]
- 124.Recurrent Respiratory Papillomatosis Foundation. Recurrent Respiratory Papillomatosis Patient-Led FDA Listening Session October 27, 2022 2022 [Available from: https://rrpf.org/wp-content/uploads/2023/01/RRPF-FDA-Listening-Session-Summary_FINAL_1.24.23-1.pdf.
- 125.Niyibizi J, Rodier C, Wassef M, Trottier H. Risk factors for the development and severity of juvenile-onset recurrent respiratory papillomatosis: a systematic review. Int J Pediatr Otorhinolaryngol. 2014;78:186–97. [DOI] [PubMed] [Google Scholar]
- 126.European Medicines Agency. Gardasil 9 Summary of Product Characteristics 2023 [Available from: https://www.ema.europa.eu/en/documents/product-information/gardasil-9-epar-product-information_en.pdf.
- 127.European Medicines Agency. Gardasil Summary of Product Characteristics 2022 [Available from: https://www.ema.europa.eu/en/documents/product-information/gardasil-epar-product-information_en.pdf.
- 128.Reuschenbach M, Doorbar J, Del Pino M, Joura EA, Walker C, Drury R, et al. Prophylactic HPV vaccines in patients with HPV-associated diseases and cancer. Vaccine. 2023;41(42):6194–205. [DOI] [PubMed] [Google Scholar]
- 129.Baothman AAFI, Al-Maghraby H, Algarni M, Alayed MI, Felemban LHA. The effect of bevacizumab (Avastin) in treating juvenile recurrent respiratory papillomatosis. Otolaryngol Case Rep. 2023. 10.1016/j.xocr.2023.100544. [Google Scholar]
- 130.See G, Shakri NM. Long term outcome of juvenile onset recurrent respiratory papillomatosis in university kebangsaan medical centre. Clin Res. 2020;10(1):244–50. [Google Scholar]
- 131.Lawlor C, Balakrishnan K, Bottero S, Boudewyns A, Campisi P, Carter J, et al. International pediatric otolaryngology group (IPOG): Juvenile-onset recurrent respiratory papillomatosis consensus recommendations. Int J Pediatr Otorhinolaryngol. 2020;128: 109697. [DOI] [PubMed] [Google Scholar]
- 132.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jackowska JWW, Hashimoto A, Małaczyńska B, Piersiala K, Świdziński P, Wiskirska-Woźnica B, Wierzbicka M. Voice improvement in patients with recurrent respiratory papillomatosis after combined treatment with cidofovir and CO(2) laser surgery. Lasers Med Sci. 2019;34(7):1433–40. [DOI] [PubMed] [Google Scholar]
- 135.Avelino MA, Zaiden TC, Gomes RO. Surgical treatment and adjuvant therapies of recurrent respiratory papillomatosis. Braz J Otorhinolaryngol. 2013;79(5):636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goodman E, Reuschenbach M, Kaminski A, Ronnebaum S. Human papillomavirus vaccine impact and effectiveness in six high-risk populations: a systematic literature review. Vaccines. 2022;10(9):1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goon P, Sauzet O, Schuermann M, Oppel F, Shao S, Scholtz LU, et al. Recurrent Respiratory Papillomatosis (RRP)-Meta-analyses on the use of the HPV vaccine as adjuvant therapy. NPJ Vaccines. 2023;8(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ponduri A, Azmy MC, Axler E, Lin J, Schwartz R, Chirilă M, et al. The efficacy of human papillomavirus vaccination as an adjuvant therapy in recurrent respiratory papillomatosis. Laryngoscope. 2023;133(9):2046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bedard MC, de Alarcon A, Kou YF, Lee D, Sestito A, Duggins AL, et al. HPV strain predicts severity of juvenile-onset recurrent respiratory papillomatosis with implications for disease screening. Cancers (Basel). 2021. 10.3390/cancers13112556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gandhi S, Jacob R. Remission in juvenile-onset recurrent respiratory papillomatosis. J Laryngol Voice. 2012;2(1):30–4. [Google Scholar]
- 141.Lawlor CMDND, Nuss RC, Rahbar R, Choi SS. Laryngeal web in the pediatric population: evaluation and management. Otolaryngol Head Neck Surgery (United States). 2020;162(2):234–40. [DOI] [PubMed] [Google Scholar]
- 142.Perdana RFSMV, Herawati S, Yusuf M. Risk factors recurrent respiratory papilloma (RRP) on juvenile and adult type in tertiary hospital Indonesia. J Global Pharma Technol. 2020;2(6):409–15. [Google Scholar]
- 143.Perdana R, Herawati S, Surarso B, et al. Correlation of aggressivity papilloma recurrent respiratory tract with human papillomavirus types 6 and 11. Medico Legal Update. 2020;20(1):471–6. [Google Scholar]
- 144.Rasmussen ERSDT, Jørkov AS, Raja AA, Olsen CH, Homøe P. Long-term follow-up and outcome in patients with recurrent respiratory laryngeal papillomatosis. Danish Med J. 2017;64(12):5424. [PubMed] [Google Scholar]
- 145.Ribeiro V, et al. Histopathological features of juvenile-onset laryngeal papillomatosis related to severity. Head Neck. 2019;41:1412–7. [DOI] [PubMed] [Google Scholar]
- 146.Wang JHD-M, Ma L-J, Ye J-Y, Xiao Y, Yang Q-W. Risk factors of juvenile onset recurrent respiratory papillomatosis in the lower respiratory tract. Chinese Med J. 2012;125(19):3496–9. [PubMed] [Google Scholar]
- 147.Xi Y, Wang H, Wang W, Wang X, Zhang J, Zhao J, et al. Risk factors for aggressive recurrent respiratory papillomatosis in Chinese juvenile patients. Acta Otolaryngol. 2020;140(9):779–84. [DOI] [PubMed] [Google Scholar]
- 148.Zhang C. CB, Nan B., Chen Y., Gao J., Huang S., Xiang H., Yu X., Liu X., Luo B. 2014 Juvenile onset respiratory papillomatosis: risk factors for severity. Lin chuang er bi yan hou tou jing wai ke za zhi. Journal of clinical otorhinolaryngology, head, and neck surgery. 28(23):1848–51. [PubMed]
- 149.Qu X, Xiao Y, Ma L, Niu Z, Wang J. High recurrence rate in patients with juvenile-onset respiratory papillomatosis and its risk factors. Eur Arch Otorhinolaryngol. 2022;279(8):4061–8. [DOI] [PubMed] [Google Scholar]
- 150.Formanek M, Formankova D, Hurnik P, et al. Epstein-Barr virus may contribute to the pathogenesis of adult onset recurrent respiratory papillomatosis: a preliminary study. Clin Otolaryngol. 2021;46:373–9. [DOI] [PubMed] [Google Scholar]
- 151.Hao F, Yue L, Yin X, Wang X, Shan C. Low-temperature radiofrequency coblation reduces treatment interval and post-operative pain of laryngotracheal recurrent respiratory papillomatosis. 2020. Biosci Rep. 10.1042/BSR20192005. [DOI] [PMC free article] [PubMed]
- 152.Hu LBPA, Garber D, Wang B, Amin MR, Branski RC. Laryngeal distribution of adult-onset recurrent respiratory papillomatosis: a longitudinal study. Laryngoscope. 2019;129(9):1993–7. [DOI] [PubMed] [Google Scholar]
- 153.Toma SHK, Harriess M. Recurrent respiratory papillomatosis in the larynx: Postoperative web formation improves outcomes. Clin Otolaryngol. 2012;37:121. [Google Scholar]
- 154.Loizou C, Laurell G, Lindquist D, Olofsson K. Voice and quality of life in patients with recurrent respiratory papillomatosis in a northern Sweden cohort. Acta Otolaryngol. 2014;134(4):401–6. [DOI] [PubMed] [Google Scholar]
- 155.Van Nieuwenhuizen AJRRNPM, De Bree R, Leemans CR, Verdonck-de Leeuw IM. Patient reported voice outcome in recurrent respiratory papillomatosis. Laryngoscope. 2010;120(1):188–92. [DOI] [PubMed] [Google Scholar]
- 156.Rees CJPGN, Koufman JA. Cost savings of unsedated office-based laser surgery for laryngeal papillomas. Ann Otol Rhinol Laryngol. 2007;116(1):45–8. [DOI] [PubMed] [Google Scholar]
- 157.Zheng M. AN, Chambers T., O'Dell K., Johns M.M. Comparison of Treatment for Recurrent Respiratory Papillomatosis at a Public County Versus Private Academic Hospital. Journal of Voice. 2022. [DOI] [PubMed]
- 158.Chesson HWFSE, Gottlieb SL, Markowitz LE. The potential health and economic benefits of preventing recurrent respiratory papillomatosis through quadrivalent human papillomavirus vaccination. Vaccine. 2008;26(35):4513–8. [DOI] [PubMed] [Google Scholar]
- 159.Pasquale KWB, Woolley A, Lewis L. Microdebrider versus CO(2) laser removal of recurrent respiratory papillomas: a prospective analysis. Laryngoscope. 2003;113(1):139–43. [DOI] [PubMed] [Google Scholar]
- 160.Ding WMY, Ma C, Ma A, Tang W. PIN26 Lifetime cost estimation of human papillomavirus related diseases in China: a modelling study using real-world data. Value in Health. 2021;24:S110. [Google Scholar]
- 161.Bresse XAM, Largeron N, Roze S, Marty R. A comparative analysis of the epidemiological impact and disease cost-savings of HPV vaccines in France. Hum Vaccin Immunother. 2013;9(4):823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harrison A, Montgomery J, Macgregor FB. Economic impact of recurrent respiratory papillomas in a UK adult population. J Laryngol Otol. 2016;130(7):645–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hao F, Yue L, Yin X, Wang X, Shan C. Low-temperature radiofrequency coblation reduces treatment interval and post-operative pain of laryngotracheal recurrent respiratory papillomatosis. 2020. Biosci Rep. 10.1042/BSR20192005. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.