Abstract
Background
Increased global trade, while beneficial economically, can also increase the spread of vector-borne diseases, particularly those transmitted by Aedes mosquitoes spreading via trade routes. Given the heightened trade-induced activity at ports of entry, it is particularly crucial to assess the risk of mosquito-borne diseases in these settings. This study compared the risks of Aedes-borne disease in and around the eastern Tanzanian seaport of Tanga.
Methods
A 200 m × 200 m grid-based system was used to sample mosquitoes within the port area, and in surrounding areas at 2 km, 2.5 km, and 5 km away, between June and December 2023. We characterized mosquito breeding habitats, collected mosquito larvae using standard dippers and tested susceptibility of raised adult Aedes aegypti populations to different insecticides. Adult mosquitoes were collected using BG sentinel traps (daytime) and Centers for Disease Control (CDC) light traps (night-time). Additionally, more than 200 port users and neighboring residents were surveyed to assess their experiences with and perceptions of mosquito biting and disease risks.
Results
There were 2931 breeding sites, with (60.8%, n = 1782) positive for Aedes larvae. The percentage of water-holding containers infested with Aedes immatures, i.e., the container index (CI), was highest in the port area (66.2%), and lowest 5 km away (44.6%). The port area also had a greater proportion of temporary breeding sites (64.9%) than did the surrounding areas. The adult mosquito surveys revealed 20,449 mosquito species including: Culex quinquefasciatus (56.2%), Mansonia uniformis (38.6%), Ae. aegypti (5.1%), Anopheles gambiae (0.1%), and Anopheles funestus. Ae. aegypti were more abundant in the port area than in the surrounding areas (P < 0.001), whereas Culex sp., and Mansonia sp., were significantly outside (P < 0.001). Adult Anopheles sp., were found only in the port area, but Anopheles larvae were found both within and outside the port areas. Tests on Ae. aegypti sp., revealed susceptibility to bendiocarb and DDT, and resistance to permethrin. Awareness of mosquito-borne diseases among respondents was high for malaria (64.8%), but low for dengue (26.3%) and Chikungunya (1.7%). Most respondents reported being bothered by mosquitoes mostly at night (53.4%) or in the evening (40.7%). In addition to insecticidal bednets, which are used primarily against malaria, preventive measures for Aedes-borne diseases are limited.
Conclusions
This study identified significant potential risk of Aedes species, specifically Ae. aegypti sp., and associated diseases, but low perception of risk and inadequate personal protection measures in the study area. This low perception of risk highlights the need to improve public knowledge of the transmission and control of Aedes-borne diseases.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-024-06586-x.
Keywords: Aedes aegypti, Mosquito-borne, Habitat characterization, Malaria, Insecticide susceptibility, Tanga port, Tanzania
Background
In urban Africa, mosquito-borne diseases including dengue, chikungunya, and yellow fever are becoming a major health issue [1]. In addition to malaria, which causes 250 million cases and more than 600,000 deaths annually [2], the World Health Organization (WHO), has indicated that global dengue incidence rates have also been surged dramatically in recent years [3, 4]. The official WHO estimates suggest that 390 million infections occur annually, of which 96 million manifest clinically, even though only a very small proportion of these cases are officially recorded and reported [5]. It has also been estimated approximately 3.9 billion people across 128 countries are at risk of dengue infection [6]. This extensive burden, the multiplicity of mosquito-borne diseases—with half of the world’s population at risk of two or more of the diseases [7] and the close correlation with human factors such as urbanization and trade [8, 9], all underscore the urgent need for effective surveillance and control measures to mitigate the impact of dengue, especially in vulnerable regions.
In Tanzania, while the burden of malaria is closely tracked by research groups and the Ministry of Health, [10–12] the full burden of other mosquito-borne diseases, notably Aedes-borne diseases is largely unknown, except for isolated research activities and reviews. For example, while dengue fever is known to occur in Tanzania in all four serotypes alongside Chikungunya and potentially other Aedes-borne diseases [13, 14], the overall burden of dengue in the whole country remains uncertain. One previous review indicated that seroprevalence estimates range from 11% to 50.6% [15], and that rates can be particularly high among patients presenting themselves with fever to health facilities. Despite repeated outbreaks in recent years, comprehensive data on the epidemiology of dengue or the ecology of its vector species are very limited, although there is a growing interest in Aedes studies in the country [16–19]. This gap in knowledge is particularly concerning given the potential for dengue outbreaks to exacerbate public health challenges in a country already burdened by other mosquito-borne diseases such as malaria. Moreover, ecological, environmental and climate change concerns such as excessive temperature and frequent flooding [20], as well as poor waste management and uncontrolled water storage practices in urban areas [21, 22], will likely increase the burden and potentially disrupt health services. Indeed, it is now estimated that with warming climate, the dengue vector mosquitoes are expected to survive these warmer temperatures better than malaria vectors are expected to survive [23], meaning that Africa might see significant increases in Aedes-borne diseases as a direct result of climate change.
The ecology of Aedes mosquitoes is indeed intricately linked to urban environments, where they thrive in a variety of habitats especially human-made containers [24]. Aedes aegypti, the primary vector for dengue, chikungunya, and Zika, prefers to breed in artificial containers such as discarded vehicle tires, plastic containers, flowerpots, and water storage tanks [25], commonly abundant in urban settings. Like other container-breeding mosquito species, Ae. aegypti can thrive almost independently of rainfall seasons, as they exploit water sources that are continuously available in human communities. This ability allows them to maintain their population densities year-round, and therefore presents a persistent risk of disease transmission. Despite the clear importance of understanding Aedes ecology for public health, surveys and studies on these mosquitoes remain limited in Tanzania, even in large cities and small towns.
Global travel, trade, and urbanization are key drivers of the spread of vector-borne diseases [26], with ports playing a critical role in the dissemination of mosquito vectors such as Ae. aegypti, Ae. albopictus [27–29], and Culex species [30]. These vectors often establish themselves in port areas, where invasions tend to be higher due to abundant breeding habitats [31–33]. Ports along the Indian Ocean are particularly vulnerable, with recent invasions of Anopheles stephensi [34], Ae. albopictus, and re-invasions of Ae. aegypti being documented [35–38]. The WHO recommends vector surveillance at ports to keep mosquito densities below national thresholds in line with the International Health Regulations (IHR) [39], but many sub-Saharan African countries, including Tanzania, face challenges such as inadequate surveillance infrastructure [40]. A 2019 dengue outbreak along Kenya’s coast, linked to the DENV-3 serotype from Pakistan, demonstrates the risks posed by global travel [41], while studies in Tanzania indicate a serotype shift from DENV-3 to DENV-1 between 2017 and 2019, increasing the risk of severe symptoms due to antibody-dependent enhancement [42]. Surveys also show widespread transmission of all four dengue serotypes and chikungunya inland [43], highlighting the need for comprehensive vector surveillance in ports.
It is also widely recognized that community awareness and preparedness play a crucial role in the control and prevention of mosquito-borne diseases. In Tanzania, it is often assumed that there is already a high degree of awareness of dengue and other mosquito-borne diseases among communities due to extensive public health campaigns and education efforts [44]. However, knowledge about Aedes-borne diseases, such as dengue, chikungunya, and yellow fever, has not been adequately investigated. Yet insufficient knowledge poses a significant challenge for effective disease control, as communities may not take the necessary precautions to protect themselves from Aedes mosquitoes. Enhancing public awareness about Aedes-borne diseases and improving community preparedness through targeted education and outreach programs are essential steps in mitigating the risks posed by these diseases. This is particularly important in port areas, where the influx of travelers and goods increases the potential for disease spread.
The objective of this current study was to assess and compare the risks of disease-transmitting mosquitoes within the eastern Tanzanian seaport of Tanga and its surrounding urban areas along the Indian Ocean focusing especially on Ae. aegypti mosquitoes. We sought to establish the mosquito species composition, abundance, insecticide resistance status and their aquatic breeding preferences; and to use this information to evaluate and address the risks of mosquito-borne infections at this critical point of entry. Additionally, we assessed the perceptions of port users and residents of the surrounding urban areas to understand how such perceptions might inform effective control strategies including social and behaviour change communication strategies.
Methods
Study area
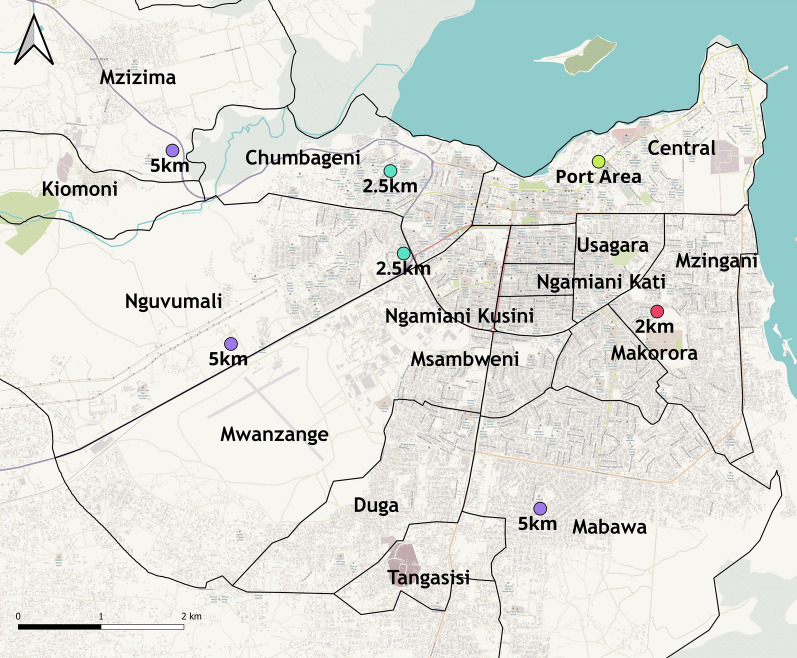
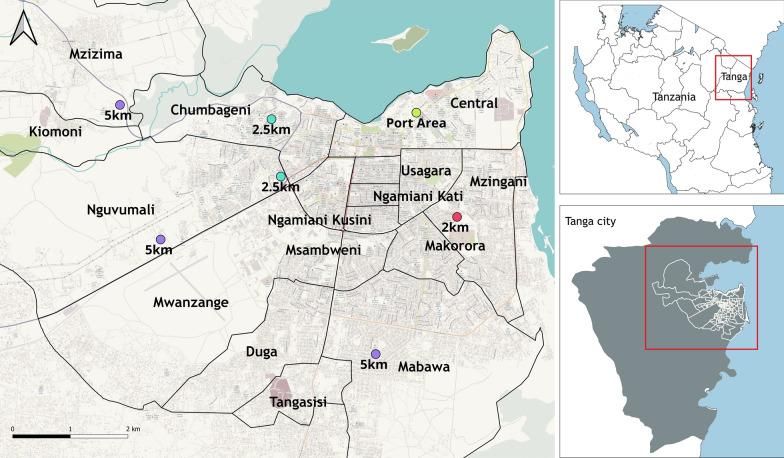
The study was conducted in the northeastern Tanzanian port city of Tanga (Fig. 1). The central location was the Tanga seaport, with additional sampling in eight surrounding wards within the Tanga City Council: Central (5° 04′ 19.26'' S, 39° 06′ 06.59'' E), Chumbageni (5° 04′ 14.66'' S, 39° 05′ 21.30'' E), Kiomoni (5° 03′ 13.24'' S, 38° 56′ 57.24'' E), Mzizima (5° 02′ 51.26'' S, 39° 02′ 37.72'' E), Nguvumali (5° 04′ 40.80'' S, 39° 04′ 39.87'' E), Mnyanjani (5° 05′ 56.22'' S, 39° 07′ 13.79'' E), Ngamiani Kusini (5° 05′ 00.42'' S, 39° 06′ 06.14'' E), and Magaoni (5° 06′ 12.49'' S, 39° 06′ 07.06'' E). Tanga District is a border district in the north-eastern part of Tanzania. It is bordered by the Mkinga District Council to the north, the Indian Ocean to the east, Muheza District to the south and west, and the country of Kenya to the north. The district seat is the city of Tanga, which also serves as the administrative and economic center of the region, major economic activities in this area including business, industrial activities, tourism, fishing and agriculture. According to the 2022 census, Tanga District had a total population of just fewer than 400,000 people and some ~100,000 households.
Fig. 1.
Map of study areas, showing the port area and surrounding wards. Map of the study area contains information from OpenStreetMap contributors, available under the Open Database License. https://help.openstreetmap.org/questions/83255/how-do-i-cite-osm-in-an-academic-paper
The Tanga seaport is the second largest international point of entry in Tanzania. It was the focal point of the study, and is located in the Central ward, which has an area of ~3.8 km2 and an altitude of 10 m above sea level. All sampling sites across all the 8 wards located within 5 km of the port area (Fig. 1).
Sampling design and site selection
A cross-sectional entomological survey was conducted between June and December 2023 (the dry season). The reference sampling was within the Tanga seaport in the Central ward (within 500 m of the seaport) but additional surveys were conducted at purposively selected sites 2.5 km and 5 km away from the port along three main roads: Pangani, Segera, and Mombasa Road, to understand mosquito distribution across the study area and expansion away from the port. An extra comparison site was selected 2 km south of the port area in the Mnyanjani ward. All the sites were located in the Tanga City Council (Fig. 1).
Sampling was conducted using a 200 m × 200 m grid-based system created in ArcGIS 11.1 environment (ESRI, USA), following the methodology used in a previous Aedes survey by Kahamba et al. [16]. Preference was given to grids with human habitation and a total of 23 grids used for the sampling. Each grid was assigned a unique identifier (grid ID) consisting of letters and numbers, with letters representing longitudes and numbers representing latitudes (e.g., A1, A2). The randomization and grid selection procedures were consistent across all locations. An initial survey of the study area was conducted with the assistance of volunteer community resource persons. During the survey, features conducive to mosquito breeding, such as water wells, drains, containers, pots, flowerpots, tins, waste collection points, discarded tires, and drums, were identified. The coordinates of these features were recorded using a handheld GPS (Magellan eXplorist GC, USA). In the central reference area around the Tanga seaport, all the grids within a 500 m buffer zone were selected but no data were collected inside the shipping vessels.
Sampling of adult mosquitoes
Adult mosquitoes were collected using two types of traps: BG-Sentinel traps or Aedes species, and Centers for Disease Control (CDC) light traps for Anopheles mosquitoes and culicines.
Five CDC light traps were deployed indoors in selected bedrooms within each grid at night. The traps were set from 18:00 PM to 6:00 AM for six consecutive days before being moved to the next grid in a random fashion. Mosquitoes were retrieved daily from 7:00 AM to 10:00 AM. The retrieved mosquitoes were sorted and identified using taxonomic keys, then preserved in Eppendorf® tubes with silica gel. Concurrently, the BG-Sentinel traps, which were used for outdoor and day-time collections, were positioned in shaded areas in strategic locations near waste collection points, houses, garages, night clubs, and other areas considered to have high mosquito activity zones, and the traps were supplemented with BG lure and molasses mixed with yeast to increase mosquito attraction. Each grid had four traps operating from 6:00 AM to 18:00 PM the following morning for six consecutive days. Mosquitoes were retrieved from the traps daily from 18:00 PM to 19:00 PM, sorted, and identified using a dissecting microscope and taxonomic keys as described above.
For each collection, either by CDC light traps or BG Sentinel Traps, the additional metadata recorded included the grid ID, date, distance from port area, GPS location, and house number. After the samples were sorted, all Anopheles and Aedes species mosquitoes were sent to Ifakara Health Institute for further analysis.
Sampling of immature mosquitoes and characterization of their aquatic habitats
Surveys for immature mosquitoes and characterization of breeding habitats were conducted in all selected grids where adult mosquito trapping was being conducted. The search for mosquito larvae focused on various natural and human-made water-holding objects, including used tires, surface drains, wells, discarded containers, animal feeding containers, flowerpots, and buckets. Additional habitats such as tree holes (holes on tree stems), discarded boots, plant leaves, and other small objects that could hold water for more than three days were also examined. Breeding sites with all mosquito larvae or pupae were geo-referenced using handheld GPS devices. The breeding sites were further characterized by assessing the habitat type, location (i.e., present inside the houses or building or outdoors), algae quantity, water color, presence of vegetation, water movement, water source, habitat mobility, and the surrounding environmental and social activities. All information was recorded on breeding site characterization forms.
To collect the mosquito (larvae and pupae) we used a 350 ml dipper or a smaller 70 ml dipper for smaller habitats as adopted from Claudia [45]. The collected larvae and pupae were placed in white trays for morphological identification using pictorial keys [46–48]. The immature mosquitoes were sorted, counted, and data recorded by habitat type, location, and date.
Rearing of Aedes Aegypti larvae for susceptibility testing
After the mosquito larvae were collected in the field, the mosquito species were separated such that only mosquitoes belonging to Aedes species were only selectedAedes larvae were reared to adulthood for susceptibility testing to establish resistance profiles. Collected immatures were maintained to the adult stage using water from their known breeding habitats mixed with tap water in plastic containers. For rearing, the immatures were placed in labeled basins and were fed with Tetramine® baby fish food. The water was changed every three days to facilitate growth. The rearing was conducted at 26 °C ± 2 °C and 82% ± 10% relative humidity. Pupae were collected daily, counted, and transferred to 30 cm × 30 cm × 30 cm net cages. Emerged adults were fed with 10% glucose and identified morphologically using identification keys [49].
Testing for susceptibility status of Aedes aegypti to insecticides
The susceptibility tests were conducted according to the WHO guidelines for Anopheles mosquitoes [50] since we did not have access to appropriate insecticide-impregnated papers for use on Ae. aegypti. Adult female Ae. aegypti were obtained from the rearing cages to be used for these tests after 3–5 days. The mosquitoes were tested against commonly used insecticides, including pyrethroids (deltamethrin at 0.05% and 0.5% doses and permethrin at 0.75% and 3.75%), an organophosphate (pirimiphos-methyl at 0.25% and 1.25% doses), an organochlorine (DDT 4%), and carbamates (bendiocarb 0.5% and 1%). The higher doses for pyrethroid insecticides were included so as to test the intensity of resistance.
In each bioassay experiment, 120 mosquitoes were distributed into six holding tubes, with 20 mosquitoes per tube. Four tubes were exposed to the test insecticide, and two tubes were served as controls. Mosquitoes in the control tubes were exposed to untreated filter papers, whereas those in the treatment tubes were exposed to filter papers impregnated with the respective doses of the candidate insecticides. The test and control mosquitoes were observed for 60 min. After this, the mosquitoes were transferred back to holding tubes, provided with 10% glucose solution, and then maintained for a day at 28 °C ± 1 °C and 80% ± 10% relative humidity. Mortality was assessed 24-h post-exposure in both the control and treatment arms. A mosquito was considered alive if it could fly, regardless of the number of legs remaining; or dead if they were immobile, unable to stand or take off or obviously moribund.
Survey of the opinions and perceptions of port users and residents of neighboring wards
To complete the entomological surveys, a comprehensive quantitative survey was conducted to gauge the opinions and perceptions of port users and residents of the neighboring wards regarding Aedes mosquito-related risks and disease transmission at entry point and in their areas of residence. We deployed the survey via the Kobo Collect toolkit programmed in PC tablets and used a structured questionnaire for the interviews. All participants were recruited voluntarily and were all aged 18 years and above. They included locals, port workers, office staff, entrepreneurs, passengers, drivers, security guards, market vendors, and fishermen, representing diverse groups across the study areas.
Recruitment was performed by visiting workplaces and homes and participants were recruited and surveyed to understand their views on mosquito-related risks at the points of entry. A total of 236 participants voluntarily consented and were recruited. The structured questionnaire assessed key issues such as (i) knowledge of diseases transmitted by mosquitoes in the Tanga port area and surrounding wards, (ii) opinions on groups at greater risk of infection, (iii) mosquito biting experiences, and (iv) personal protection methods used against Aedes mosquito bites. Responses were analyzed to identify trends on the basis of occupational status and education levels, providing valuable insights into community awareness and preparedness regarding mosquito-borne diseases.
Statistical analysis and presentation
Analysis of the quantitative data was conducted using open-source software, R version 3.6.2 [51], and ArcGIS [52]. The data were initially entered into MS Excel and then exported to R in a csv format for cleaning and coding. Initially, a descriptive analysis was performed, where we calculated the abundance and distribution of mosquitoes (both adult and immature stages) across sampling locations. A generalized linear model (GLM) with Poisson variate was then employed to determine the associations of mosquito larvae in breeding habitats with habitat characteristics such as water color and movement, the presence of vegetation, and whether the habitat was natural or artificial included as the main predictors. Another GLM with a negative binomial distribution was used to assess the relative comparison of mosquito abundance between sampling areas, trap types, and indoor-outdoor settings at the point of entry, with the grid ID as a random variable to identify significant factors.
To assess the Aedes insecticide resistance profile, mosquito populations were classified as susceptible if the mortality rate was greater than 98%, suggestive or unconfirmed resistance that need further investigation if between 90 and 98%, and resistant if less than 90% [50]. All the visualization and spatial analyses was performed in the GIS Environment using QGIS software to analyze mosquito species diversity, abundance, and distribution by interpolation techniques. The environmental data, such as the number of mosquitoes per species, location of collection, and grid ID, trap type, were extracted and compared with Cx. quinquefasciatus, Ma. uniformis and Ae. aegypti abundance. Hotspot analysis using inverse distance weighted (IDW) interpolation was used to identify grids with high mosquito abundance, which were statistically significant and crucial for targeting control measures. IDW was chosen as interpolation method since it estimates cell values by averaging the values of sample data points in the neighborhood of each processing cell. The data were interpolated by means of IDW and transformed from vector into raster.
Lastly, the questionnaire data from the survey of port users and residents were analyzed using descriptive statistics to summarize the participants' knowledge, opinions, and practices regarding mosquito-related risks and disease transmission.
Results
Diversity and abundance of adult mosquitoes in the port area and surrounding wards
A total of 20,449 mosquitoes were collected between June and December 2023 (Table 1). Among these: 56.2%, n = 11,488 were identified as Culex quinquefasciatus, 38.7%, n = 7919 as Mansonia uniformis, 5.1%, n = 1042 as Aedes Aegypti, 0.1%, n = 16 as An. gambiae s.l., and 0.0%, n = 3 as An. funestus s.l. Outdoor collections using BG sentinel traps accounted for 53.8%, n = 11,028, of the total, while indoor collections using CDC light traps comprised 46.1%, n = 9421.
Table 1.
Mean number of mosquitoes caught at different distances from the port area using the CDC light traps (placed indoors for nighttime collections) and the BG sentinel taps (placed outdoors for daytime collections)
| Species | Distance (km) from port |
CDC light traps (indoors) | BG sentinel traps (outdoors) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Mean [95% CI]* | RR [95% CI] | P values | Total | Mean [95% CI]* | RR [95% CI] | p values | ||
| Culex quinquefasciatus | 0 (port area) | 1932 | 8.99 [7.10, 11.37] | 1 | 1938 | 5.63 [4.72, 6.73] | 1 | ||
| 2 | 856 | 14.27 [9.17, 22.20] | 1.59 [0.96, 2.62] | 0.070 | 1035 | 10.78 [7.75, 15.00] | 1.91 [1.32, 2.79] | < 0.0001 | |
| 2.5 | 1,279 | 9.84 [7.27, 13.31] | 1.10 [0.75, 1.61] | 0.643 | 2,234 | 10.74 [8.58, 13.44] | 1.91 [1.43, 2.54] | < 0.0001 | |
| 5 | 652 | 5.02 [3.69, 6.82] | 0.56 [0.38, 0.82] | < 0.01 | 1,562 | 7.51 [5.99, 9.42] | 1.33 [1.00, 1.78] | < 0.05 | |
| Aedes aegypti | 0 (port area) | 14 | 0.065 [0.04, 0.12] | 1 | 682 | 1.98 [1.49, 2.64] | 1 | ||
| 2 | 0 | 0 | NA | 41 | 0.43 [0.23, 0.78] | 0.22 [0.11, 0.42] | < 0.0001 | ||
| 2.5 | 6 | 0.046 [0.02, 0.11] | 0.71 [0.24, 2.06] | 0.526 | 160 | 0.77 [0.52, 1.14] | 0.39 [0.24, 0.63] | < 0.0001 | |
| 5 | 3 | 0.023 [0.01, 0.08] | 0.35 [0.09, 1.34] | 0.127 | 136 | 0.65 [0.44, 0.97] | 0.33 [0.20, 0.54] | < 0.0001 | |
| Mansonia uniformis | 0 (port area) | 1129 | 5.25 [3.45, 7.99] | 1 | 1411 | 4.10 [2.98, 5.65] | 1 | ||
| 2 | 1 | 0.02 [0.002, 0.14] | 0.01 [0.00, 0.03] | < 0.0001 | 14 | 0.45 [0.07, 0.32] | 0.04 [0.02, 0.08] | < 0.0001 | |
| 2.5 | 2,179 | 16.76 [9.81, 28.64] | 3.19 [1.62, 6.30] | < 0.0001 | 1231 | 5.92 [3.93, 8.91] | 1.44 [0.86, 2.43] | 0.167 | |
| 5 | 1,370 | 10.54 [6.16, 18.02] | 2.00 [1.02, 3.97] | < 0.05 | 584 | 2.81 [1.87, 4.25] | 0.66 [0.41, 1.16] | 0.155 | |
RR, relative risk; CI, confidence interval
* Predicted means from Generalized Linear Model
Most of Ae. aegypti mosquitoes were collected outdoors (97.8%, n = 1019) on the other hand, half of the Cx. quinquefasciatus (58.9%, n = 6769) were outdoors. Full summaries are found in Table 1. The highest abundances of Aedes mosquitoes collected outdoor were observed in the port area, with populations significantly declining in surrounding areas. Outdoor bite of Aedes Aegypti was much lower at 2 km (z = −4.47, p < 0.001), 2.5 km (z = − 3.84, p < 0.001) and 5 km (z = − 4.45, p < 0.001) distance compared with the port area (0 km), as seen in Table 1, with non-linear trends relative to port area. A total of 37 blood-fed Ae. aegypti were obtained from port area and 2 at 5 km while none were captured at 2 and 2.5 km distance from the port.
For Cx. quinquefasciatus, the indoor bite was lower at 2 km (z = 1.81, p = 0.07), 5 km (z = −2.96, p < 0.01) distance compared to the port area (0 km), though bites were the same at 2.5 km (z = 1.81, p = 0.643), as seen in Table 1. Outdoor bite of Cx. quinquefasciatus was found to be lower at 2 km (z = 3.39, p < 0.001) and 5 km (z = 1.96, p < 0.05), but higher at 2.5 km (z = 4.42, p < 0.001) compared with the port area (Table 1).
For Ma. uniformis very low indoor collection was found at 2 km (z = −5.24, p < 0.01) and higher collections at 2.5 km (z = 3.34, p < 0.001) and 5 km (z = 2.00, p < 0.05) compared with the port area (Table 1). On the other hand, lower catches were observed at 2 km (z = −7.64, p < 0.001) while similar catches were observed at 2.5 km (z = 1.38, p = 0.167) and 5 km (z = −1.42, p = 0.155) compared with port area for outdoor.
Spatial distribution of adult Aedes mosquitoes
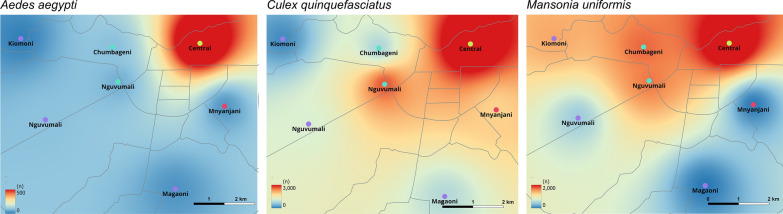
The distribution of adult Aedes mosquitoes varied across the surveyed administrative wards. The results from interpolation techniques by inverse distance weighted (IDW) interpolation of a point vector layer revealed a higher density of Aedes mosquitoes in the port area located in the Central ward, indicating these as hotspots. Conversely, areas such as Mnyanjani (2 km from the port), and Magaoni and Kiomoni (5 km from the port) were identified as having lower densities of Aedes mosquitoes as shown in Fig. 2.
Fig. 2.
Comparison of mosquito catches in the port area and the surrounding area
Diversity and abundance of immature stages and their aquatic habitats in the port area and surrounding wards
The larval searches and habitat characterization activities identified immature stages of Anopheles, Culex, and Aedes mosquitoes in several habitats across the study area. Out of 2934 sampled mosquitoes, 60.7% were Aedes, 39.1% were Culex, and 0.1% were Anopheles (Table 2). High densities of Aedes immatures were found in Central ward within the port area (68.3%), followed by Nguvumali ward (11.9%), with the lowest density in Kiomoni ward (1.1%). Notably, Anopheles larvae were only detected in Magaoni ward. Culex larvae were most prevalent in Central ward (60.4%), followed by Nguvumali (12.9%).
Table 2.
Sampled populations of Aedes, Anopheles and Culex larvae
| Wards | Aedes | Culex | Anopheles | Total |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Central (location of port) | 1,21,768.3 | 69,460.4 | 00 | 1911 |
| Chumbageni | 1327.4 | 776.7 | 00 | 209 |
| Kiomoni | 201.1 | 847.3 | 00 | 104 |
| Magaoni | 522.9 | 282.4 | 4100 | 84 |
| Mnyanjani | 693.9 | 363.1 | 00 | 105 |
| Mzizima | 321.8 | 564.9 | 00 | 88 |
| Ng/Kusini | 472.6 | 262.3 | 00 | 73 |
| Nguvumali | 21,211.9 | 14,812.9 | 00 | 360 |
| Total | 1781 (60.7%) | 1149 (39.1%) | 4 (0.1%) | 2934 (100%) |
N, number of larvae collected, and %, percentage of larvae by ward surveyed
Larval indices—for Aedes mosquitoes
A total of 2931 breeding sites were identified in 416 households. Of these, 60.8% (1,781/2,931) were positive for Aedes immatures stages. Various indices were calculated, including the container index (CI), house index (HI), and Breteaux index (BI). The highest CI values were observed in the port area (66.2%), 2.5 km away (51.5%), and 5 km away (44.6%). For the HI, the highest values were at 5 km (61.5%) and 2 km (60%) from the port, while the port area had the lowest HI (44%). Lastly, the highest BI values were found at 2 km (45.7%) and 5 km (30.3%) from the port, with the port area having the lowest BI (7.7%), as shown in Table 3.
Table 3.
Summary of Ae. aegypti larval survey indices
| Name of area | No. of premises | No. of positive premises | House index (HI) | No. of containers surveyed | No. of positive containers | Container index (CI) | Breteaux index (BI) |
|---|---|---|---|---|---|---|---|
| Port area | 150 | 66 | 44 | 1748 | 1158 | 66.2 | 7.7 |
| 2 km away | 35 | 21 | 60 | 195 | 16 | 8.2 | 45.7 |
| 2.5 km away | 122 | 66 | 54.1 | 68 | 35 | 51.5 | 28.9 |
| 5 km away | 109 | 67 | 61.5 | 74 | 33 | 44.6 | 30.3 |
Container Index (CI), ratio of larval infested containers to total inspected containers; house index (HI), ratio of larval infested houses to all inspected houses; Breteaux index (BI), ratio of positive containers per 100 houses inspected
Habitat characterization
During the habitat search for Aedes mosquitoes, the breeding sites were characterized on the basis of physical parameters such as algal quantity, water source, watercolor, water movement, water type, and vegetation quantity (Table 4). A total of 2931 breeding sites were identified, of which 22.7%, 664/2931, were used tires, 71.1%, 2085/2931, were containers (both plastic and metal), 1.3%, 38/2931, were surface drains, 4.5%, 132/2931, were flowerpots, and 0.4%, 12/2931, was coconut shells. The majority of positive breeding habitats for Aedes mosquitoes had clear water (62.9%, 1,242/1973), moderate vegetation (66.9%, 696/1041), water resulting from rainfall (60.9%, 1529/2525), scarce algal quantity (60.8%, 1033/1698), and stagnant water (61%, 1777/2911). The different habitat types of Aedes mosquitoes observed in the study area are shown in Fig. S1 and Fig. S2.
Table 4.
Physical characteristics of the Aedes mosquitoes’ breeding habitats identified in the study area
| Parameter | Category | Positive, n (%) | Negative, n (%) | Total |
|---|---|---|---|---|
| Habitat type | Disposed containers* | 1158 (55.5) | 927 (44.5) | 2085 |
| Coconut shell | 12 (100) | 0 | 12 | |
| Surface drains | 15 (39.5) | 23 (60.5) | 38 | |
| Flowerpots | 100 (75.8) | 32 (24.2) | 132 | |
| Tires | 496 (74.8) | 168 (25.2) | 664 | |
| Algae quantity | None | 360 (57.4) | 267 (42.6) | 627 |
| Moderate | 372 (65.1) | 199 (34.9) | 571 | |
| Scarce | 1033 (60.8) | 665 (39.2) | 1698 | |
| Abundant | 16 (47.1) | 18 (52.9) | 34 | |
| Water source | Domestic | 252 (62.2) | 153 (37.8) | 405 |
| Rainwater | 1529 (60.6) | 996 (39.4) | 2525 | |
| Water color | Clear | 1242 (62.9) | 731 (37.1) | 1973 |
| Polluted | 539 (56.3) | 418 (43.7) | 957 | |
| Water movement | Stagnant | 1777 (61.1) | 1134 (38.9) | 2911 |
| Slow | 4 (21.1) | 15 (78.9) | 19 | |
| Water type | Permanent | 530 (52.8) | 473 (47.2) | 1003 |
| Temporary | 1251 (64.9) | 676 (35.1) | 1927 | |
| Vegetation quantity | None | 452 (51.9) | 419 (48.1) | 871 |
| Moderate | 696 (66.9) | 345 (33.1) | 1041 | |
| Scarce | 454 (61.1) | 289 (38.9) | 743 | |
| Abundant | 179 (65.1) | 96 (34.9) | 275 | |
| Total | 1781 | 1150 | 2931 |
(*): The disposed containers included plastic drums, buckets, tins, plastic barrels, basins, metal drums, jerrycans, and tanks
Association between habitat characteristics and mosquito densities
Disposed containers were the primary habitat for both Aedes and Culex mosquitoes, with a high number of mosquitoes collected (65.6%; n = 1158). Overall, the likelihood of capturing Aedes mosquitoes in disposed tires was about three times higher compared to disposed containers (z = 7.401, p < 0.001, Table 5). Aedes species also preferred habitats with scarce algae level (z = 1.77, p < 0.05), turbid/polluted water (z = −2.66, p < 0.01), and temporary aquatic habitats (z = −9.120, p < 0.001, Table 5) with slow moving water (z = − 3.35, p < 0.01).
Table 5.
Mean number of mosquitoes collected in different habitat types (and 95% CI)
| Habitat characteristics | Total | Mean ± 2SE* | RR [95% CI] | p values | ||
|---|---|---|---|---|---|---|
| Aedes spp. | Habitat type | Disposed containers | 1158 | 4.3 ± 1.0 | 1 | |
| Coconut shell | 12 | 2.4 ± 1.2 | 0.9 [0.6, 2.4] | = 0.846 | ||
| Surface drains | 15 | 1.2 ± 0.8 | 0.8 [0.4, 1.6] | = 0.528 | ||
| Flowerpots | 100 | 3.0 ± 1.2 | 1.3 [0.9, 2.0] | = 0.187 | ||
| Tires | 496 | 7.1 ± 2.1 | 2.8 [2.1, 3.6] | < 0.001 | ||
| Algae quantity | None | 360 | 13.0 ± 5.9 | 1 | ||
| Moderate | 372 | 3.0 ± 0.9 | 0.6 [0.4, 0.9] | < 0.05 | ||
| Scarce | 1033 | 4.5 ± 1.0 | 0.7 [0.4, 1.0] | < 0.05 | ||
| Abundant | 16 | 3.2 ± 2.6 | 0.6 [0.2, 1.7] | = 0.348 | ||
| Water source | Domestic | 252 | 3.1 ± 0.8 | 1 | ||
| Rainwater | 1529 | 5.0 ± 1.0 | 0.8 [0.6, 1.1] | = 0.156 | ||
| Water color | Clear | 1242 | 5.1 ± 1.0 | 1 | ||
| Turbid | 539 | 3.7 ± 1.3 | 0.7 [0.6, 0.9] | < 0.01 | ||
| Water type | Permanent or semi-permanent | 530 | 23.0 ± 8.0 | 1 | ||
| Temporary | 1251 | 3.4 ± 0.5 | 0.1 [0.1, 0.2] | < 0.001 | ||
| Vegetation quantity | None | 452 | 14.6 ± 5.8 | 1 | ||
| Moderate | 696 | 3.6 ± 0.6 | 1.1 [0.8, 1.6] | = 0.638 | ||
| Scarce | 454 | 3.7 ± 1.5 | 1.1 [0.6, 2.0] | = 0.746 | ||
| Abundant | 179 | 4.2 ± 2.2 | 0.9 [0.6, 1.3] | = 0.414 | ||
| Culex spp. | Habitat type | Disposed containers | 927 | 3.5 ± 0.8 | 1 | |
| Coconut shell | 0 | 0 | 0.0 [0.0, 0.0] | 0.999 | ||
| Surface drains | 23 | 1.8 ± 0.6 | 0.8 [0.4, 1.7] | 0.591 | ||
| Flowerpots | 32 | 1.0 ± 0.6 | 0.5 [0.3, 0.8] | < 0.01 | ||
| Tires | 167 | 2.4 ± 1.0 | 1.1 [0.8, 1.4] | 0.629 | ||
| Algae quantity | None | 267 | 9.5 ± 5.0 | 1 | ||
| Moderate | 199 | 1.6 ± 0.3 | 0.5 [0.3, 0.9] | < 0.05 | ||
| Scarce | 665 | 2.9 ± 0.7 | 0.7 [0.4, 1.0] | = 0.067 | ||
| Abundant | 18 | 3.6 ± 2.0 | 1.6 [0.6, 4.5] | = 0.401 | ||
| Water source | Domestic | 153 | 1.9 ± 0.7 | 1 | ||
| Rainwater | 996 | 3.2 ± 0.7 | 1.3 [1.0, 1.8] | = 0.064 | ||
| Water color | Clear | 731 | 3.0 ± 0.8 | 1 | ||
| Turbid | 418 | 2.9 ± 1.0 | 1.3 [1.1, 1.7] | < 0.05 | ||
| Water type | Permanent or semi-permanent | 473 | 20.6 ± 5.5 | 1 | ||
| Temporary | 676 | 1.9 ± 0.3 | 0.2 [0.1, 0.2] | < 0.001 | ||
| Vegetation quantity | None | 419 | 13.5 ± 5.0 | 1 | ||
| Moderate | 345 | 1.8 ± 0.3 | 0.6 [0.4, 1.1] | = 0.057 | ||
| Scarce | 289 | 2.4 ± 0.9 | 0.6 [0.4, 1.0] | < 0.05 | ||
| Abundant | 96 | 2.2 ± 0.5 | 0.6 [0.4, 1.1] | = 0.095 | ||
*2SE; Refers to two standard errors
For the Culex mosquitoes, the likelihood of capturing Culex species in flower pots was 0.5 times lower compared with disposed containers (z = −2.96, p < 0.01, Table 5). Similarly, Culex species prefer moderate amount of algae (z = −2.55, p < 0.05, Table 5). Similar to Aedes, the Culex species also prefer temporary aquatic habitats (z = −8.50, p < 0.001, Table 5) with polluted water (z = 2.05, p < 0.05, Table 5). A full detailed summary is found in Table 5.
Susceptibility of Aedes aegypti mosquitoes to public health insecticides
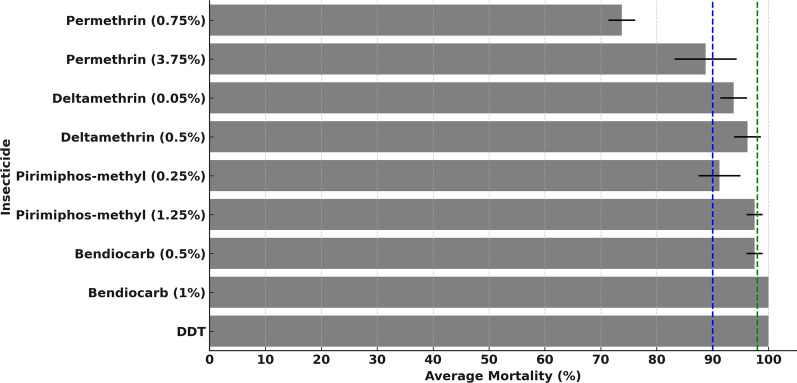
Susceptibility tests for female Ae. aegypti mosquitoes aged 3–5 days were conducted against five insecticides at various concentrations, including permethrin (0.75%, 3.75%), deltamethrin (0.05%, 0.5%), pirimiphos-methyl (0.25%, 1.25%), DDT (4%), and bendiocarb (0.5%, 1%). At 24 h post-exposure, the average mortality rates for Ae. aegypti exposed against bendiocarb (1%) and DDT (4%) were 98.8% and 100%, respectively, indicating full susceptibility. The average mortalities associated with pirimiphos-methyl (0.25%) and deltamethrin (0.05%) were 94.4%, 93.8%, respectively, suggesting possible or unconfirmed resistance. Increasing the dose of deltamethrin to 0.5% increased the 24-h mortality to 96.3%, suggesting that even at this dose the mosquitoes were still slightly resistant. Tests against permethrin showed average mortality rates of 73.8% and 88.8%, indicating clearly confirmed resistance even at the five times higher dose of 3.75%. In contrast, the Ae. aegypti mosquitoes were fully susceptible to DDT and bendiocarb (Fig. 3).
Fig. 3.
Results of the susceptibility tests for female Ae. aegypti mosquitoes showing mean mortality after 24 h of monitoring post-exposure to the candidate insecticides. The dotted green lines (≥ 98% mortality) indicate full susceptibility, while the dotted blue lines (90–98% mortality) indicate possible resistance or unconfirmed resistance requiring confirmation
Awareness and perceptions of the risk of Aedes-borne diseases
Demographic characteristics of survey respondents
A total of 236 respondents participated in the survey within the port area and the nearby community (Table 6). Among the participants, 58.1%, n = 137, were male and 41.9%, n = 99, were female. The majority had a secondary level of education (47.0%, n = 111), with the age interval ranging from 36–45 years (36.4%, n = 86). Primary economic activities among respondents were self-employment including entrepreneurship (73.3%, n = 173), formal employment including government employee (21.2%, n = 50), fishing (3.0%, n = 7), unemployed (1.7%, n = 4), and other were farmers (0.8%, n = 2).
Table 6.
Characteristics of study respondents at Tanga point of entry (N = 236)
| Category | Variable | n (%) |
|---|---|---|
| Sex | Female | 99 (41.9) |
| Male | 137 (58.1) | |
| Occupation | Self-employed | 173 (73.3) |
| Fisherman | 7 (3.0) | |
| Public services (health and other workers) | 50 (21.2) | |
| Farmer | 2 (0.8) | |
| Unemployed | 4 (1.7) | |
| Age | 16–25 | 45 (19.1) |
| 26–35 | 84 (35.6) | |
| 36–45 | 86 (36.4) | |
| 46–55 | 20 (8.5) | |
| 56 and above | 1 (0.4) | |
| Education level | Illiterate | 3 (1.3) |
| Primary | 47 (19.9) | |
| Secondary | 111 (47.0) | |
| University | 75 (31.8) | |
| Marital status | Married | 131 (55.5) |
| Unmarried | 104 (44.1) | |
| Widow | 1 (0.4) |
Awareness, perception, and experience of mosquito-borne diseases among residents of Tanga seaport
The survey results revealed that approximately two thirds of the respondent reported that only female mosquitoes bite humans and transmit diseases (69.8%, n = 134) while about one third reported that both sexes of mosquito bite human and transmit diseases (27.6%, n = 53). Overall, about two-third (64.8%, n = 153) of all respondents were aware of mosquitoes transmitting only malaria, and less than one third (26.3%, n = 62) were aware that mosquitoes also transmit dengue fever viruses. Chikungunya is far less recognized and was identified as a mosquito-borne disease by only 1.7%, n = 4, of respondents.
Approximately 97.9%, n = 231, of the respondents perceived that there is a risk of contracting mosquito-borne diseases or infections at the seaport where only 2.1%, n = 5, respondents reported no perceived risk. Additionally, about two thirds of all respondents reported that workers at the port are perceived to be at greatest risk of mosquito-borne infections (61.4%, n = 145) followed by community living nearby port area (28%, n = 67), indicating that people who reside around the seaport area, have highest perception of risk than those living away from the seaport area. Lastly, the majority of the survey respondents reported being bothered by mosquito bites at night (53.4%, n = 126) and in the evening (40.7%, n = 96). Other than insecticide treated nets (ITNs), which were widely used for malaria prevention, only a smalls percentage of respondents reported using other interventions against mosquito-borne diseases, these other interventions included the use of long clothing (19.1%, n = 45), topical repellents (12.3%, n = 29), and swatting with bare hands (11.9%, n = 28) as shown in Table 7.
Table 7.
Awareness, perception, and experience of mosquito-borne diseases among residents of Tanga seaport
| Question asked | Variables | n (%) |
|---|---|---|
| Do you know the sex of mosquito that bite human? | Yes | 192 (81.4) |
| No | 44 (18.6) | |
| Which sex of mosquito biting human? | Female | 134 (69.8) |
| Male | 5 (0.6) | |
| Both sexes | 53 (27.6) | |
| Which diseases you know are transmitted by mosquitoes? | Malaria | 153 (64.8) |
| Chikungunya | 4 (1.7) | |
| Dengue | 62 (26.3) | |
| Malaria, dengue, chikungunya, and others | 17 (7.2) | |
| Is there any risk of contracting mosquito-borne diseases or infections while at seaport? | Yes | 231 (97.9) |
| No | 5 (2.1) | |
| Which group is at greater risk of mosquito-borne infection in seaport? | All people | 4 (1.7) |
| Nearby community | 67 (28.4) | |
| Fishers | 2 (0.8) | |
| Passengers/travelers | 13 (5.5) | |
| Undecided | 5 (2.1) | |
| Workers at port | 145 (61.4) | |
| Time at which mosquito bite more while at seaport | Evening | 96 (40.70 |
| Morning | 5 (2.1) | |
| All the time | 7 (3) | |
| Night | 126 (53.4) | |
| Noon | 2 (0.8) | |
| Personal protection methods used against mosquito biting at seaport | Swatting | 28 (11.9) |
| Nothing | 134 (56.8) | |
| Use of long clothing | 45 (19.1) | |
| Use of repellents | 29 (12.3) |
Discussion
Most studies on arbovirus vectors in Africa have been reactive to outbreaks or focused on large urban populations, neglecting high-risk areas such as international points of entry, which can be hotspots for mosquito-borne viruses [53–57]. Despite being of significant economic value, international shipping activities at ports can facilitate the migration of vectors, and can pose significant threats in the absence of effective surveillance systems [26]. For this reason, strengthening the basic entomological surveillance systems at all major international points of entry has been recommended both by the IHR [58] and Tanzania’s National Strategy for Vector Control (2019–2024) [40]. The aim of this study was therefore to compare the risks of disease-transmitting mosquitoes, with a focus on Aedes mosquitoes within and in the surrounding urban areas of the eastern Tanzanian seaport of Tanga along the Indian Ocean. To complement these entomological surveys, we also assessed the knowledge and perceptions of port users and residents of surrounding wards regarding the risk and control of mosquito-borne infections in the area.
The entomological study identified five mosquito species: An. gambiae s.l., An. funestus, Ae. aegypti, Cx. quinquefasciatus, and Mansonia uniformis. The high abundance of Ae. aegypti in the port area suggests it is a hotspot for this species, which is consistent with findings from other studies in Mumbai International Seaport (India) and the port of Abidjan [31, 59]. The presence of discarded tires and containers likely contributes to this high density. While most Aedes mosquitoes were caught in outdoor BG sentinel traps during the day, indicating significant outdoor biting activity, Culex species were commonly found both indoors and outdoors. Notably, the low numbers of Anopheles mosquitoes in the port area aligned with their ecological preference for rural areas and limited breeding opportunities in urban settings of Tanga as evidenced in previous studies in which malaria vectors, including An. funestus, preferred to breed along rivers with slow-moving clear waters and emergent vegetation by Nambunga et al. (2020) [60] and swamps or large drain by Sattler et al. (2005) in Dar es salaam [61], resulting in low levels of malaria transmission. Overall, the findings of this study highlight the significant presence and distribution of key disease-transmitting mosquitoes, particularly Ae. aegypti, within the Tanga port area and its surrounding wards. The high composition of Ae. aegypti in the present study correspond with the findings of survey studies in urban regions of (Dar es Salaam, Coast, Tanga and Arusha) by Philbert et al. (2020), where the composition was dominated by Aedes genera despite the use of traps different from those used in the present study [62].
The Aedes species preferred breeding in discarded tires and containers, such as water buckets and drums, which is consistent with findings from earlier studies of deendayal seaport, kandla, gujarat in india, and the state of Maranhao in Brazil [63, 64]. These elevated densities underscore the importance of ports as critical points for mosquito surveillance and control to address the risk of diseases such as dengue, chikungunya, and Zika. Field data from both the port and its surrounding wards clearly revealed that, indeed, Ae. aegypti populations decreased with increasing distance from the port, confirming that the port area is likely a hotspot, potentially because of factors such as stagnant water in containers and increased human activity during the day.
Interestingly, while malaria remains the most recognized mosquito-borne disease among the respondents, awareness of dengue and other Aedes-transmitted diseases is considerably lower than in the study conducted in Pwani in 2019, where the majority seemed to be aware of dengue fever virus (DFV) [65], the raised awareness about DFV influenced by the major outbreak of dengue occurred in Dar es salaam in 2019 [66].
Malaria is indeed also a significant public health problem in Tanzania though the risk in these urban settings is lower. The latest survey showed that malaria prevalence in Tanga was 4% [11], and in this survey, we found only a negligible number of Anopheles mosquitoes in the survey. According to the latest Demographic Health and Malaria Indicator Survey (TDHS and MIS 2022), household bed net use in Tanga was 84.2%, the percentage of children who had fevers in the preceding 2 weeks was 8.5%, and the overall malaria prevalence in children was 4% [11]. It is understandable why communities continue with an emphasis on malaria but the neglect of Aedes-borne diseases is particularly concerning given the rising global incidence of dengue and the presence of Ae. aegypti in the region [35, 38, 54, 62, 67]. The limited use of personal protective measures against Aedes mosquitoes, as indicated by our survey, further exacerbates the risk of Aedes-borne diseases. Other than bed nets, which protect people mostly at night-time, the majority of respondents did not use any form of protection against day-biting mosquito bites such as Ae. aegypti, with only a small fraction using methods such as long clothing or repellents. This highlights the urgent need for public health education campaigns to improve awareness and encourage the adoption of protective measures against Aedes mosquitoes (day-biters) among port users and surrounding communities.
Susceptibility tests showed that Ae. aegypti were susceptible to Bendiocarb, but full susceptibility to DDT was observed, consistent with previous research in Ifakara, Tanzania [16]. However, reduced susceptibility to deltamethrin was detected consistent, which is consistent with the findings of a previous study in Dar Es salaam [68], indicating possible resistance. The potential resistance to deltamethrin in our study may have resulted from repeated chemical use in the area. Additionally, Ae. aegypti showed reduced susceptibility to pirimiphos-methyl, contradicting recent studies in Ifakara [16] and Nigeria [69], which reported full susceptibility. These differences might be due to either ecological differences or seasonal variations. Furthermore, the present study confirmed the resistance of Ae. aegypti to permethrin, similar to the findings of a previous study in Dar Es salaam [68], one of the most common pyrethroids used in both agriculture and public health in the area. Overall, these investigations show that the available chemical options are increasingly limited and that greater vigilance is required. These tests should therefore be periodically repeated, preferably using more standardized tests with protocols designed specifically for Ae. aegypti mosquitoes as recommended by the WHO [70] to best determine the range of options for control.
This study also revealed significant gaps in knowledge among port users and neighboring residents regarding mosquito-borne diseases. While most participants were aware that malaria is a major public health problem, very small proportions, consisting predominantly of people with tertiary education were aware of other mosquito borne diseases such as dengue and chikungunya viruses. This is a common phenomenon in malaria-endemic areas, where awareness of mosquito bites and their role in malaria transmission is commonly found to be relative high, whereas knowledge about arboviral diseases transmitted by Aedes mosquitoes is notably limited [71]. For instance, in one study in the Central African Republic, most respondents identified malaria as the sole mosquito-borne disease despite evidence of dengue and other mosquito-borne diseases in circulation [72]. However, as these arboviruses become more significant public health challenges, greater efforts, including social and behavior change communication strategies should be adopted to improve community knowledge of the disease prevention and their control management.
In the assessment of community perceptions regarding groups at risk, a significant proportion of respondents acknowledged that port users and nearby communities are at risk of mosquito bites and infections, even though they did not distinguish between the different diseases. Moreover, people working at the port were considered to be at greater risk than the rest of the communities. The respondents also reported frequent mosquito bites, especially in the evening and at night, highlighting their exposure. This study aligns with previous research indicating that behavioral responses to infection risk are shaped by perceived exposure and bite frequency [73]. Despite recognizing their vulnerability, respondents showed poor adoption of personal protection measures. This mismatch of risk perception and self-protection was a surprise as previous studies have shown a direct correlation of these variables-people with high awareness being correlated with higher engagement in protective behaviors [74].
Although the main objectives were successfully achieved, this study had some limitations. First, the limited number of Aedes traps used for collecting adult mosquitoes may not have captured the full distribution effectively. Second, larvae and pupae collections were restricted to selected grids with human occupations or buildings, potentially leading to an underestimation of overall densities and distribution. Additionally, in the tests for insecticide susceptibility, WHO standard doses for Anopheles mosquitoes were used due to the lack of appropriate papers for testing Aedes mosquitoes. However, some of these insecticides, such as pirimiphos methyl, permethrin, and deltamethrin, already have diagnostic concentrations specific for Aedes mosquitoes, whereas other insecticides have different diagnostic concentrations specific for Aedes mosquitoes, which if used, might have yielded different results.
Conclusions
This study highlights the significant risk of Ae. aegypti and associated diseases within the Tanga port area, emphasizing the critical role of ports in the surveillance and control of mosquito-borne diseases, particularly those transmitted by Aedes mosquitoes. Despite the community being more concerned with malaria, awareness of other mosquito-borne diseases remains limited and should be increased given the observed risk. High abundance of Ae. aegypti in the port area, especially in discarded tires and containers, underscores the need for targeted interventions and more broadly, environmental sanitation to reduce the risk. Insecticide susceptibility tests revealed full susceptibility to bendiocarb and DDT but emerging resistance to pirimiphos-methyl and deltamethrin, suggesting that options for effective control may be limited; therefore, there is a need to for diversified control strategies. The study also revealed that the central port area, compared with other sites, is likely a hotspot for mosquito breeding, with Aedes populations decreasing with distance from the port. Since this study was the first dedicated mosquito survey to target this international point of entry, the results will form a basis for future research on pathogen transmission and control programs. The identification of key breeding habitats offers potential targets for Aedes control, emphasizing the need for integrated vector management involving community engagement, environmental management, and periodic insecticide rotation. The key recommendations are as follows: a) authorities should integrate environmental management, insecticide use, and community engagement to address discarded habitats; b) port health authorities should implement robust Aedes mosquito control measures to prevent potential outbreaks; c) future insecticide susceptibility studies should incorporate specific guidelines and appropriate concentrations for Aedes mosquitoes; d) port workers and nearby communities should be educated on mosquito control and prevention; and e) year-round studies should be conducted to understand seasonal variations in mosquito densities and resistance profiles. In conclusion, this study identified a relatively high potential risk of Ae. aegypti and associated diseases, but a low perception of risk and inadequate personal protection measures in the study area. This low perception of risk highlights the need to improve public knowledge of the transmission and control of Aedes-borne diseases.
Supplementary Information
Supplementary material 1: Fig. S1: Common habitat types observed in the study area: a shallow well, b surface drain c discarded bowl holding water, d root hole of banana tree, e discarded car tires, f fire hydrant
Supplementary material 2: Fig. S2: Common habitat types observed in the study area: a flowerpot, b plastic buckets for animal drinking, c container for feeding hens, d inspection chamber, e plastic container holding water placed under charcoal burner, f stream pool
Acknowledgements
We would like to thank the team of the Mosquito Mobility Project of Ifakara Health Institute, Outdoor Mosquito Control (OMC) team, as well as our colleagues from the international project “Mobile Mosquitoes – understanding the entangled mobilities of Aedes mosquitoes and humans in India, Mexico, Tanzania and Germany” for their invaluable feedback and support throughout this survey. Special thanks is due to Richard Lumuli, Hussein Mwaiswelo, Julius Kihonda, Agnes Falle, Rukia Ahmed, Ridhia Mkabala, Chiku S. Mulula, Mwanamvua Jalala, Mwanamkasi Ally, Jumaa Jumaa, Hadija Kombo, Salma Kalovya, Shabani Dossa, Mwindadi Mohamed, Mwanamwaya Nyoro, Juma Mohamed, Thabiti Mitwango, Amina Kassim, Magesa B. Kusaga, Hussein J. Hussein, Maryam Kaguta, and Iddi H. Amani for their invaluable contributions to conducting the surveys, administering questionnaires, grids searching, habitat searches, and laboratory procedures. We extend special thanks to colleagues MSc. students undertaking Public Health Research from Nelson Mandela African Institution of Science and Technology, including but not limited to Hassan Matimbwa, Salumu Chiamba, Aidi Lugenge, Fahad Mwakalebela, Muwonge Mukisa, Ampuriire Patience, and Sperancia Coelestine. Additionally, we extend our thanks to Maiko E. Kiberiti, the in-charge of Muheza Vector laboratory, students, and staff at Muheza College of Health and Allied Sciences who contributed to this study.
Author contributions
Conceptualization: A.S.A., E.G.K., H.S.N., and F.O.O. Data curation: A.S.A, H.S.N, I.H.N., and D.S.M. Formal analysis: A.S.A, H.S.N., I.H.N., D.S.M., A.R.K., and F.O.O. Funding acquisition: F.O.O., N.F.K., and H.S.N. Investigation: A.S.A., HSN., N.F.K., E.G.K., and F.O.O. Methodology: A.S.A., A. J.S., Y.PM., N.F.K., M.L.F., M.B.K., A.S., H.S.N., E.E.K., and F.O.O. Project administration: A.S.A., H.S.N., R.M.N. Resources: A.S.A, R.A., H.J.H., F.O.O., H.S.N., and E.G.K. Supervision: F.O.O., and H.S.N. Validation: H.S.N., F.O.O., and E.G.K. Visualization: A.S.A, H.S.N., E.G.K., and F.O.O. Writing original draft: A.S.A., H.S.N., F.O.O., and E.G.K. Writing review and editing: All authors.
Funding
Masters’ scholarship in Public Health Research awarded to the lead author funded by Ifakara Health Institute (IHI). Material, equipment and field activities were supported by Volkswagen Foundation (Grant Number 9B366) awarded to Ifakara Health Institute (IHI).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethical approvals for this project were obtained from Ifakara Health Institute’s Institutional Review Board (Protocol ID: IHI/IRB/No:39-2023) and the Medical Research Coordinating Committee (MRCC) at the National Institute for Medical Research, in Tanzania (Protocol ID: NIMR/HQ/R.8a/Vol.IX/4178). Written consents were sought from all participants of this study, after they had understood the purpose and procedure of the study.
Consent for publication
Permission to publish this study was obtained from National Institute for Medical Research, in Tanzania ref. no. BD.242/437/01C/26.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Esther G. Kimaro, Halfan S. Ngowo and Fredros O. Okumu are contributed equally as senior authors.
Contributor Information
Amri S. Abas, Email: aabas@ihi.or.tz
Fredros O. Okumu, Email: fredros@ihi.or.tz
References
- 1.Bayissa Chala FH. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: a review. Front Public Heal. 2021;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World malaria report. Geneva: World Health Organization; 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023
- 3.WHO. Dengue-global situation. World Heal. Organ. 2024. https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518#: Accessed 30 May 2024.
- 4.WHO. Dengue and severe dengue. World Heal. Organ. 2024. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessec 23 Apr 2024.
- 5.WHO. Vector-borne dieases. World Heal. Organ. 2020. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Accessed 23 Jul 2024.
- 6.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012. 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golding N, Wilson AL, Moyes CL, Cano J, Pigott DM, Velayudhan R, et al. Integrating vector control across diseases. BMC Med. 2015;13:1–6. 10.1186/s12916-015-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilcox BA, Gubler DJ, Pizer HF. Urbanization and the social ecology of emerging infectious diseases. Soc Ecol Infect Dis. 2008(pp. 113–137). 10.1016/B978-012370466-5.50009-1. [Google Scholar]
- 9.Chen Y, Yang Z, Jing Q, Huang J, Guo C, Yang K, et al. Effects of natural and socioeconomic factors on dengue transmission in two cities of China from 2006 to 2017. Sci Total Environ. 2020;724:138200. [DOI] [PubMed] [Google Scholar]
- 10.Kitojo C, Gutman JR, Chacky F, Kigadye E, Mkude S, Mandike R, et al. Estimating malaria burden among pregnant women using data from antenatal care centres in Tanzania: a population-based study. Lancet Glob Heal. 2019;7:e1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.TDHS. Demographic and Health Survey and Malaria Indicator Survey. Pap Knowl Towar a Media Hist Doc. 2022; 1–23.
- 12.Thawer SG, Golumbeanu M, Munisi K, Aaron S, Chacky F, Lazaro S, et al. The use of routine health facility data for micro - stratification of malaria risk in mainland Tanzania. Malar J. 2022;21:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mwanyika GO, Mboera LEG, Rugarabamu S, Makange M, Sindato C, Lutwama JJ, et al. Circulation of dengue serotype 1 viruses during the 2019 outbreak in Dar es Salaam. Tanzania Pathog Glob Health. 2021;115:467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallau GL, Abanda NN, Abbud A, Abdella S, Abera A, Ahuka-Mundeke S, et al. Arbovirus researchers unite: expanding genomic surveillance for an urgent global need. Lancet Glob Heal. 2023;11:e1501–2. [DOI] [PubMed] [Google Scholar]
- 15.Ward T, Samuel M, Maoz D, Runge-Ranzinger S, Boyce R, Toledo J, et al. Dengue data and surveillance in Tanzania: a systematic literature review. Trop Med Int Heal. 2017;22:960–70. [DOI] [PubMed] [Google Scholar]
- 16.Kahamba NF, Limwagu AJ, Mapua SA, Msugupakulya BJ, Msaky DS, Kaindoa EW, et al. Habitat characteristics and insecticide susceptibility of Aedes aegypti in the Ifakara area, south—eastern Tanzania. Parasit Vectors. 2020;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ijumba AP, JN, Mkwawa. Preferred breeding habitats of Aedes Aegypti (Diptera-Culicidae) Mosquito and its public health implications in Dares Salaam, Tanzania. J Environ Res Manag. 2017;4:344. [Google Scholar]
- 18.Mgeni MT, et al. Understanding alternative control methods and their mode of action for the control of outdoor biting mosquitoes. Int J Technol. 2023;47:100950. [Google Scholar]
- 19.Msellemu D, Tanner M, Yadav R, Moore SJ. Occupational exposure to malaria, leishmaniasis and arbovirus vectors in endemic regions: a systematic review. ScienceDirect. 2024;6:100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson MC, Stanberry LR. Climate change and vectorborne diseases. N Engl J Med. 2022;387:1969–78. [DOI] [PubMed] [Google Scholar]
- 21.Rosser JI, Tarpenning MS, Bramante JT, Tamhane A, Chamberlin AJ, Mutuku PS, et al. Development of a trash classification system to map potential Aedes aegypti breeding grounds using unmanned aerial vehicle imaging. Environ Sci Pollut Res. 2024;31:41107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karisa J, Muriu S, Omuoyo D, Karia B, Ngari M, Nyamwaya D, et al. Urban ecology of arboviral mosquito vectors along the kenyan coast. J Med Entomol. 2021;58:428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, et al. Thermal biology of mosquito-borne disease. Ecol Lett. 2019;22:1690–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratnasari A, Jabal AR, Rahma N, Rahmi SN, Karmila M, Wahid I. The ecology of aedes aegypti and Aedes albopictus larvae habitat in coastal areas of South Sulawesi. Ind Biodiv. 2020;21:4648–54. [Google Scholar]
- 25.Ojukwu KC, Chabi J, Frempong K, et al. ecology, distribution and risk of transmission of viral haemorrhagic fevers by Aedes mosquitoes around the port areas of Tema in Southern Ghana. Sci Res Publ. 2022;10:135–48. [Google Scholar]
- 26.Hulme PE. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth. 2021;4:666–79. [Google Scholar]
- 27.Jeannin C, Perrin Y, Cornelie S, Gloria-Soria A, Gauchet J-D, Robert V. An alien in Marseille: investigations on a single Aedes aegypti mosquito likely introduced by a merchant ship from tropical Africa to Europe. Parasite J. 2022;29:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guagliardo SA, Morrison AC, Barboza JL, Requena E, Astete H, Vazquez-prokopec G, et al. River boats contribute to the regional spread of the dengue vector Aedes aegypti in the Peruvian Amazon. Neglected Trop Dis. 2015;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ammar SE, Mclntyre M, Swan T, Kasper J, Derraik JGB, Baker MG, et al. Intercepted mosquitoes at New Zealand’s ports of entry, 2001 to 2018: current status and future concerns. Trop Med Infect Dis. 2019;2019:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bataille A, Cunningham AA, Cedeño V, Cruz M, Eastwood G, Fonseca DM, et al. Evidence for regular ongoing introductions of mosquito disease vectors into the Galápagos Islands. Proc R Soc B Biol Sci. 2009;276:3769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar K, Sharma AK, Sarkar M, Chauhan A, Sharma R. Surveillance of Aedes aegypti (L.) mosquitoes in Mumbai international seaport (India) to monitor potential global health risks. J Insects. 2014;2014:1–5. [Google Scholar]
- 32.Webb Cameron E, Doggett SL. Exotic mosquito threats require strategic surveillance and response planning. Publichealth Res. 2016;26:1–4. [DOI] [PubMed] [Google Scholar]
- 33.Elahee B, Munglee N, Latchooman N, Puryag S. A regional one health approach to the risk of invasion by Anopheles stephensi in Mauritius. bioRxiv. 2023;24:1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa : predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Environ Sci. 2020;117:24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longbottom J, Walekhwa AW, Mwingira V, Kijanga O, Mramba F, Lord JS. Aedes albopictus invasion across Africa: the time is now for cross-country collaboration and control. Lancet Glob Heal. 2023;11:e623–8. [DOI] [PubMed] [Google Scholar]
- 36.Obame-Nkoghe J, Roiz D, Ngangue MF, Costantini C, Rahola N, Jiolle D, et al. Towards the invasion of wild and rural forested areas in Gabon (Central Africa) by the Asian tiger mosquito Aedes albopictus: Potential risks from the one health perspective. PLoS Negl Trop Dis. 2023;17:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kampango A, Abílio AP. The Asian tiger hunts in Maputo city - The first confirmed report of Aedes (Stegomyia) albopictus (Skuse, 1895) in Mozambique. Parasit Vectors. 2016;9:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritz M, Taty Taty R, Portella C, Guimbi C, Mankou M, Leroy EM, et al. Re-emergence of chikungunya in the Republic of the Congo in 2019 associated with a possible vector-host switch. Int J Infect Dis. 2019;84:99–101. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Vector surveillance and control at ports, airports, and ground crossings. Int Heal Regul. 2016;92. http://apps.who.int/iris/bitstream/10665/204660/1/9789241549592_eng.pdf
- 40.The United Republic of Tanzania. National Strategy for Vector Control 2019–2024. Minist Heal Tanzania. 2019;
- 41.Muthanje EM, Kimita G, Nyataya J, Njue W, Mulili C, Mugweru J, et al. March 2019 dengue fever outbreak at the Kenyan south coast involving dengue virus serotype 3, genotypes III and V. PLOS Glob Public Heal. 2022;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly ME, Msafiri F, Affara M, Gehre F, Moremi N, Mghamba J, et al. Molecular characterization and phylogenetic analysis of dengue fever viruses in three outbreaks in Tanzania between 2017 and 2019. PLoS Negl Trop Dis. 2023;17:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chipwaza B, Sumaye RD, Weisser M, Gingo W, Yeo NKW, Amrun SN, et al. Occurrence of 4 dengue virus serotypes and chikungunya virus in Kilombero valley, Tanzania, during the dengue outbreak in 2018. Open Forum Infect Dis. 2021;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Msellemu D, Gavana T, Ngonyani H, Mlacha YP, Chaki P, Moore SJ. Knowledge, attitudes and bite prevention practices and estimation of productivity of vector breeding sites using a habitat suitability score (Hss) among households with confirmed dengue in the 2014 outbreak in dar es salaam, tanzania. PLoS Negl Trop Dis. 2020;14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Malley C. Seven ways to a successful dipping career. Wing Beats. 1995;6:23–4. [Google Scholar]
- 46.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. South African Inst Med Res. 1987;55:1. [Google Scholar]
- 48.Simsaa MAA, Harbach RE, Ali Almalik AM, Ahmed EM, Eisa AA, Mohamed AH, et al. Culex mosquitoes (Diptera: Culicidae) recorded along the Nile River in central and northern Sudan, with a key for the identification of all species of the genus known to occur in the country. Zootaxa. 2021;4963:401–11. [DOI] [PubMed] [Google Scholar]
- 49.Van Handel E. Rapid determination of glycogen and sugars in mosquitoes. J Am Mosq Control Assoc. 1985;1:299–301. [PubMed] [Google Scholar]
- 50.WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes Second edition. World Heal Organ. 2016;Second edi. https://iris.who.int/bitstream/handle/10665/250677/9789241511575-eng.pdf
- 51.Team RC. R: A language and environment for statistical computing. MSOR Connect. 2014;1.
- 52.Sithiprasasna R, Linthicum KJ, Liu GJ, Jones JW, Singhasivanon P. Some entomological observations on temporal and spatial distribution of malaria vectors in three villages in northwestern Thailand using a geographic information system. Southeast Asian J Trop Med Public Health. 2003;34:505–16. [PubMed] [Google Scholar]
- 53.Kayiwa JT, Nankya AM, Ataliba IJ, Mossel EC, Crabtree MB, Lutwama JJ, et al. Confirmation of Zika virus infection through hospital-based sentinel surveillance of acute febrile illness in Uganda, 2014–2017. Microbiol Soc. 2018;99:1248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obonyo M, Fidhow A, Ofula V. Investigation of laboratory confirmed dengue outbreak in north-eastern Kenya, 2011. PLoS ONE. 2018;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mboera LEG, Mweya CN, Rumisha SF, Tungu PK, Stanley G, Makange MR, et al. The risk of dengue virus transmission in Dar es Salaam, Tanzania during an epidemic period of 2014. PLoS Negl Trop Dis. 2016;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilder, et al. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17:e101–6. [DOI] [PubMed] [Google Scholar]
- 57.Kelvin AA. Outbreak of chikungunya in the Republic of Congo and the global picture. J Infect Dev Ctries. 2011;5:441–4. [PubMed] [Google Scholar]
- 58.IHR. Vector surveillance and management at Points of Entry International Health Regulations (2005). 2018;
- 59.Konan YL, Coulibaly ZI, Kone AB, Ekra KD, Doannio JM, Dosso M, et al. Species composition and population dynamics of Aedes mosquitoes, potential vectors of arboviruses, at the container ˆ te d ’ Ivoire terminal of the autonomous port of Abidjan. Co Parasite. 2013;20. 10.1051/parasite/2013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nambunga IH, Ngowo HS, Mapua SA, Hape EE, Msugupakulya BJ, Msaky DS, et al. Aquatic habitats of the malaria vector Anopheles funestus in rural south—eastern Tanzania. Malar J. 2020;19:1–1. 10.1186/s12936-020-03295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, Killeen GF, et al. Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar J. 2005;15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philbert, et al. Dengue vector distribution and their infection status in selected regions in Tanzania. Tanzania J Sci. 2020;46:636–46. [Google Scholar]
- 63.Bhadauriya AS, Dhan S, Ramteke PU, Bhan S, Lalthazuali Kumawat R, et al. Entomological survey for aedes species at deendayal seaport, kandla, gujarat india during pre-monsoon period, 2018. J Commun Dis. 2020;52:35–8. [Google Scholar]
- 64.Rodrigues GO, Pereira BGV, Pereira MAF, Trindade-Bezerra JM, Guimaraes-E-silva AS, Soares-Pinheiro VC, et al. Potential breeding containers of Aedes aegypti (Linnaeus, 1762) and Aedes albopictus (Skuse, 1894) at strategic points in a city in the eastern region of Maranhao. Brazilian J Biol. 2023;83:1–10. [DOI] [PubMed] [Google Scholar]
- 65.Kazaura M. Knowledge, attitude and practices about dengue fever among adults living in Pwani region, Tanzania in 2019. Afr Health Sci. 2020;20:1601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mwanyika GO, Mboera LEG, Rugarabamu S, Sindato C, Lutwama JJ, Paweska JT, et al. Circulation of dengue serotype 1 viruses during the 2019 outbreak in Dar es Salaam,Tanzania. Pathog Glob Health. 2021; [DOI] [PMC free article] [PubMed]
- 67.Weetman D, Kamgang B, Badolo A, Moyes CL, Shearer FM, Coulibaly M, et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int J Environ Res Public Health. 2018;15:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathias L, Baraka V, Philbert A, Innocent E, Francis F, Nkwengulila G, et al. Habitat productivity and pyrethroid susceptibility status of Aedes aegypti mosquitoes in Dar es Salaam. Tanzania Infect Dis Poverty. 2017;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mkpola O, Mathias C, Author C. Insecticide susceptibility status of Aedes Aegypti in Umudike, Ikwuano Lga Abia State. Nigeria Anim Res Int. 2018;15:3082–9. [Google Scholar]
- 70.WHO. Framework for National Surveillance & Control Plans for Aedes Vectors in the Pacific. World Heal Organ. 2023;1–60.
- 71.Duval P, Aschan-Leygonie C, Valiente MC. A review of knowledge, attitudes and practices regarding mosquitoes and mosquito-borne infectious diseases in nonendemic regions. Front Public Heal. 2023;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serengbe GB, Moyen J, Fioboy R, Beyam EN, Kango C, Bangue C, et al. Knowledge and perceptions about malaria in communities in four districts of the Central African Republic. Bio Med Central. 2015;8:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raude J, Chinfatt K, Huang P, Betansedi CO, Katumba K, Vernazza N, et al. Public perceptions and behaviours related to the risk of infection with Aedes mosquito-borne diseases: a cross-sectional study in Southeastern France. BMJ Open. 2012;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kusuma YS, Goswami AK, Babu BV. Dengue awareness, preventive behaviours and Aedes breeding opportunities among slums and slum-like pockets in Delhi, India: a formative assessment. Trans R Soc Trop Med Hyg. 2021;115:653–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1: Fig. S1: Common habitat types observed in the study area: a shallow well, b surface drain c discarded bowl holding water, d root hole of banana tree, e discarded car tires, f fire hydrant
Supplementary material 2: Fig. S2: Common habitat types observed in the study area: a flowerpot, b plastic buckets for animal drinking, c container for feeding hens, d inspection chamber, e plastic container holding water placed under charcoal burner, f stream pool
Data Availability Statement
No datasets were generated or analysed during the current study.