Abstract
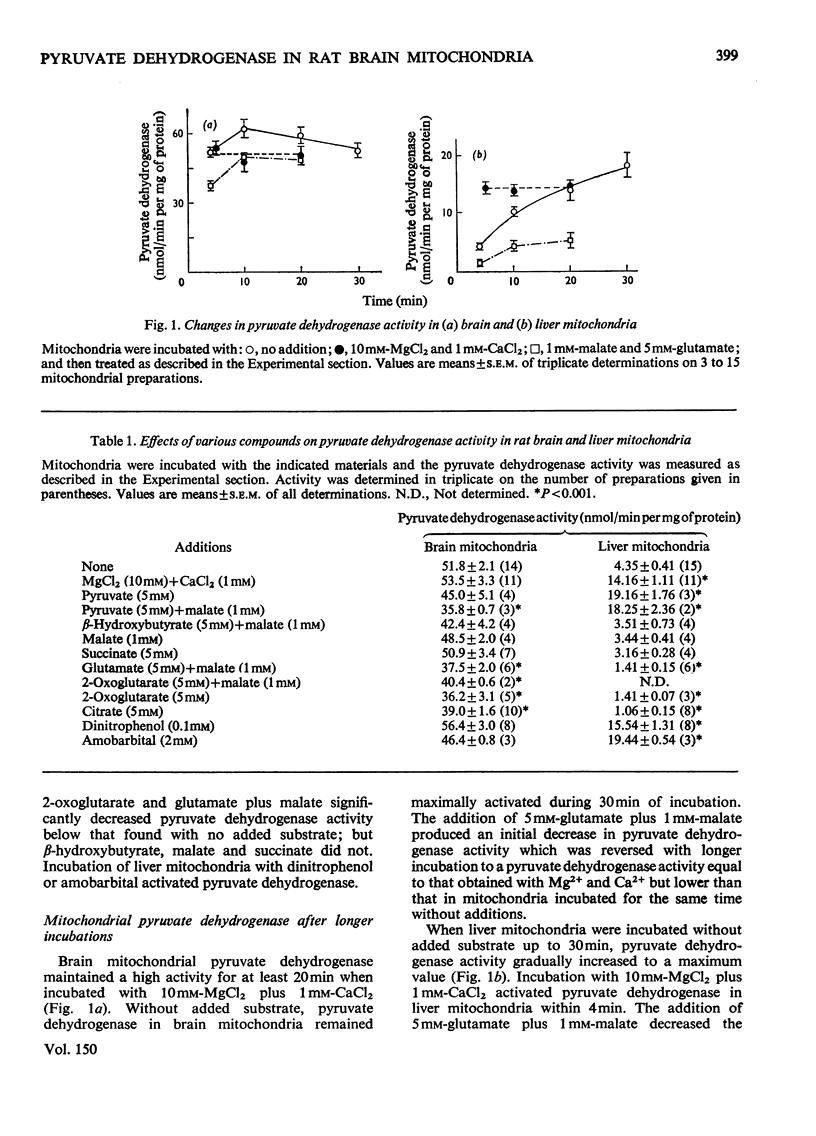
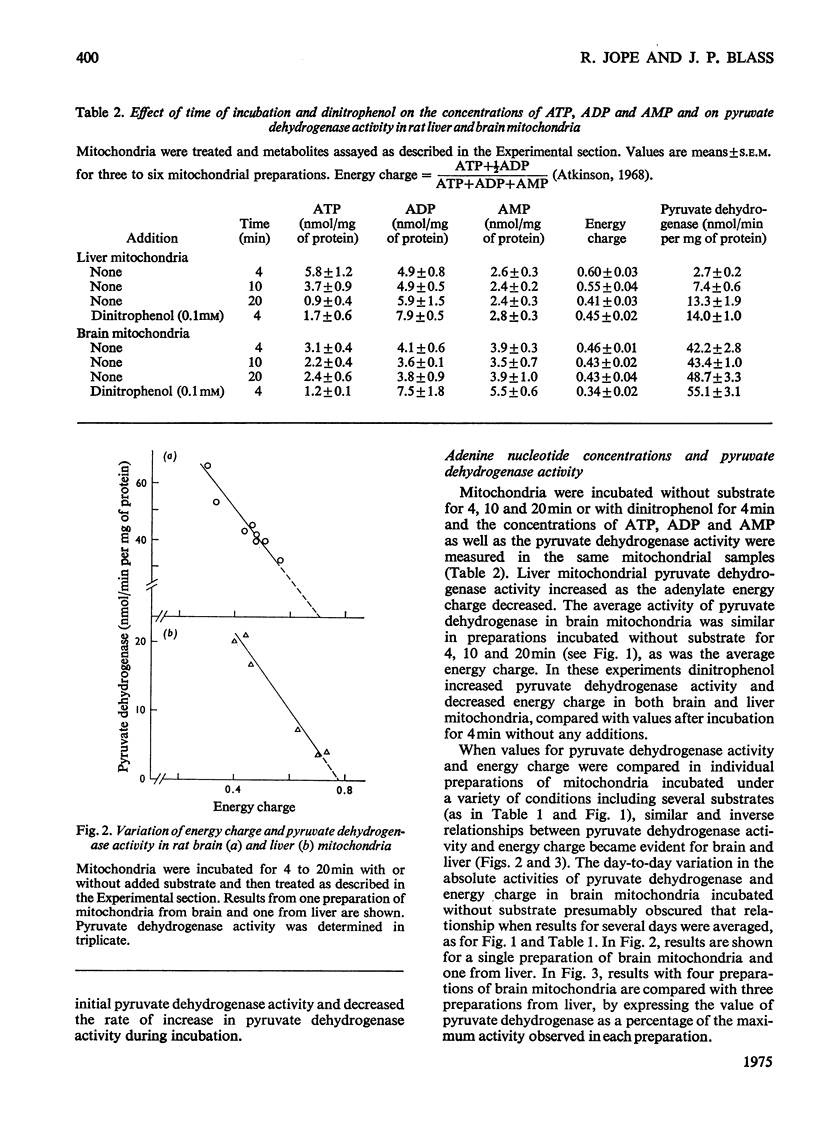
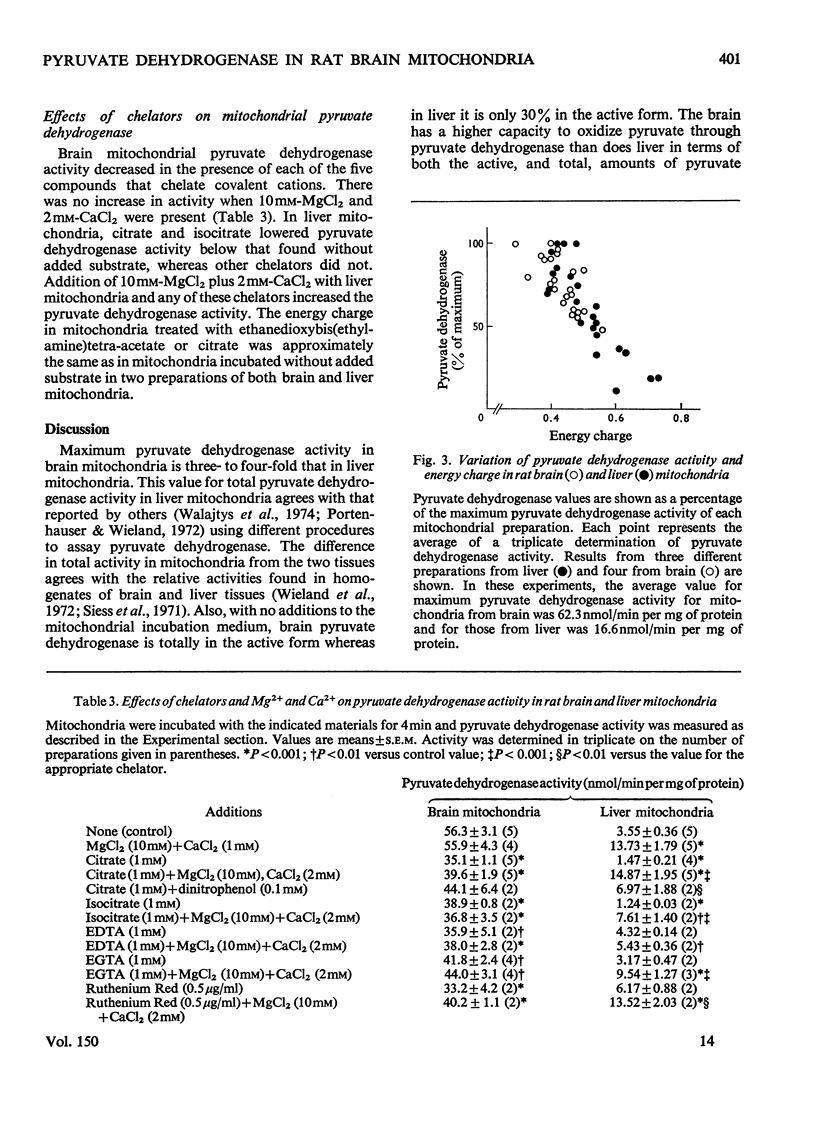
The total activity of pyruvate dehydrogenase in mitochondria isolated from rat brain and liver was 53.5 and 14.2nmol/min per mg of protein respectively. Pyruvate dehydrogenase in liver mitochondria incubated for 4 min at 37 degrees C with no additions was 30% in the active form and this activity increased with longer incubations until it was completely in the active form after 20 min. Brain mitochondrial pyruvate dehydrogenase activity was initially high and did not increase with addition of Mg2+ plus Ca2+ or partially purified pyruvate dehydrogenase phosphatase or with longer incubations. The proportion of pyruvate dehydrogenase in the active form in both brain and liver mitochondria changed inversely with changes in mitochondrial energy charge, whereas total pyruvate dehydrogenase did not change. The chelators citrate, isocitrate, EDTA, ethanedioxybis(ethylamine)tetra-acetic acid and Ruthenium Red each lowered pyruvate dehydrogenase activity in brain mitochondria, but only citrate and isocitrate did so in liver mitochondria. These chelators did not affect the energy charge of the mitochondria. Mg2+ plus Ca2+ reversed the pyruvate dehydrogenase inactivation in liver, but not brain, mitochondria. The regulation of the activation-inactivation of pyruvate dehydrogenase in mitochondria from rat brain and liver with respect to energy charge is similar and may be at least partially regulated by this parameter, and the effects of chelators differ in the two types of mitochondria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Avigan J., Uhlendorf B. W. A defect in pyruvate decarboxylase in a child with an intermittent movement disorder. J Clin Invest. 1970 Mar;49(3):423–432. doi: 10.1172/JCI106251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass J. P., Lewis C. A. Kinetic properties of the partially purified pyruvate dehydrogenase complex of ox brain. Biochem J. 1973 Jan;131(1):31–37. doi: 10.1042/bj1310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. B., Nicklas W. J. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970 Sep 25;245(18):4724–4731. [PubMed] [Google Scholar]
- Cremer J. E., Teal H. M. The activity of pyruvate dehydrogenase in rat brain during postnatal development. FEBS Lett. 1974 Feb 1;39(1):17–20. doi: 10.1016/0014-5793(74)80006-2. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdall M. J., Boyne A. F., Whittaker V. P. Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J. 1974 Apr;140(1):1–12. doi: 10.1042/bj1400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. E., Jope R., Blass J. P. Decreased synthesis of acetylcholine accompanying impaired oxidation of pyruvic acid in rat brain minces. Biochem J. 1975 Apr;148(1):17–23. doi: 10.1042/bj1480017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörikofer-Zwez S., Kunin A. S., Walter P. Effects of calcium and sucrose on pyruvate carboxylase activity in intact rat liver mitochondria. J Biol Chem. 1973 Nov 10;248(21):7588–7594. [PubMed] [Google Scholar]
- Patel M. S., Tilghman S. M. Regulation of pyruvate metabolism via pyruvate carboxylase in rat brain mitochondria. Biochem J. 1973 Feb;132(2):185–192. doi: 10.1042/bj1320185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenhauser R., Wieland O. Regulation of pyruvate dehydrogenase in mitochondria of rat liver. Eur J Biochem. 1972 Dec 4;31(2):308–314. doi: 10.1111/j.1432-1033.1972.tb02534.x. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. C., Fall L., Walton G. M., Atkinson D. E. Interaction between energy charge and metabolite modulation in the regulation of enzymes of amphibolic sequences. Phosphofructokinase and pyruvate dehydrogenase. Biochemistry. 1968 Nov;7(11):4041–4045. doi: 10.1021/bi00851a035. [DOI] [PubMed] [Google Scholar]
- Siess E., Wittmann J., Wieland O. Interconversion and kinetic properties of pyruvate dehydrogenase from brain. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):447–452. doi: 10.1515/bchm2.1971.352.1.447. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Taylor S. I., Mukherjee C., Jungas R. L. Studies on the mechanism of activation of adipose tissue pyruvate dehydrogenase by insulin. J Biol Chem. 1973 Jan 10;248(1):73–81. [PubMed] [Google Scholar]
- Taylor W. M., Halperin M. L. Regulation of pyruvate dehydrogenase in muscle. Inhibition by citrate. J Biol Chem. 1973 Sep 10;248(17):6080–6083. [PubMed] [Google Scholar]
- Walajtys E. I., Gottesman D. P., Williamson J. R. Regulation of pyruvate dehydrogenase in rat liver mitochondria by phosphorylation-dephosphorylation. J Biol Chem. 1974 Mar 25;249(6):1857–1865. [PubMed] [Google Scholar]
- Wieland O. H., Patzelt C., Löffler G. Active and inactive forms of pyruvate dehydrogenase in rat liver. Effect of starvation and refeeding and of insulin treatment on pyruvate-dehydrogenase interconversion. Eur J Biochem. 1972 Apr 11;26(3):426–433. doi: 10.1111/j.1432-1033.1972.tb01783.x. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E., Schulze-Wethmar F. H., von Funcke H. G., Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971 Apr;143(2):593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]


