Abstract
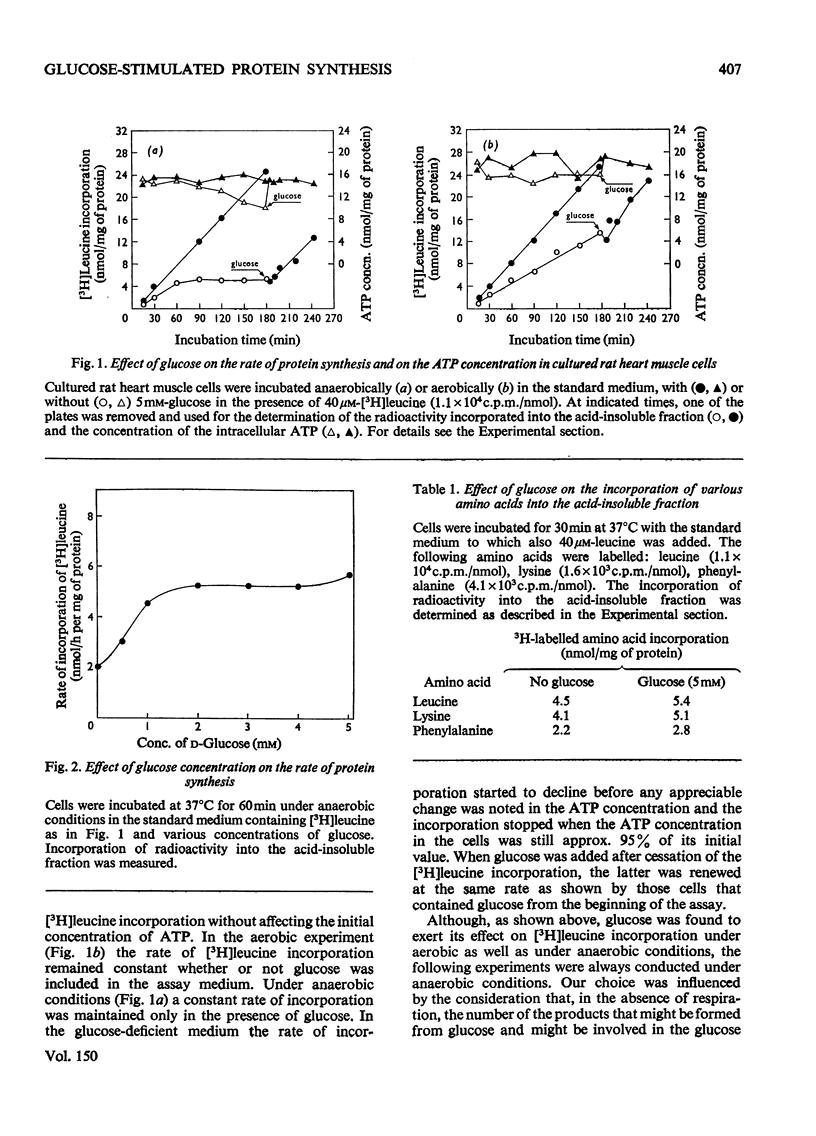
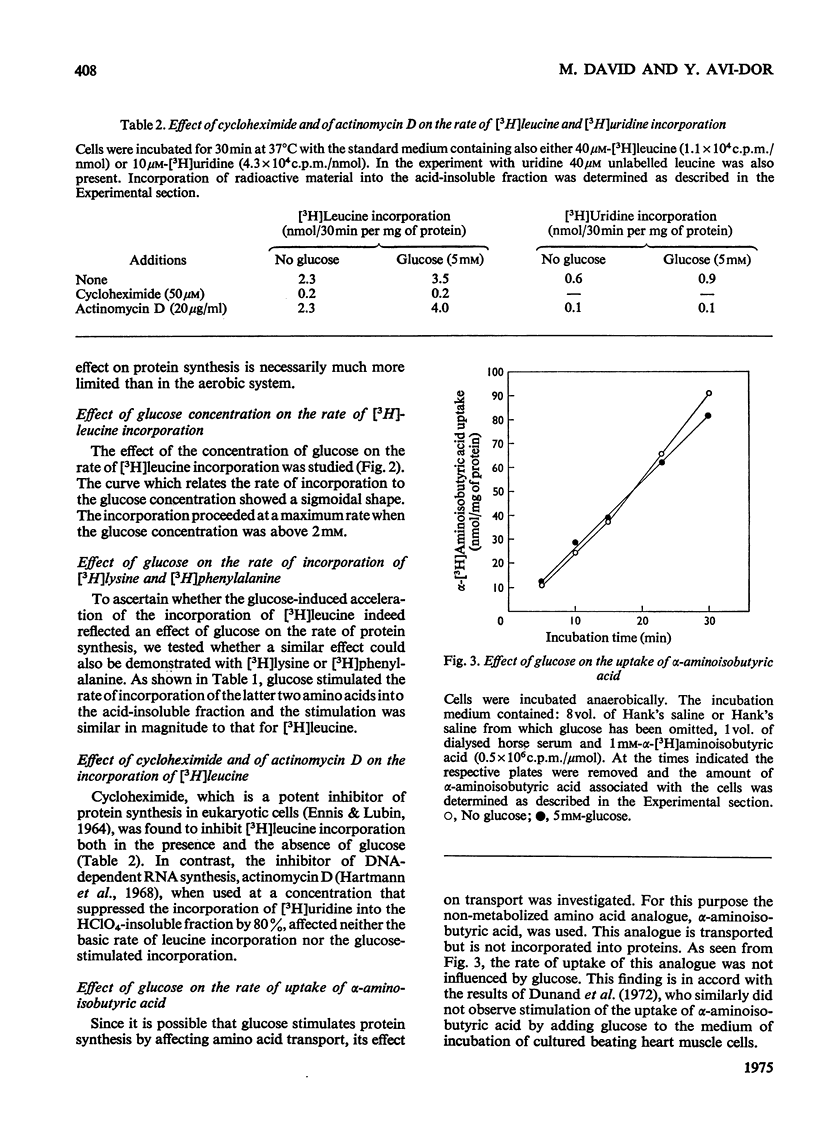
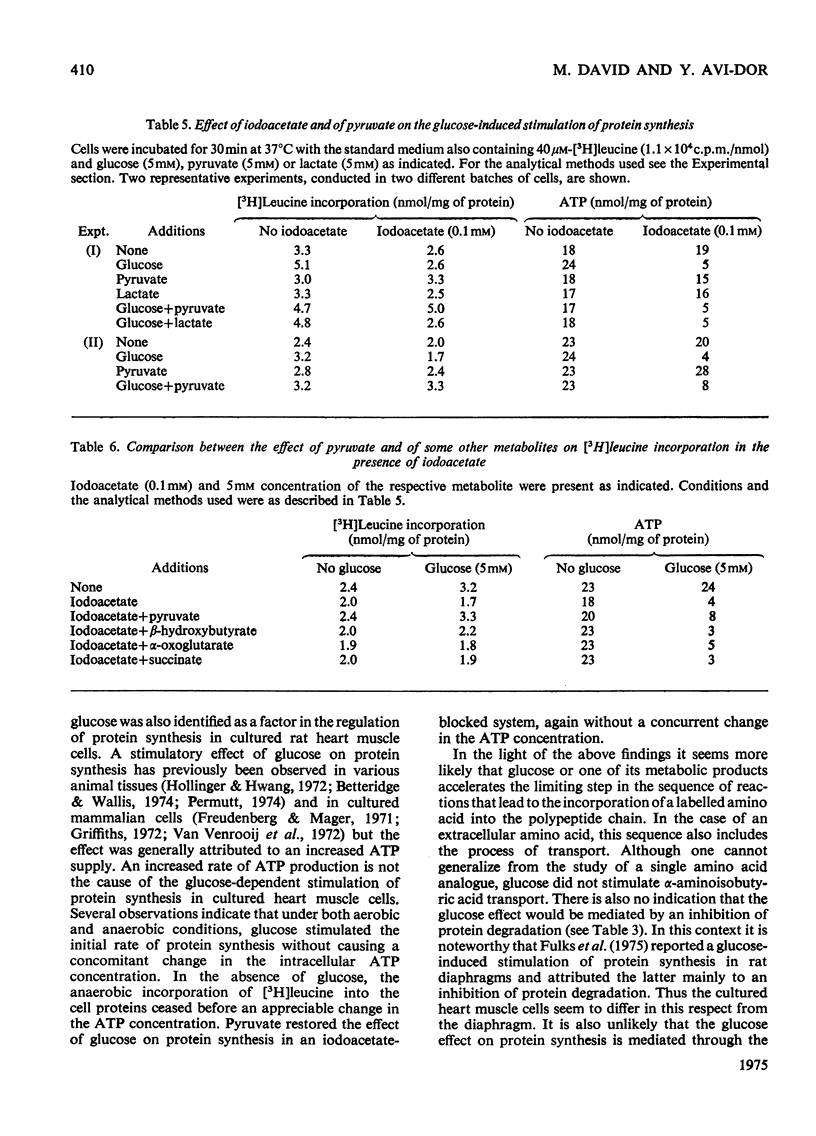
Glucose stimulated the rate of incorporation of [3H]leucine into HCLO4-insoluble fraction of cultured rat heart muscle cells under both aerobic and anaerobic conditions. In the aerobic system the incorporation proceeded at a constant rate during 3h of incubation with and without glucose whereas in the anaeorbic system the incorporation ceased after approx. 60 min and could be renewed only by the addition of glucose. No correlation was found to exist between the above effect of glucose on protein synthesis and glucose-dependent changes in the intracellular ATP concentration. The extent of the stimulation of protein synthesis was related to the concentration of glucose. The effect of glucose was suppressed by cycloheximide but was not affected by actinomycin D. Glucose had no effect on the rate of transport of alpha-aminoisobutyric acid. Mannose also stimulated [3H]leucine incorporation. Substances that did not produce lactate were ineffective. Iodoacetate inhibited the stimulatory effect of glucose, but pyruvate, which by itself had no apprecialbe stimulatory action, relieved the inhibition induced by iodoacetate. There was no concomitant change in the concentration of ATP when iodoacetate inhibition was reversed by pyruvate. L-Lactate or other intermediates of energy metabolism could not relieve the inhibitory effect of iodoacetate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betteridge A., Wallis M. Biosynthesis of growth hormone in the rat anterior pituitary gland. Control of biosynthesis in vitro by glucose. Biochim Biophys Acta. 1974 Aug 7;362(1):66–74. doi: 10.1016/0304-4165(74)90027-0. [DOI] [PubMed] [Google Scholar]
- Dunand P., Blondel B., Girardier L., Jeanrenaud B. Alpha-aminoisobutyric acid uptake by cultured beating heart cells. Biochim Biophys Acta. 1972 Feb 11;255(2):462–478. doi: 10.1016/0005-2736(72)90150-2. [DOI] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. CYCLOHEXIMIDE: ASPECTS OF INHIBITION OF PROTEIN SYNTHESIS IN MAMMALIAN CELLS. Science. 1964 Dec 11;146(3650):1474–1476. doi: 10.1126/science.146.3650.1474. [DOI] [PubMed] [Google Scholar]
- Freudenberg H., Mager J. Studies on the mechanism of the inhibition of protein synthesis induced by intracellular ATP depletion. Biochim Biophys Acta. 1971 Mar 25;232(3):537–555. doi: 10.1016/0005-2787(71)90608-3. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Griffiths J. B. The effect of cell population density on nutrient uptake and cell metabolism: a comparative study of human diploid and heteroploid cell lines. J Cell Sci. 1972 Mar;10(2):515–524. doi: 10.1242/jcs.10.2.515. [DOI] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res. 1963 Feb;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Hartmann G., Behr W., Beissner K. A., Honikel K., Sippel A. Antibiotics as inhibitors of nucleic acid and protein synthesis. Angew Chem Int Ed Engl. 1968 Sep;7(9):693–701. doi: 10.1002/anie.196806931. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Chain E. B. The role of glucose in the survival and 'recovery' of the anoxic isolated perfused rat heart. Biochem J. 1972 Aug;128(5):1125–1133. doi: 10.1042/bj1281125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger M. A., Hwang F. Effect of puromycin and actinomycin D on glucose-stimulated protein and RNA labeling, in vitro, in rat testis. Biochim Biophys Acta. 1972 Mar 24;262(3):336–343. doi: 10.1016/0005-2787(72)90271-7. [DOI] [PubMed] [Google Scholar]
- Jennings R. B. Early phase of myocardial ischemic injury and infarction. Am J Cardiol. 1969 Dec;24(6):753–765. doi: 10.1016/0002-9149(69)90464-0. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Sommers H. M., Herdson P. B., Kaltenbach J. P. Ischemic injury of myocardium. Ann N Y Acad Sci. 1969 Jan 31;156(1):61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. Dissociation of the insulin releasing and the metabolic functions of hexoses in islets of Langerhans. Biochem Biophys Res Commun. 1973 Jan 23;50(2):193–199. doi: 10.1016/0006-291x(73)90826-7. [DOI] [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Permutt M. A. Effect of glucose on initiation and elongation rates in isolated rat pancreatic islets. J Biol Chem. 1974 May 10;249(9):2738–2742. [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Weissler A. M., Kruger F. A., Baba N., Scarpelli D. G., Leighton R. F., Gallimore J. K. Role of anaerobic metabolism in the preservation of functional capacity and structure of anoxic myocardium. J Clin Invest. 1968 Feb;47(2):403–416. doi: 10.1172/JCI105737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenthal K. Studies of foetal mouse hearts in organ culture: metabolic requirements for prolonged function in vitro and the influence of cardiac maturation on substrate utilization. J Mol Cell Cardiol. 1973 Feb;5(1):87–99. doi: 10.1016/0022-2828(73)90038-2. [DOI] [PubMed] [Google Scholar]
- Wollenberger A., Onnen K., Hinterberger U., Rabitzsch G., Kleitke B. Myocardial protein synthesis in acute myocardial hypoxia and ischemia. Cardiology. 1971;56(1):48–64. doi: 10.1159/000169341. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Henshaw E. C., Hirsch C. A. Effects of deprival of glucose or individual amino acids on polyribosome distribution and rate of protein synthesis in cultured mammalian cells. Biochim Biophys Acta. 1972 Jan 18;259(1):127–137. doi: 10.1016/0005-2787(72)90480-7. [DOI] [PubMed] [Google Scholar]


