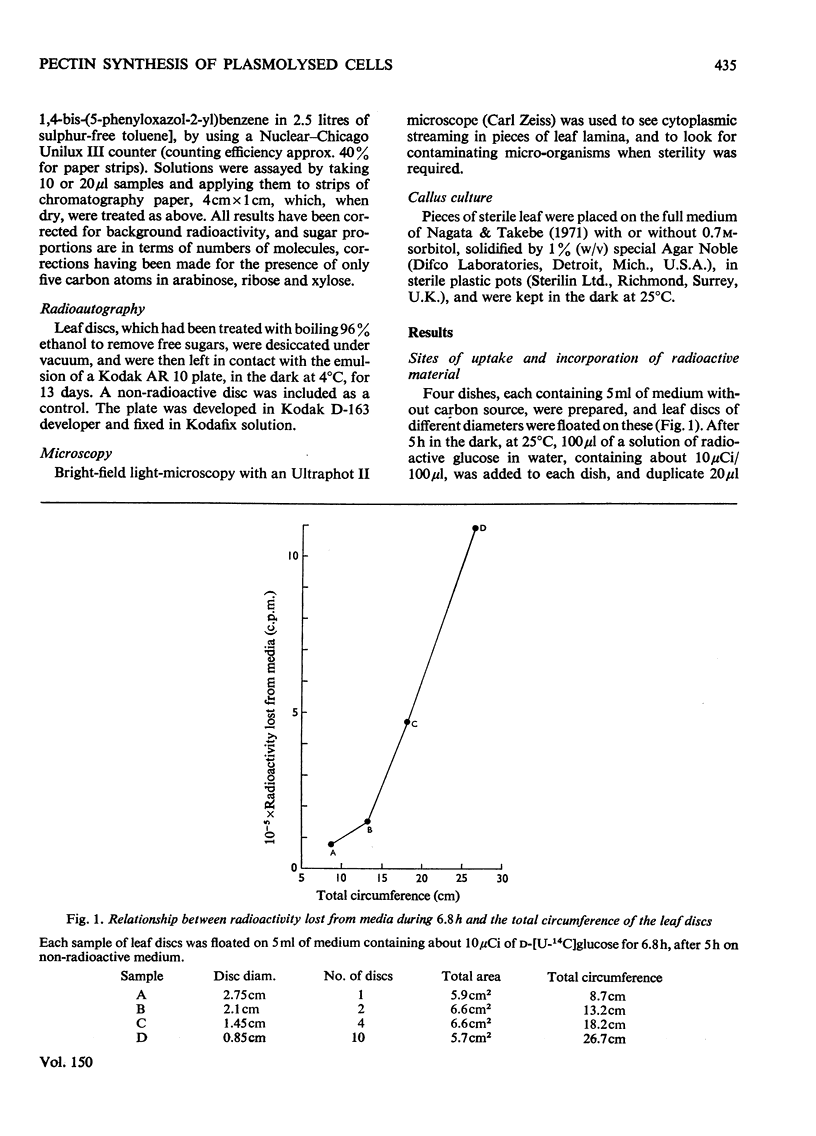
Abstract
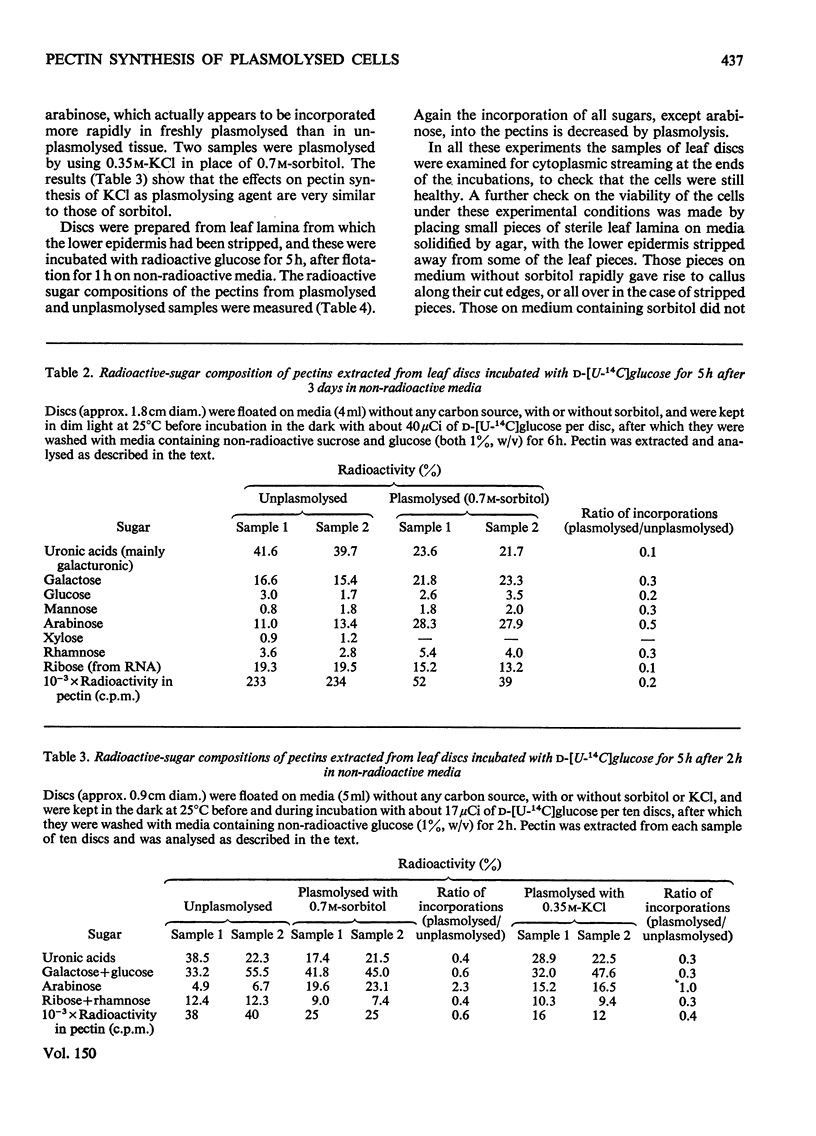
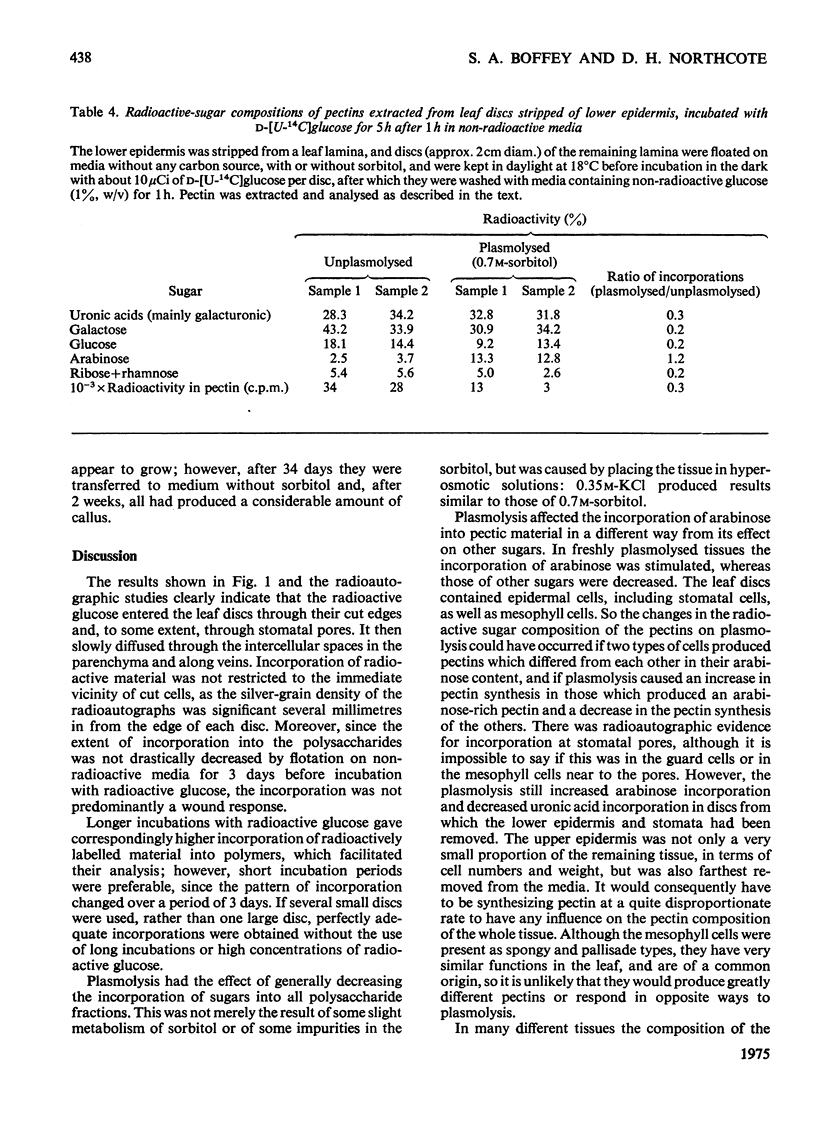
1. Discs of tobacco leaf lamina were floated on media containing D-[U-14C]glucose. Glucose uptake was mainly through the cut edges, and diffusion through the veins and intracellular spaces was slow. 2. Radioactivity was detected in all polysaccharide fractions extracted from the discs, including those associated with the wall. 3. Plasmolysis in sorbitol or KCL decreased the incorporation of radioactive material into all fractions. 4. Incorporation of arabinose into pectins was increased or unaffected by plasmolysis, but the incorporations of other sugars were decreased. Removal of the lower epidermis did not affect this result. 5. Seperate mechanisms for arabinan and polygalacturonorhamman syntheses must exist, and these must differ in their responses to the physiochemical, structural and organizational changes that accompany plasmolysis.
Full text
PDF

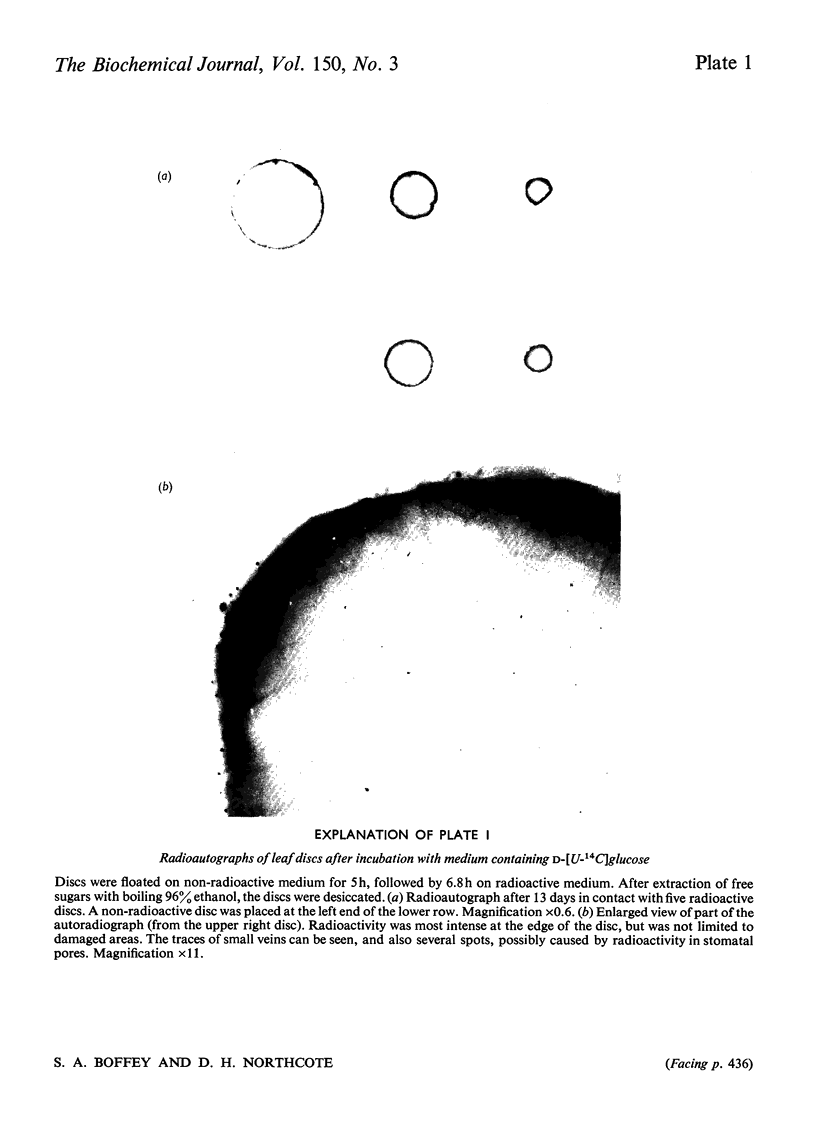


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Northcote D. H. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochem J. 1972 Dec;130(4):1133–1145. doi: 10.1042/bj1301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess J., Fleming E. N. Ultrastructural observations of cell wall regeneration around isolated tobacco protoplasts. J Cell Sci. 1974 Mar;14(2):439–449. doi: 10.1242/jcs.14.2.439. [DOI] [PubMed] [Google Scholar]
- Greenway H., Leahy M. Effects of rapidly and slowly permeating osmotica on metabolism. Plant Physiol. 1970 Aug;46(2):259–262. doi: 10.1104/pp.46.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke D. E., Northcote D. H. Cell wall formation by soybean callus protoplasts. J Cell Sci. 1974 Jan;14(1):29–50. doi: 10.1242/jcs.14.1.29. [DOI] [PubMed] [Google Scholar]
- Northcote D. H., Pickett-Heaps J. D. A function of the Golgi apparatus in polysaccharide synthesis and transport in the root-cap cells of wheat. Biochem J. 1966 Jan;98(1):159–167. doi: 10.1042/bj0980159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLAITAN S. A., NORTHCOTE D. H. Polysaccharides of Chlorella pyrenoidosa. Biochem J. 1962 Mar;82:509–519. doi: 10.1042/bj0820509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem. 1969;24:267–332. doi: 10.1016/s0065-2318(08)60352-2. [DOI] [PubMed] [Google Scholar]
- Rees D. A., Wight N. J. Molecular cohesion in plant cell walls. Methylation analysis of pectic polysaccharides from the cotyledons of white mustard. Biochem J. 1969 Nov;115(3):431–439. doi: 10.1042/bj1150431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery P. H., Northcote D. H. The effect of auxin (2,4-dichlorophenoxyacetic acid) on the synthesis of cell wall polysaccharides in cultured sycamore cells. Biochim Biophys Acta. 1970 Oct 27;222(1):95–108. doi: 10.1016/0304-4165(70)90355-7. [DOI] [PubMed] [Google Scholar]
- Stoddart R. W., Barrett A. J., Northcote D. H. Pectic polysaccharides of growing plant tissues. Biochem J. 1967 Jan;102(1):194–204. doi: 10.1042/bj1020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart R. W., Northcote D. H. Metabolic relationships of the isolated fractions of the pectic substances of actively growing sycamore cells. Biochem J. 1967 Oct;105(1):45–59. doi: 10.1042/bj1050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. II. Carbohydrate constituents of each main component. Biochem J. 1961 Dec;81:455–464. doi: 10.1042/bj0810455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers L. A., Cocking E. C. Fine-structural studies on spontaneous and induced fusion of higher plant protoplasts. J Cell Sci. 1972 Jul;11(1):59–75. doi: 10.1242/jcs.11.1.59. [DOI] [PubMed] [Google Scholar]
- Wright K., Northcote D. H. Differences in ploidy and degree of intercellular contact in differentiating and non-differentiating sycamore calluses. J Cell Sci. 1973 Jan;12(1):37–53. doi: 10.1242/jcs.12.1.37. [DOI] [PubMed] [Google Scholar]
- Wright K., Northcote D. H. The relationship of root-cap slimes to pectins. Biochem J. 1974 Jun;139(3):525–534. doi: 10.1042/bj1390525. [DOI] [PMC free article] [PubMed] [Google Scholar]