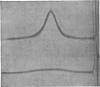
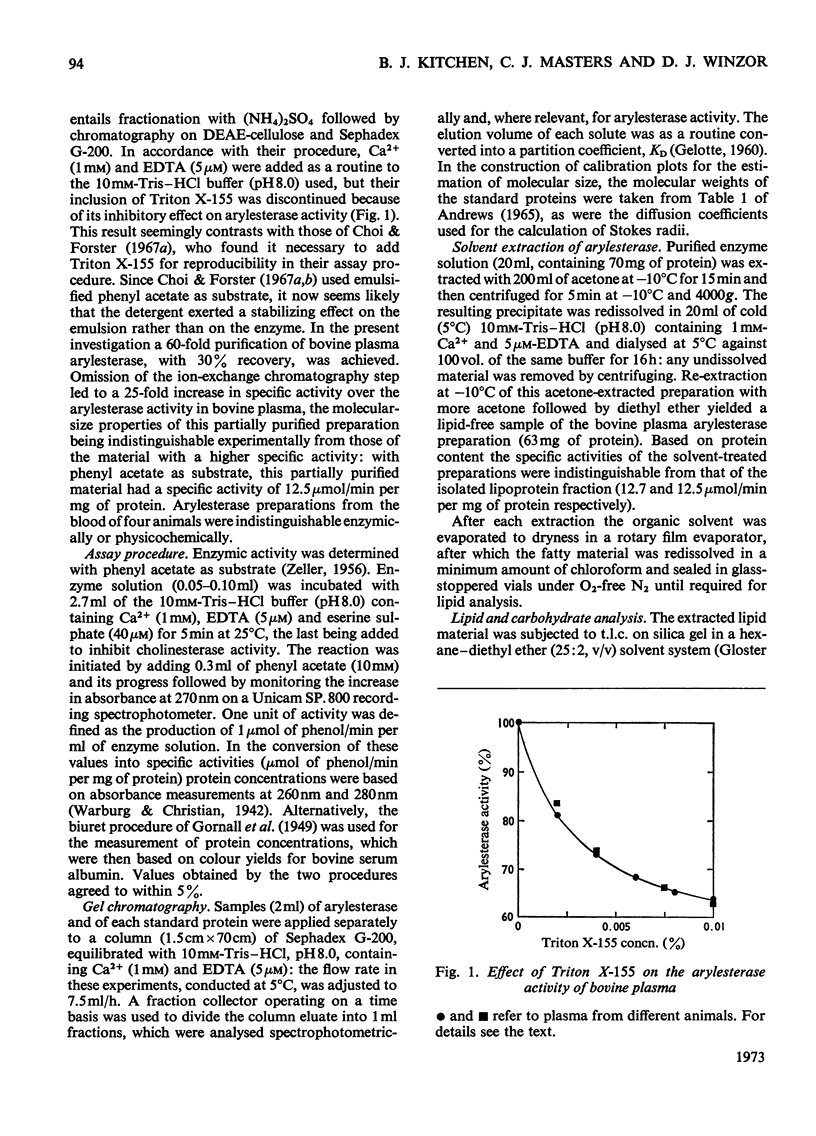
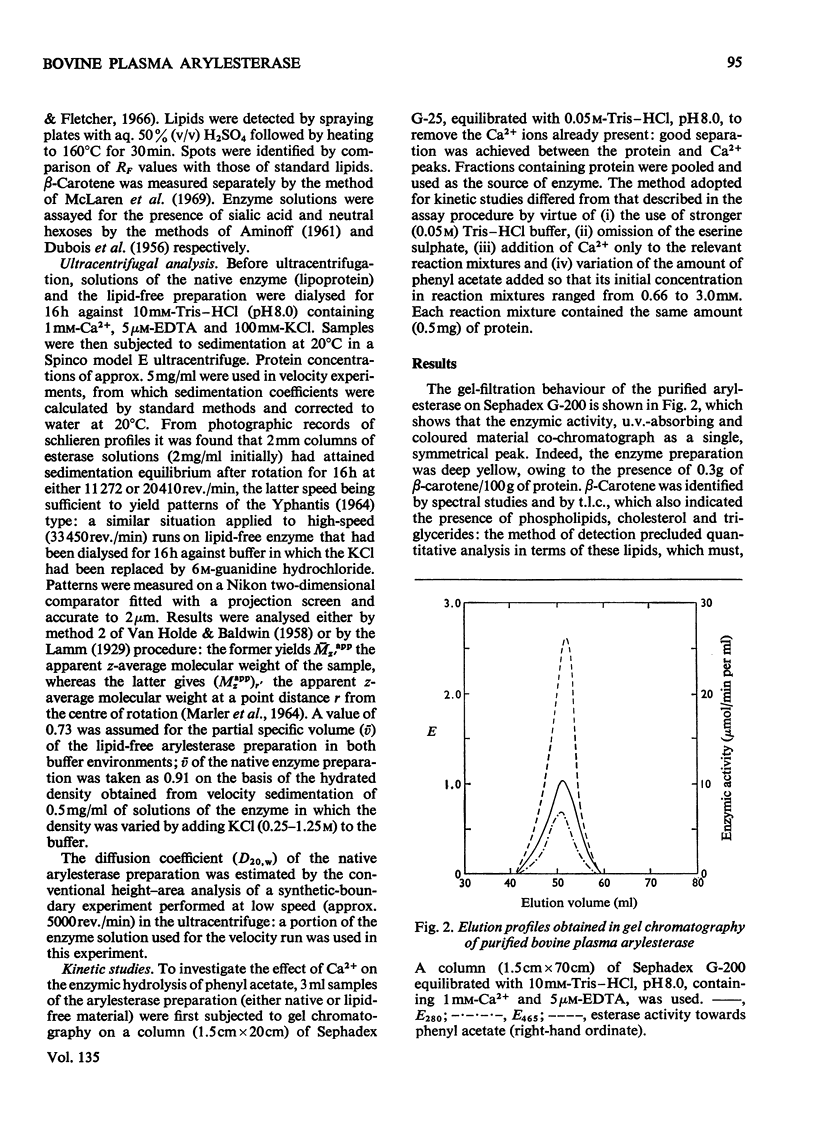
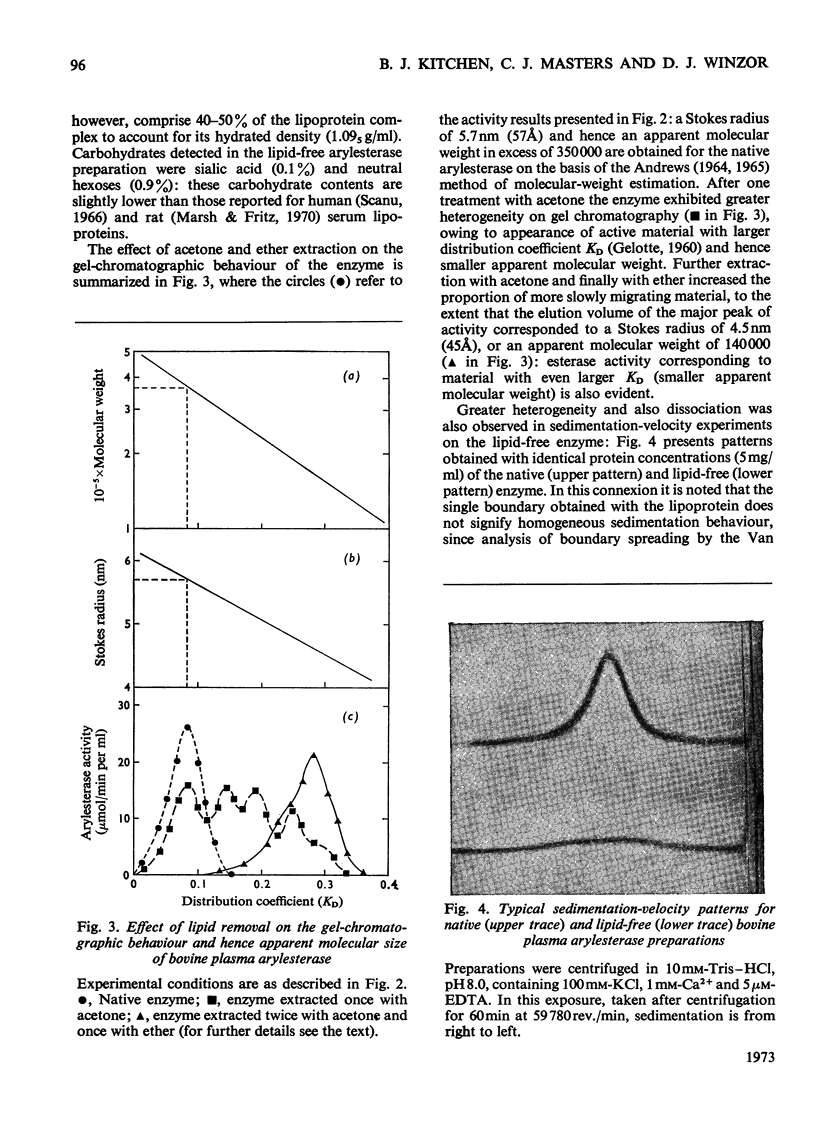
Abstract
A purified arylesterase preparation from bovine plasma was characterized to the extent that it has a partial specific volume of 0.91ml/g and an apparent z-average molecular weight of 440000. The relatively large magnitude of the former reflects the presence of phospholipids, cholesterol, triglycerides and β-carotene, the last-named being responsible for the pronounced yellow colour of the preparation. Removal of the lipid material is accompanied by a decrease in the apparent z-average molecular weight to 120000, the size of the smallest species detected by high-speed sedimentation equilibrium being in the vicinity of 70000 daltons: denaturation of the lipid-free preparation with 6m-guanidine hydrochloride caused essentially complete breakdown into subunits of this size. In kinetic studies on the enzyme the maximal velocity for the hydrolysis of phenyl acetate was found to increase by 60% on addition of 1 mm-Ca2+, with the Km showing a concomitant decrease from 6.6 to 2.1 mm. Removal of lipid had no detectable effect on Vmax. or Km in either the presence or the absence of Ca2+. It is concluded that the bovine plasma arylesterase preparation is either a lipoprotein or an enzyme–lipoprotein complex with properties very similar to those of the α1-lipoprotein or high-density lipoprotein (HDL2) fraction of serum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem J. 1953 Jan;53(1):110–117. doi: 10.1042/bj0530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERDOS E. G., DEBAY C. R., WESTERMAN M. P. Activation and inhibition of the arylesterase of human serum. Nature. 1959 Aug 8;184:430–431. doi: 10.1038/184430a0. [DOI] [PubMed] [Google Scholar]
- ERDOS E. G., DEBAY C. R., WESTERMAN M. P. Arylesterases in blood: effect of calcium and inhibitors. Biochem Pharmacol. 1960 Nov;5:173–186. doi: 10.1016/0006-2952(60)90061-7. [DOI] [PubMed] [Google Scholar]
- Gloster J., Fletcher R. F. Quantitative analysis of serum lipids with thin-layer chromatography. Clin Chim Acta. 1966 Feb;13(2):235–240. doi: 10.1016/0009-8981(66)90298-1. [DOI] [PubMed] [Google Scholar]
- Kaminski M., Dubois P. Are serum lipoproteins esterases? Esterase activity of the immunoprecipitates of duck-serum high-density lipoprotein in agar immunoelectrophoresis and immunodiffusion. Eur J Biochem. 1972 Aug 18;29(1):175–183. doi: 10.1111/j.1432-1033.1972.tb01972.x. [DOI] [PubMed] [Google Scholar]
- Kingsbury N., Masters C. J. Molecular weight interrelationships in the vertebrate esterases. Biochim Biophys Acta. 1970 Jan 20;200(1):58–69. doi: 10.1016/0005-2795(70)90043-7. [DOI] [PubMed] [Google Scholar]
- MARLER E., NELSON C. A., TANFORD C. THE POLYPEPTIDE CHAINS OF RABBIT GAMMA-GLOBULIN AND ITS PAPAIN-CLEAVED FRAGMENTS. Biochemistry. 1964 Feb;3:279–284. doi: 10.1021/bi00890a024. [DOI] [PubMed] [Google Scholar]
- MARTON A. V., KALOW W. Interaction between aromatic esterase of human serum and bivalent metal ions. Can J Biochem Physiol. 1962 Mar;40:319–324. [PubMed] [Google Scholar]
- Marsh J. B., Fritz R. The carbohydrate components of rat serum lipoproteins. Proc Soc Exp Biol Med. 1970 Jan;133(1):9–10. doi: 10.3181/00379727-133-34395. [DOI] [PubMed] [Google Scholar]
- McLaren D. S., Read W. W., Awdeh Z. L., Tchalian M. Microdetermination of vitamin A and carotenoids in blood and tissue. Methods Biochem Anal. 1967;15:1–23. doi: 10.1002/9780470110331.ch1. [DOI] [PubMed] [Google Scholar]
- SCANU A., LEWIS L. A., BUMPUS F. M. Separation and characterization of the protein moiety of human alpha1-lipoprotein. Arch Biochem Biophys. 1958 Apr;74(2):390–397. doi: 10.1016/0003-9861(58)90009-2. [DOI] [PubMed] [Google Scholar]
- SHORE B. C-and N-terminal amino acids of human serum lipoproteins. Arch Biochem Biophys. 1957 Sep;71(1):1–10. doi: 10.1016/0003-9861(57)90002-4. [DOI] [PubMed] [Google Scholar]
- Scanu A. Forms of human serum high density lipoprotein protein. J Lipid Res. 1966 Mar;7(2):295–306. [PubMed] [Google Scholar]
- TALAL N., HERMANN G., VAUX S. T., de CYR, GRABAR P. Immunoelectrophoretic study of mouse serum and urinary esterases. Chromatographic separation of albumin esterase. J Immunol. 1963 Feb;90:246–256. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- ZELLER E. A. Enzymology of the refractory media of the eye. IV. Direct photometric determination of cholinesterase and aliesterase of corpus vitreum. Arch Biochem Biophys. 1956 Mar;61(1):231–240. doi: 10.1016/0003-9861(56)90335-6. [DOI] [PubMed] [Google Scholar]