Abstract
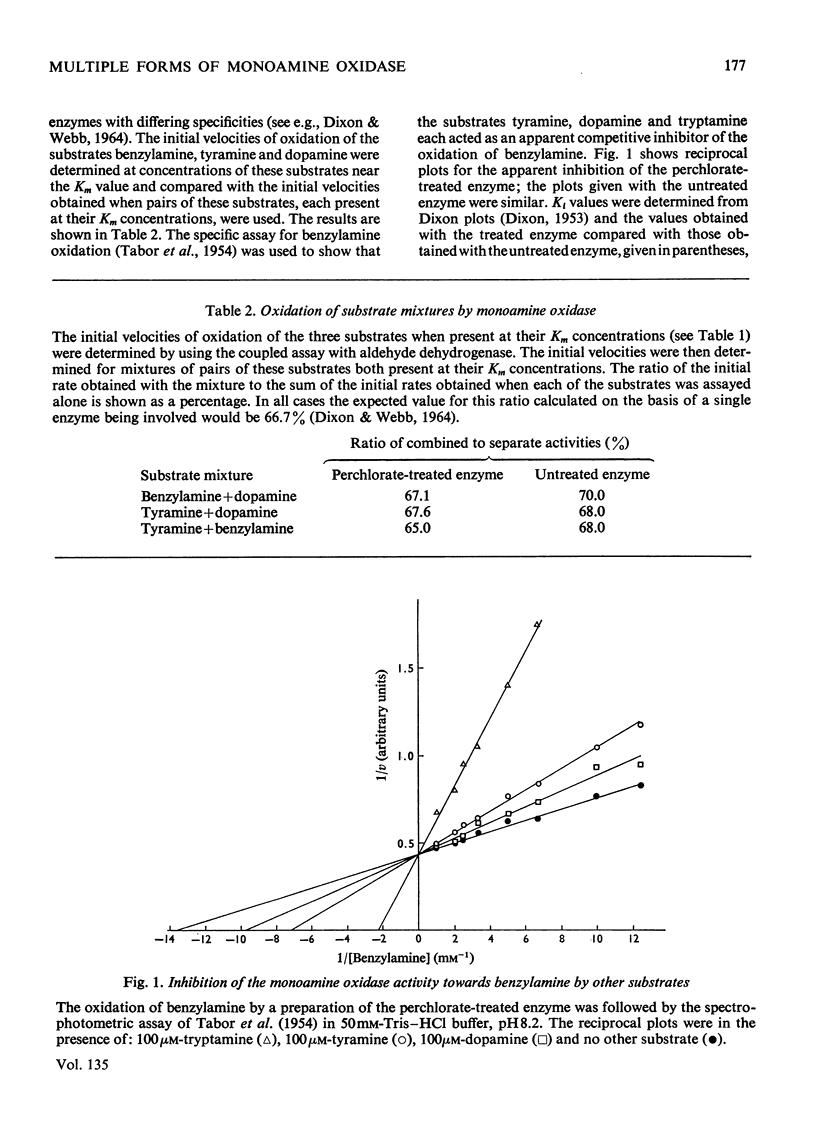
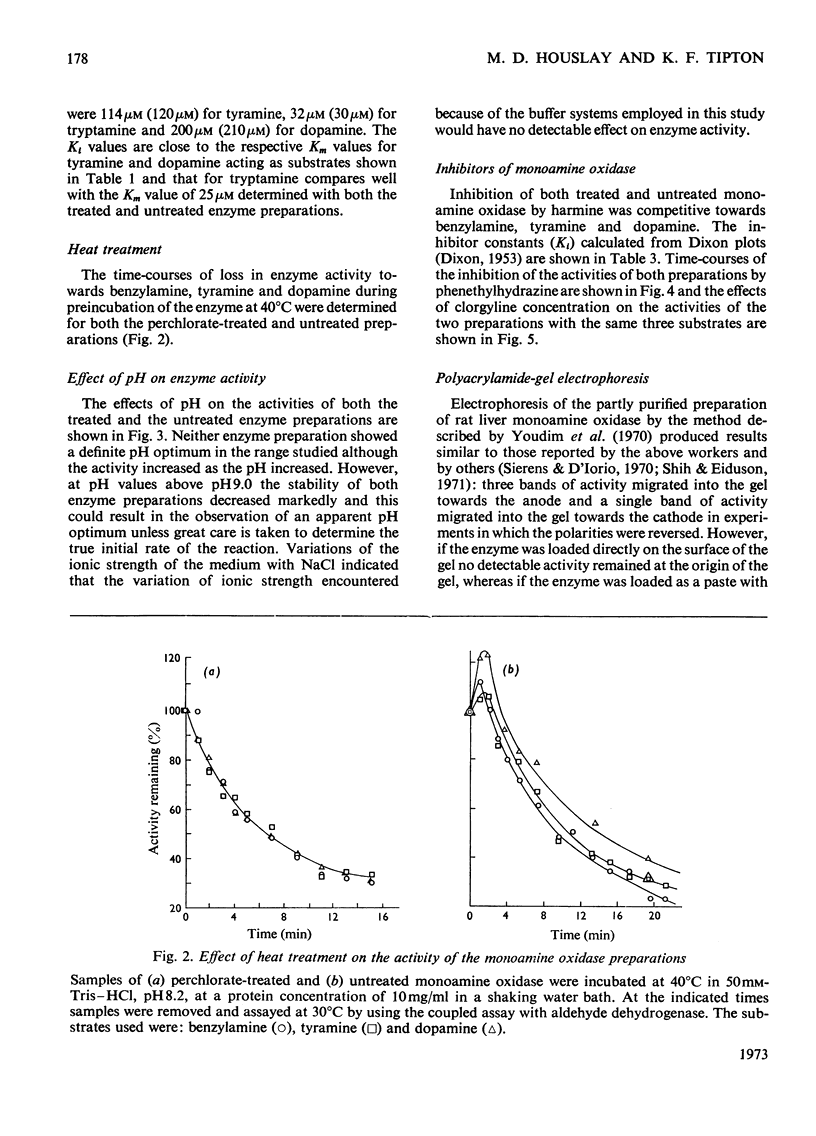
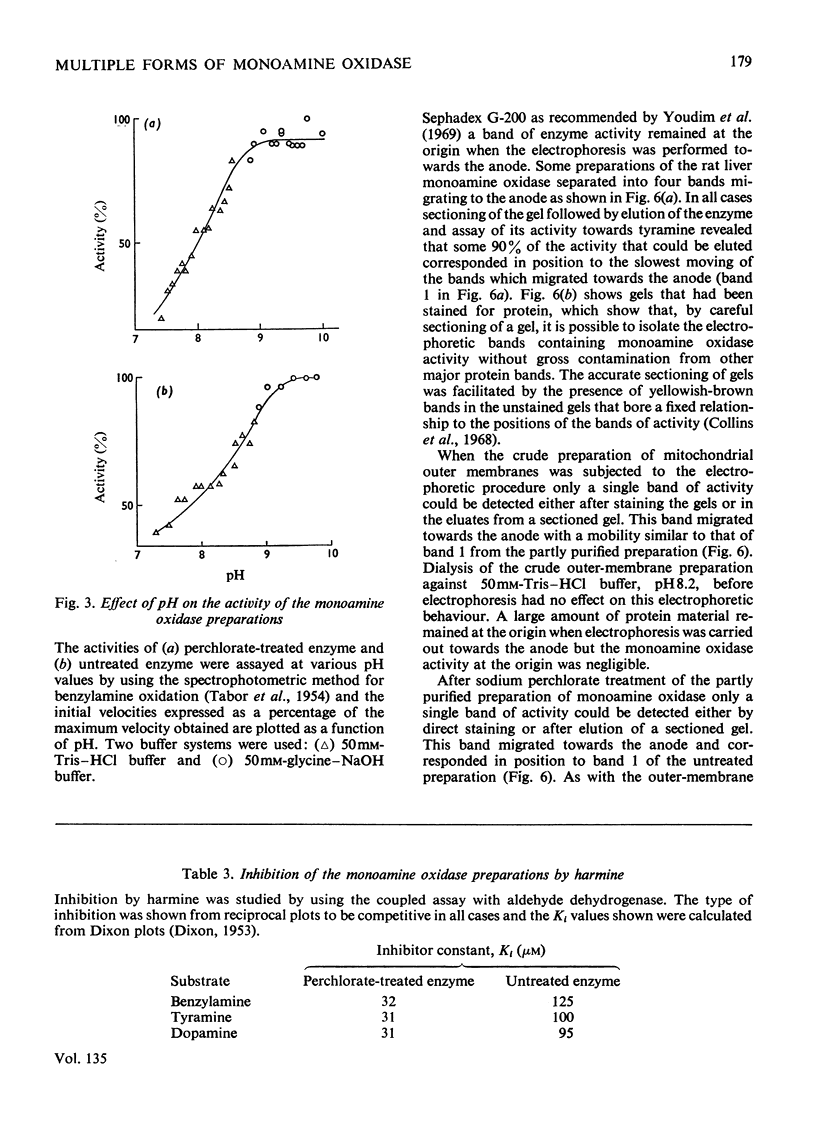
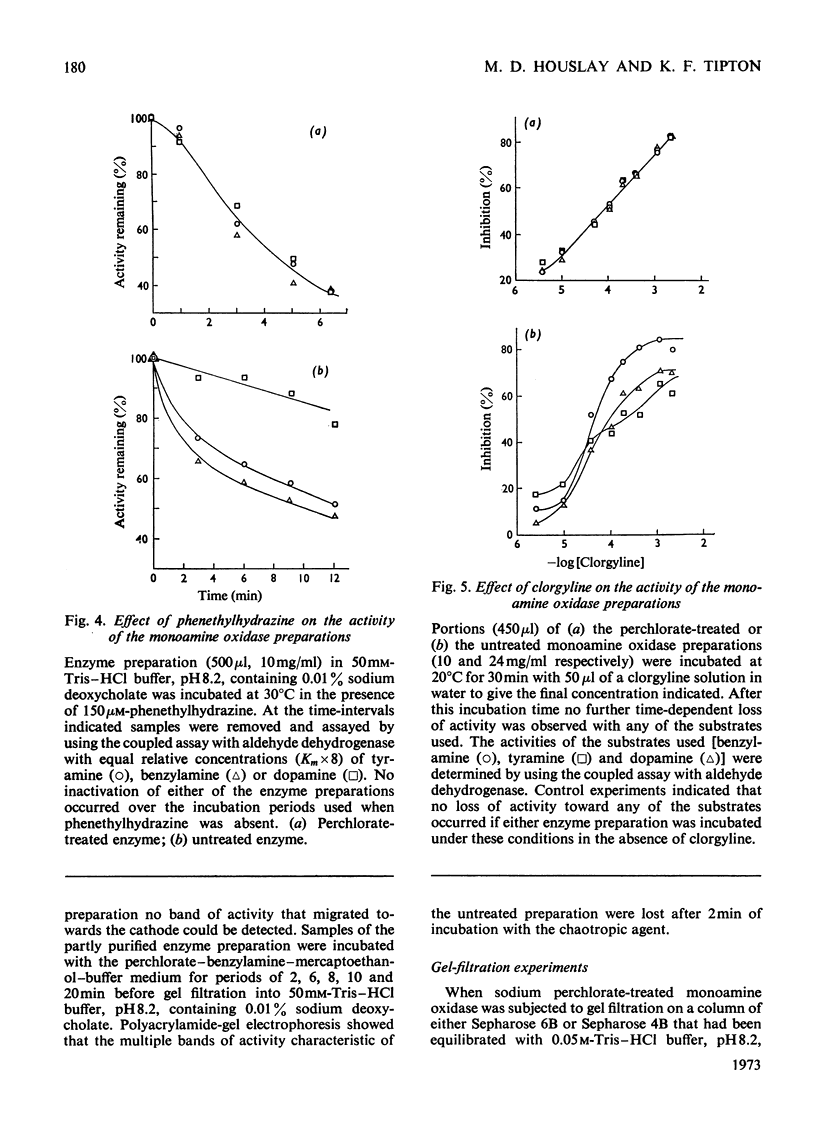
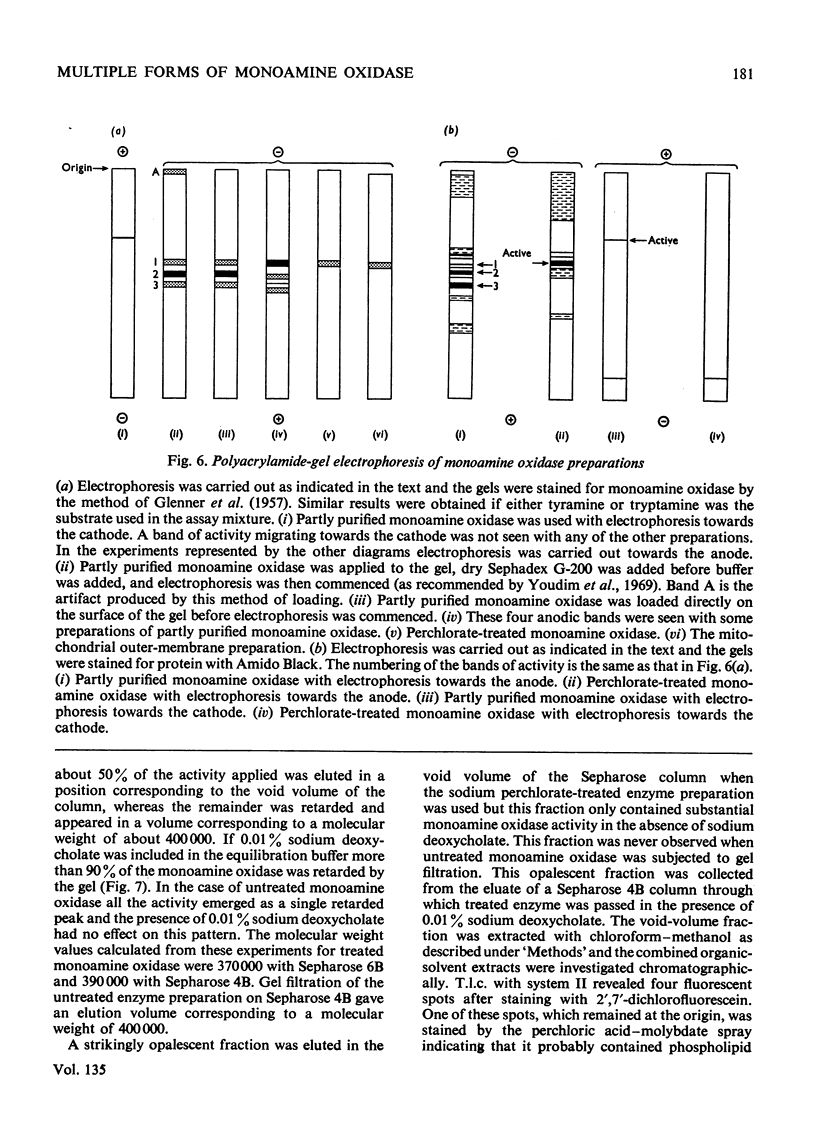
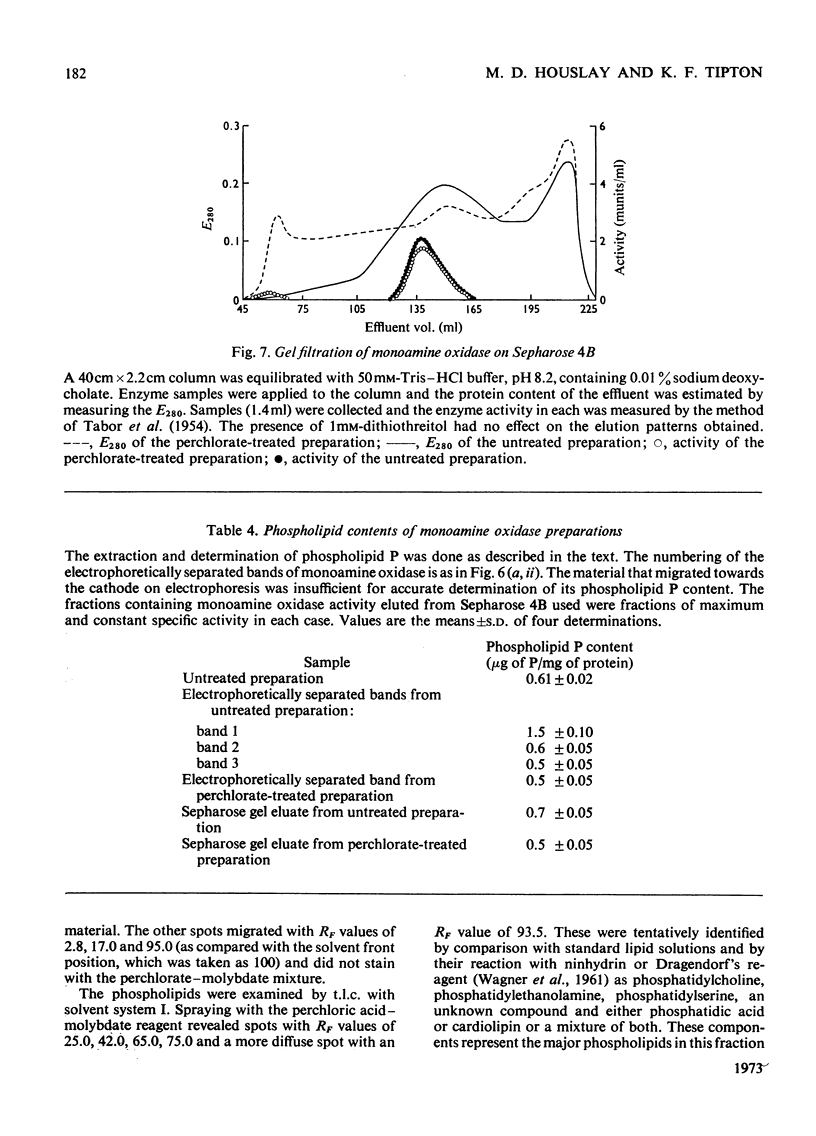
1. Treatment of a partly purified preparation of rat liver monoamine oxidase with the chaotropic agent sodium perchlorate caused the enzyme to migrate as a single band of activity of polyacrylamide-gel electrophoresis, whereas the untreated enzyme separated into a number of bands. 2. Treatment with the chaotropic agent caused no loss of enzyme activity towards benzylamine, dopamine or tyramine. 3. The activities of the untreated preparation towards different substrates were inhibited to different extents by heat treatment and by some inhibitors. No such differences could be detected after the enzyme preparation had been treated with sodium perchlorate. 4. Lipid material, which could be separated by gel filtration, was liberated from the enzyme preparation by sodium perchlorate treatment. 5. The molecular weight of the treated enzyme was found to be 380000±38000. 6. Perchlorate treatment altered the solubility of the enzyme. 7. A continuous assay method for monoamine oxidase is described.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins G. G.S., Youdim M. B.H., Sandler M. Isoenzymes of human and rat liver monoamine oxidase. FEBS Lett. 1968 Sep;1(4):215–218. doi: 10.1016/0014-5793(68)80065-1. [DOI] [PubMed] [Google Scholar]
- Collins G. G., Youdim M. B., Sandler M. Multiple forms of monoamine oxidase. Comparison of in vitro and in vivo inhibition patterns. Biochem Pharmacol. 1972 Jul 15;21(14):1995–1998. doi: 10.1016/0006-2952(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Goldblatt P. J., Basford R. E. Brain hexokinase. The preparation of inner and outer mitochondrial membranes. Biochemistry. 1969 Sep;8(9):3525–3532. doi: 10.1021/bi00837a007. [DOI] [PubMed] [Google Scholar]
- DEITRICH R. A., HELLERMAN L., WEIN J. Diphosphopyridine nucleotide-linked aldehyde dehydrogenase. I. Specificity and sigma-rho function. J Biol Chem. 1962 Feb;237:560–564. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall D. W., Klotz I. M. Protein subunits: a table (revised edition). Arch Biochem Biophys. 1972 Mar;149(1):1–14. doi: 10.1016/0003-9861(72)90293-7. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Kinetics of the resolution of complex I (reduced diphosphopyridine nucleotide-coenzyme Q reductase) of the mitochondrial electron transport system by chaotropic agents. Biochemistry. 1969 Aug;8(8):3355–3361. doi: 10.1021/bi00836a033. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Duncan R. J., Tipton K. F. The purification and properties of the NAD-linked aldehyde dehydrogenase from pig brain. Eur J Biochem. 1971 Sep 24;22(2):257–262. doi: 10.1111/j.1432-1033.1971.tb01539.x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- GLENNER G. G., BURTNER H. J., BROWN G. W., Jr The histochemical demonstration of monoamine oxidase activity by tetrazolium salts. J Histochem Cytochem. 1957 Nov;5(6):591–600. doi: 10.1177/5.6.591. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Gomes B., Igaue I., Kloepper H. G., Yasunobu K. T. Amine oxidase. XIV. Isolation and characterization of the multiple beef liver amine oxidase components. Arch Biochem Biophys. 1969 Jun;132(1):16–27. doi: 10.1016/0003-9861(69)90334-8. [DOI] [PubMed] [Google Scholar]
- Gorkin V. Z. Monoamine oxidases. Pharmacol Rev. 1966 Mar;18(1):115–120. [PubMed] [Google Scholar]
- Gorkin V. Z., Tatyanenko L. V. On the inhibition by harmine of oxidative deamination of biogenic monoamines. Life Sci. 1967 Apr 15;6(8):791–795. doi: 10.1016/0024-3205(67)90280-9. [DOI] [PubMed] [Google Scholar]
- HAWKINS J. The localization of amine oxidase in the liver cell. Biochem J. 1952 Mar;50(5):577–581. doi: 10.1042/bj0500577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. W., Logan B. W., Parsons G. H. Further studies on the inhibition of monoamine oxidase by M and B 9302 (clorgyline). I. Substrate specificity in various mammalian species. Biochem Pharmacol. 1969 Jun;18(6):1447–1454. doi: 10.1016/0006-2952(69)90258-5. [DOI] [PubMed] [Google Scholar]
- Hanstein W. G., Davis K. A., Hatefi Y. Water structure and the chaotropic properties of haloacetates. Arch Biochem Biophys. 1971 Dec;147(2):534–544. doi: 10.1016/0003-9861(71)90411-5. [DOI] [PubMed] [Google Scholar]
- Johnston J. P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968 Jul;17(7):1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- McEwen C. M., Jr, Sasaki G., Jones D. C. Human liver mitochondrial monoamine oxidase. II. Determinants of substrate and inhibitor specificities. Biochemistry. 1969 Oct;8(10):3952–3962. doi: 10.1021/bi00838a011. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Nakano G., Mizutani K., Harada M. Purification and properties of amine oxidases in brain and connective tissue (dental pulp). Adv Biochem Psychopharmacol. 1972;5:25–36. [PubMed] [Google Scholar]
- OSWALD E. O., STRITTMATTER C. F. COMPARATIVE STUDIES IN THE CHARACTERIZATION OF MONOAMINE OXIDASES. Proc Soc Exp Biol Med. 1963 Dec;114:668–673. doi: 10.3181/00379727-114-28765. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Oreland L. Reassociation of soluble monoamine oxidase with lipid-depleted mitochondria in the presence of phospholipids. Biochemistry. 1971 Jan 19;10(2):332–340. doi: 10.1021/bi00778a021. [DOI] [PubMed] [Google Scholar]
- Severina I. S., Sheremet'evskaia T. N. O substratnoi spetsifichnosti mitokhondrial'noi monoaminoksidazy pecheni krysy i sviazyvanii fermenta s substratami v ferment-substratnom komplekse. Biokhimiia. 1967 Jul-Aug;32(4):843–853. [PubMed] [Google Scholar]
- Severina I. S., Sheremet'evskaia T. N. Zavisimost' mezhdu strukturoi substrata i aktivnost'iu mitokhondrial'noi monoaminoksidazy. Biokhimiia. 1969 Jan-Feb;34(1):125–135. [PubMed] [Google Scholar]
- Shih J. H., Eiduson S. Multiple forms of monoamine oxidase in developing brain: tissue and substrate specificities. J Neurochem. 1971 Jul;18(7):1221–1227. doi: 10.1111/j.1471-4159.1971.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Sierens L., D'Iorio A. Multiple monoamine oxidases in rat liver mitochondria. Can J Biochem. 1970 Jun;48(6):659–663. doi: 10.1139/o70-105. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires R. F. Multiple forms of monoamine oxidase in intact mitochondria as characterized by selective inhibitors and thermal stability: a comparison of eight mammalian species. Adv Biochem Psychopharmacol. 1972;5:355–370. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Tipton K. F. Some properties of monoamine oxidase. Adv Biochem Psychopharmacol. 1972;5:11–24. [PubMed] [Google Scholar]
- VEREVKINA I. V., GORKIN V. Z., MITIUSHIN V. M., ELPINER Ie. O DE ISTVII UL'TRAZVUKOVYKH VOLN NA MONOAMINOKSIDAZU, SVIAZANNUIU S SUBMIKROSKOPICHESKIMI STRUKTURAMI MITOKHONDRI I. Biofizika. 1964;9:503–506. [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- WEISSBACH H., REDFIELD B. G., UDENFRIEND S. Soluble monoamine oxidase; its properties and actions on serotonin. J Biol Chem. 1957 Dec;229(2):953–963. [PubMed] [Google Scholar]
- Youdim M. B., Collins G. G., Sandler M. Multiple forms of rat brain monoamine oxidase. Nature. 1969 Aug 9;223(5206):626–628. doi: 10.1038/223626a0. [DOI] [PubMed] [Google Scholar]
- Youdim M. B. Multiple forms of monoamine oxidase and their properties. Adv Biochem Psychopharmacol. 1972;5:67–77. [PubMed] [Google Scholar]


