Abstract
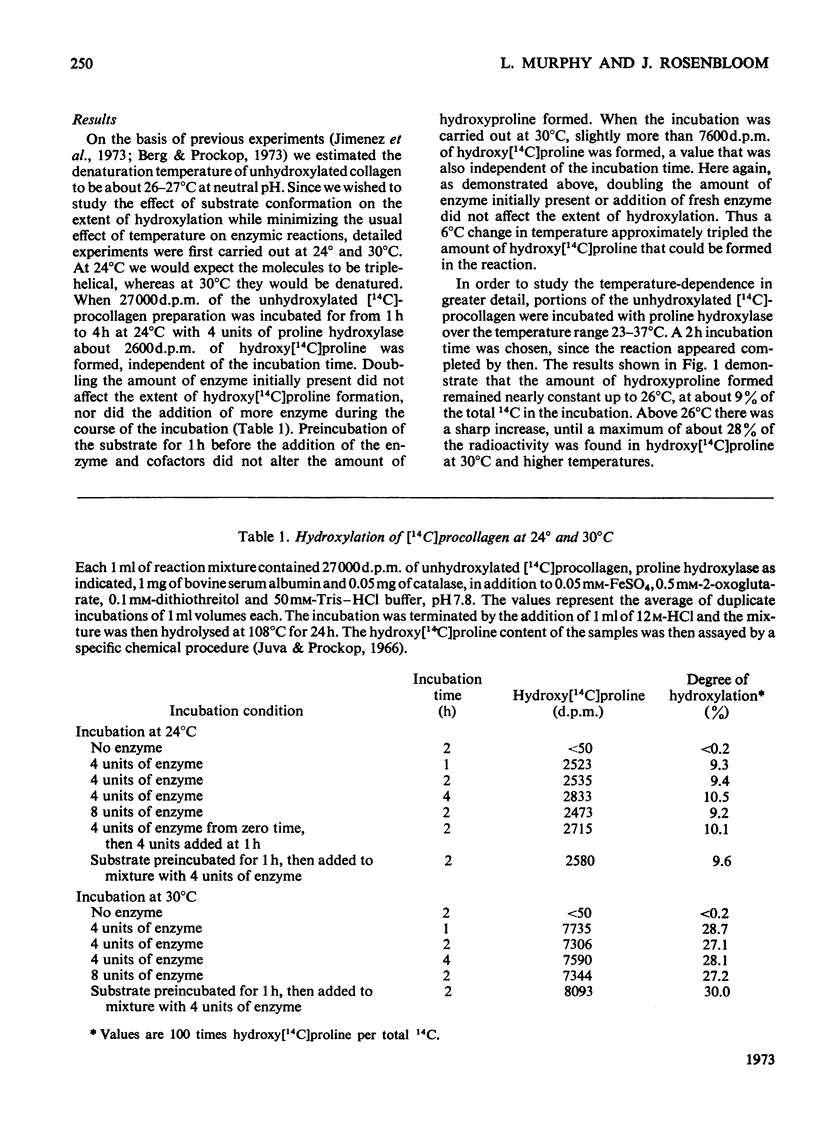
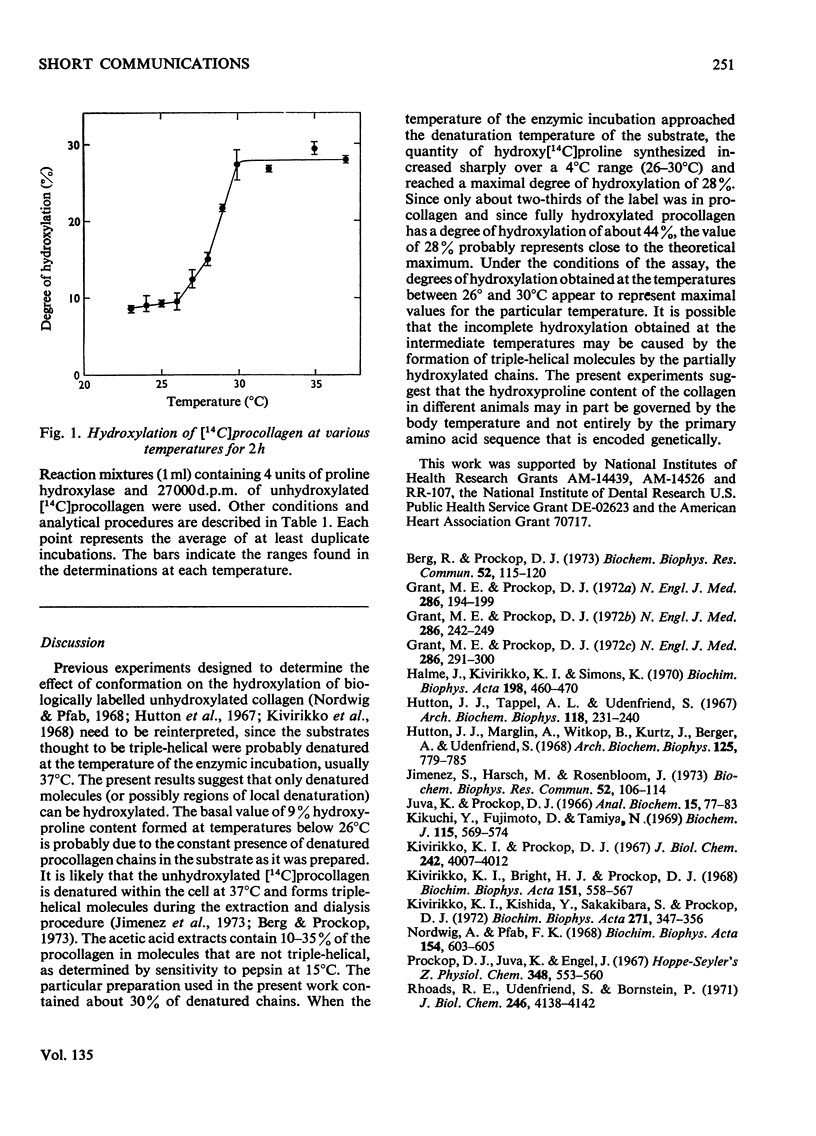
The ability of chick-embryo proline hydroxylase to hydroxylate [14C]proline-labelled procollagen was investigated between 23° and 37°C. The amount of hydroxy[14C]proline that could be formed increased sharply between 26° and 30°C. This corresponded to the temperature interval in which the [14C]procollagen substrate was thermally denatured, and the results therefore indicate that only denatured molecules can be hydroxylated.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 1. N Engl J Med. 1972 Jan 27;286(4):194–199. doi: 10.1056/NEJM197201272860406. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 2. N Engl J Med. 1972 Feb 3;286(5):242–249. doi: 10.1056/NEJM197202032860505. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 3. N Engl J Med. 1972 Feb 10;286(6):291–300. doi: 10.1056/NEJM197202102860604. [DOI] [PubMed] [Google Scholar]
- Halme J., Kivirikko K. I., Simons K. Isolation and partial characterization of highly purified protocollagen proline hydroxylase. Biochim Biophys Acta. 1970 Mar 18;198(3):460–470. doi: 10.1016/0005-2744(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Hutton J. J., Jr, Witkop B., Kurtz J., Berger A., Udenfriend S. Synthetic polypeptides as substrates and inhibitors of collagen proline hydroxylase. Arch Biochem Biophys. 1968 Jun;125(3):779–785. doi: 10.1016/0003-9861(68)90514-6. [DOI] [PubMed] [Google Scholar]
- Jimenez S., Harsch M., Rosenbloom J. Hydroxyproline stabilizes the triple helix of chick tendon collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):106–114. doi: 10.1016/0006-291x(73)90960-1. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Fujimoto D., Tamiya N. The enzymic hydroxylation of protocollagen models. Biochem J. 1969 Nov;115(3):569–574. doi: 10.1042/bj1150569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Bright H. J., Prockop D. J. Kinetic patterns of protocollagen hydroxylase and further studies on the polypeptide substrate. Biochim Biophys Acta. 1968 Mar 25;151(3):558–567. doi: 10.1016/0005-2744(68)90002-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Kishida Y., Sakakibara S., Prockop D. J. Hydroxylation of (X-Pro-Gly)n by protocollagen proline hydroxylase. Effect of chain length, helical conformation and amino acid sequence in the substrate. Biochim Biophys Acta. 1972 Jul 21;271(2):347–356. doi: 10.1016/0005-2795(72)90209-7. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Hydroxylation of proline in synthetic polypeptides with purified protocollagen hydroxylase. J Biol Chem. 1967 Sep 25;242(18):4007–4012. [PubMed] [Google Scholar]
- Nordwig A., Pfab F. K. Independence of collagen hydroxylation on the conformational state of the precursor substrate. Biochim Biophys Acta. 1968 Apr 9;154(3):603–605. doi: 10.1016/0005-2795(68)90026-3. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Juva K., Engel J. Enzymatic synthesis of hydroxyproline by the hydroxylation of poly(L-propyl-glycyl-L-prolyl). Hoppe Seylers Z Physiol Chem. 1967 May;348(5):553–560. doi: 10.1515/bchm2.1967.348.1.553. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S., Bornstein P. In vitro enzymatic hydroxylation of prolyl residues in the alpha 1-CB2 fragment of rat collagen. J Biol Chem. 1971 Jul 10;246(13):4138–4142. [PubMed] [Google Scholar]


