Abstract
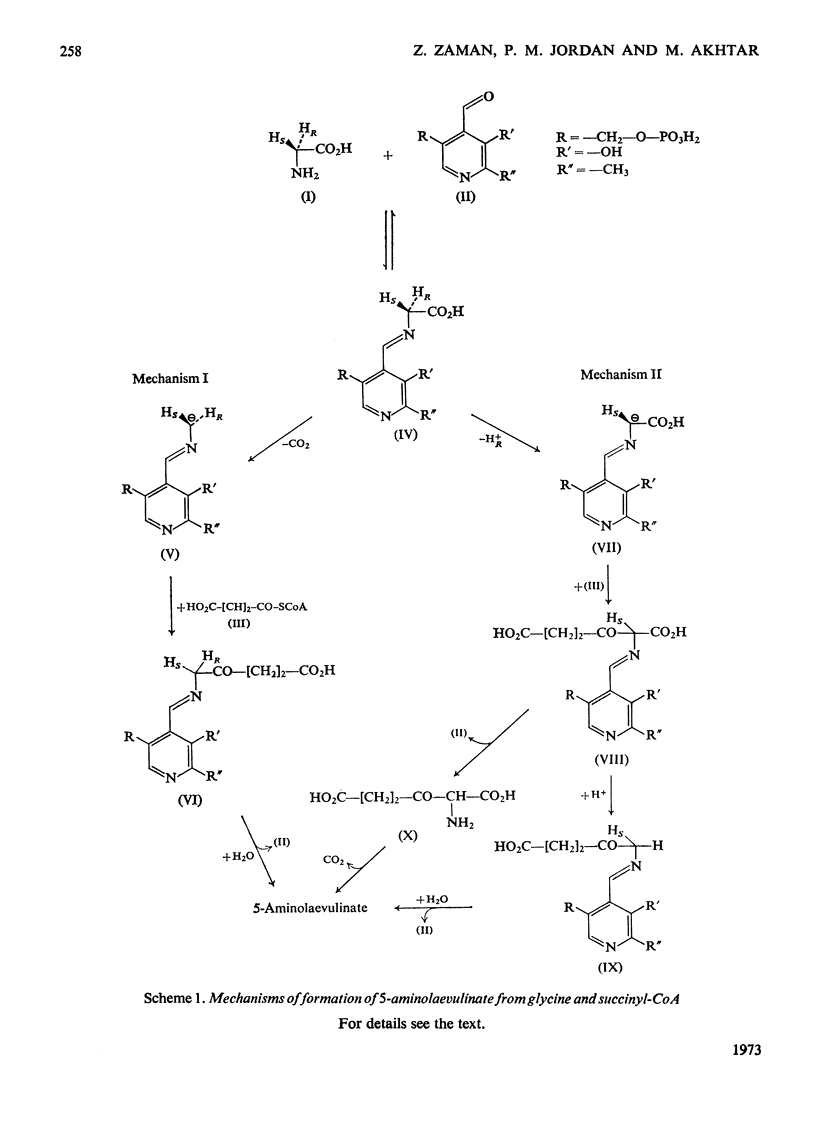
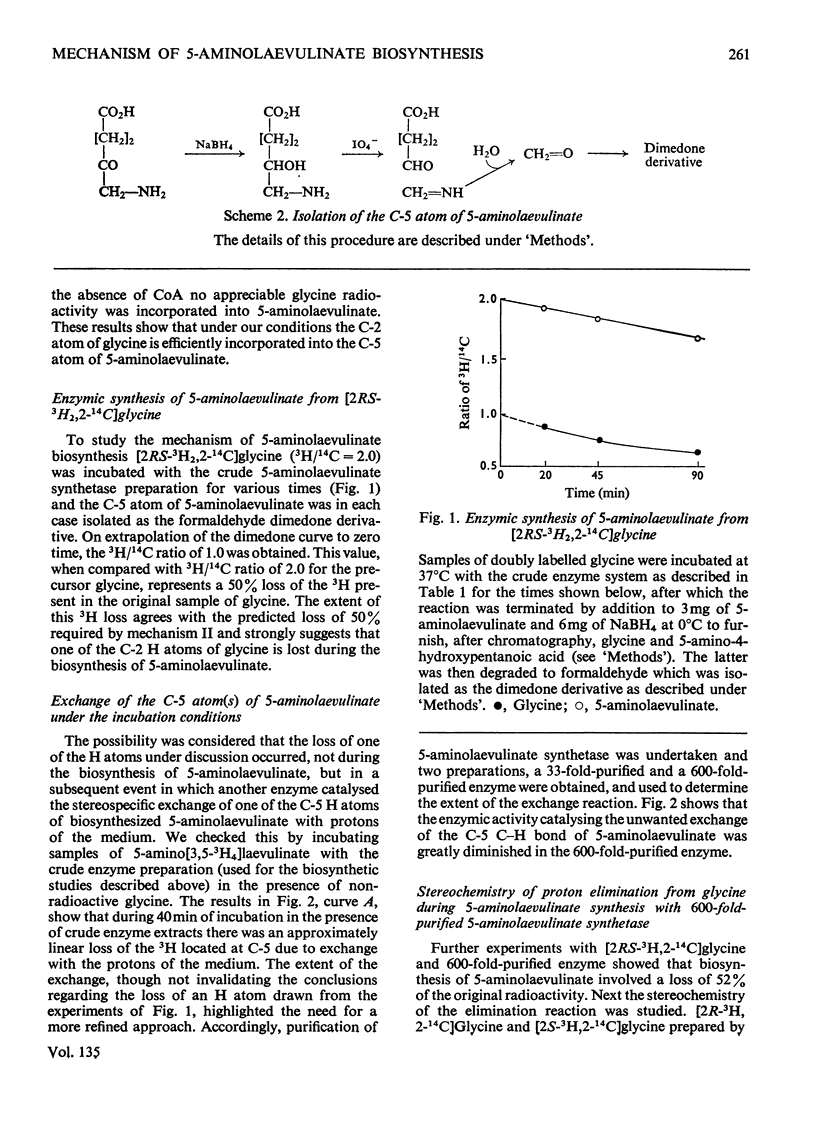
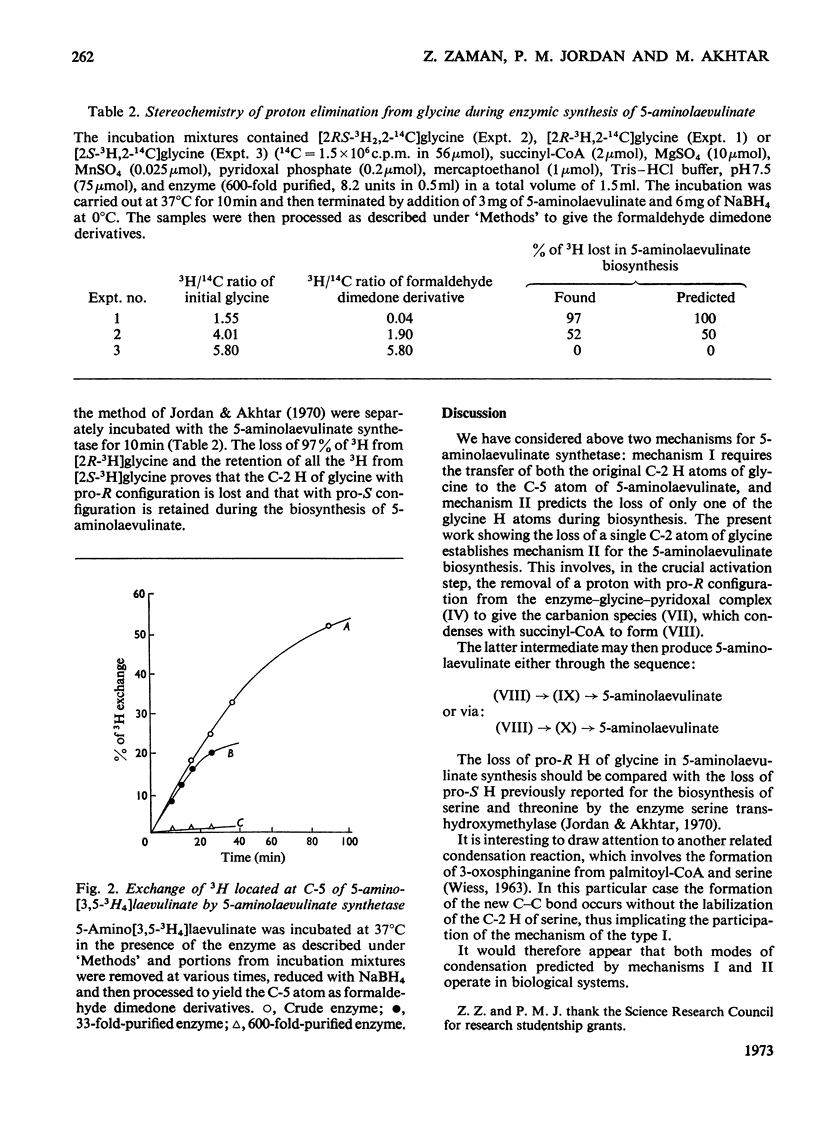
1. Two mechanisms for the biosynthesis of 5-aminolaevulinate from glycine and succinyl-CoA (3-carboxypropionyl-CoA) are considered. One of the mechanisms involves the retention of both the C-2 H atoms of glycine during the synthesis of 5-aminolaevulinate, whereas the other predicts the retention of only one of the C-2 H atoms of glycine. 2. Highly purified 5-aminolaevulinate synthetase from Rhodopseudomonas spheroides was used to show that the C-2 H atom of glycine with R configuration is specifically removed during the biosynthesis of 5-aminolaevulinate. 3. The mechanism of the condensation therefore differs from the analogous reaction of the biosynthesis of sphinganine from palmitoyl-CoA and serine, in which the C-2 H of serine is retained (Wiess, 1963).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., el-Obeid H. A. Inactivation of serine transhydroxymethylase and threonine aldolase activities. Biochim Biophys Acta. 1972 Mar 8;258(3):791–799. doi: 10.1016/0005-2744(72)90180-5. [DOI] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Akhtar M. The mechanism of action of serine transhydroxymethylase. Biochem J. 1970 Jan;116(2):277–286. doi: 10.1042/bj1160277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIKUCHI G., KUMAR A., TALMAGE P., SHEMIN D. The enzymatic synthesis of delta-aminolevulinic acid. J Biol Chem. 1958 Nov;233(5):1214–1219. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- NEUBERGER A. Aspects of the metabolism of glycine and of porphyrins. Biochem J. 1961 Jan;78:1–10. doi: 10.1042/bj0780001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuboi S., Kim H. J., Kikuchi G. Differential induction of fraction I and fraction II of delta-aminolevulinate synthetase in Rhodopseudomonas spheroides under various incubation conditions. Arch Biochem Biophys. 1970 May;138(1):155–159. doi: 10.1016/0003-9861(70)90294-8. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Kim H. J., Kikuchi G. Occurrence and properes of two types of delta-aminolevulinate synthetase in Rhodopseudomonas spheroides. Arch Biochem Biophys. 1970 May;138(1):147–154. doi: 10.1016/0003-9861(70)90293-6. [DOI] [PubMed] [Google Scholar]
- WEISS B. The biosynthesis of sphingosine. I. A study of the reaction with tritium-labeled serine. J Biol Chem. 1963 Jun;238:1953–1959. [PubMed] [Google Scholar]
- Warnick G. R., Burnham B. F. Regulation of prophyrin biosynthesis. Purification and characterization of -aminolevulinic acid synthase. J Biol Chem. 1971 Nov 25;246(22):6880–6885. [PubMed] [Google Scholar]


