Abstract
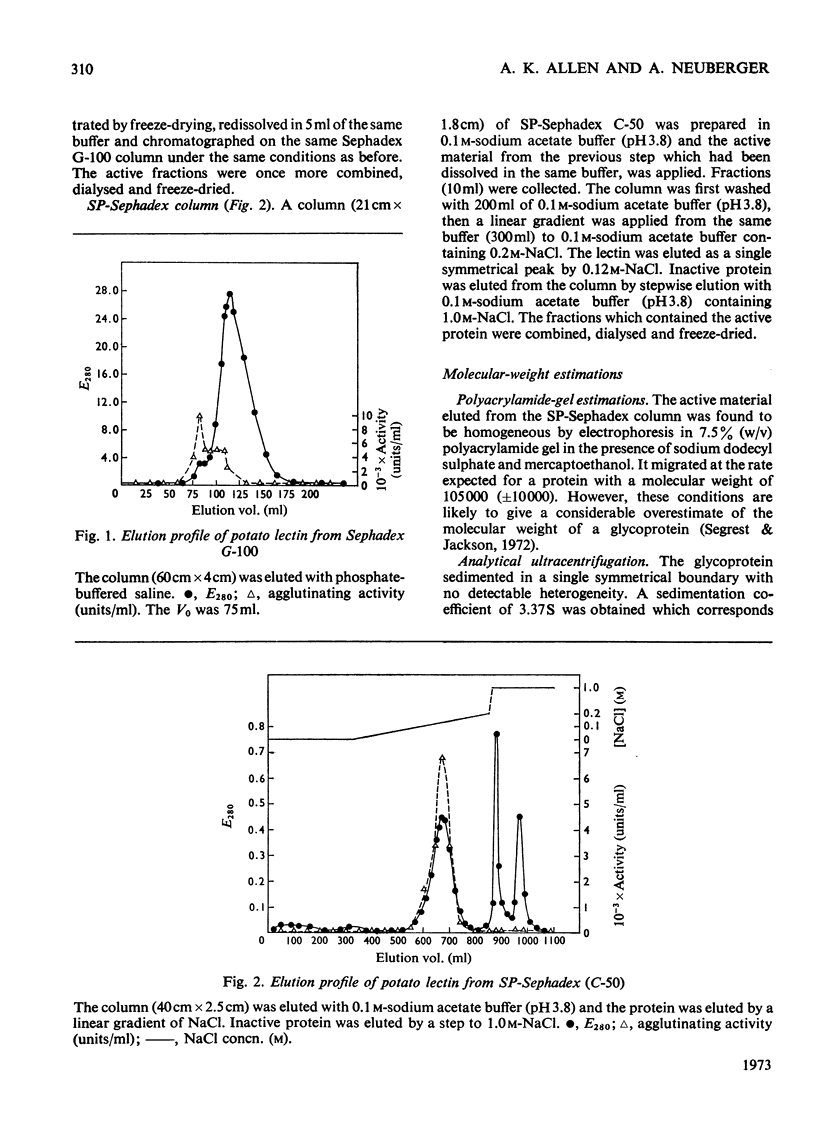
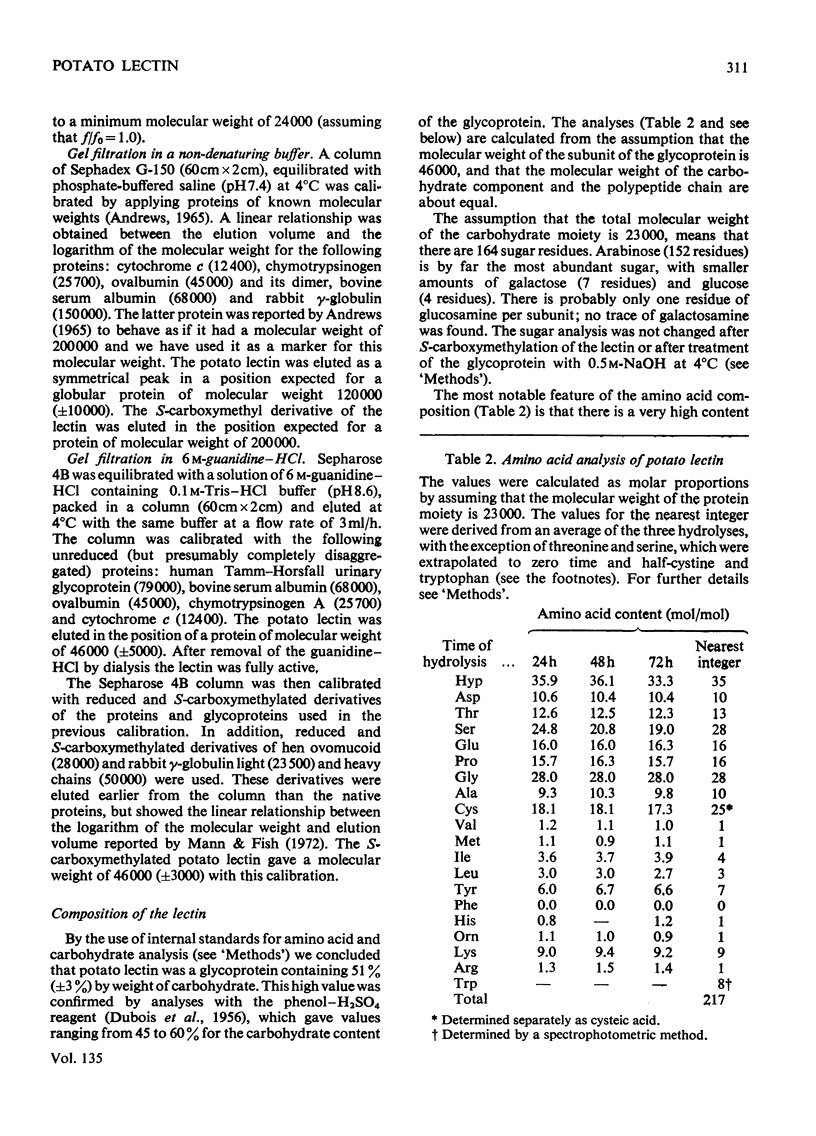
1. Potato lectin has been purified and shown to be a glycoprotein containing about 50% of carbohydrate. Most of the sugar residues (92%) are arabinose; small amounts of galactose, glucose and glucosamine are also present. 2. The most abundant amino acid is hydroxyproline (16% of the residues), 11.5% of the residues are half-cystine and phenylalanine is absent. The lectin also contains about one residue/molecule of a basic amino acid, not usually found in proteins, which has been tentatively identified as ornithine. There is indirect evidence that the components of the glycoprotein are linked through hydroxyproline and arabinose. 3. By gel filtration in 6m-guanidine–HCl on Sepharose 4B, it was found that both the native glycoprotein and its S-carboxymethylated derivative had subunit molecular weights of 46000 (±5000). In a non-denaturing solution, two of these units appear to be associated. 4. The lectin is specifically inhibited in its agglutination reaction by oligosaccharides that contain N-acetylglucosamine. Its specificity is similar to, but not identical with, that of wheat-germ agglutinin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Crumpton M. J. Solubilization of pig lymphocyte plasma membrane and fractionation of some of the components. Biochem J. 1971 Aug;123(5):967–975. doi: 10.1042/bj1230967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fletcher A. P., Neuberger A., Ratcliffe W. A. Tamm-Horsfall urinary glycoprotein. The chemical composition. Biochem J. 1970 Nov;120(2):417–424. doi: 10.1042/bj1200417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A. P., Neuberger A., Ratcliffe W. A. Tamm-Horsfall urinary glycoprotein. The subunit structure. Biochem J. 1970 Nov;120(2):425–432. doi: 10.1042/bj1200425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliard T. The enzymic deacylation of phospholipids and galactolipids in plants. Purification and properties of a lipolytic acyl-hydrolase from potato tubers. Biochem J. 1971 Feb;121(3):379–390. doi: 10.1042/bj1210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Heath M. F., Northcote D. H. Glycoprotein of the wall of sycamore tissue-culture cells. Biochem J. 1971 Dec;125(4):953–961. doi: 10.1042/bj1250953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- LeVine D., Kaplan M. J., Greenaway P. J. The purification and characterization of wheat-germ agglutinin. Biochem J. 1972 Oct;129(4):847–856. doi: 10.1042/bj1290847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H., Sela B. A., Sachs L., Sharon N. Specific inhibition by N-acetyl-D-galactosamine of the interaction between soybean agglutinin and animal cell surfaces. Biochim Biophys Acta. 1970 Sep 15;211(3):582–585. doi: 10.1016/0005-2736(70)90265-8. [DOI] [PubMed] [Google Scholar]
- MARINKOVICH V. A. PURIFICATION AND CHARACTERIZATION OF THE HEMAGGLUTININ PRESENT IN POTATOES. J Immunol. 1964 Nov;93:732–741. [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Matsumoto I., Osawa T. On the specificity of various heterologous anti-H hemagglutinins. Vox Sang. 1971 Dec;21(6):548–557. doi: 10.1111/j.1423-0410.1971.tb04814.x. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Isolation and crystallization. J Biol Chem. 1972 Apr 10;247(7):2248–2250. [PubMed] [Google Scholar]
- Pardoe G. I., Bird G. W., Uhlenbruck G. On the specificity of lectins with a broad agglutination spectrum. I. The nature of the specific receptors for Ricinus communis and Solanum tuberosum Lectins. Z Immunitatsforsch Allerg Klin Immunol. 1969 May;137(5):442–457. [PubMed] [Google Scholar]
- Poretz R. D., Goldstein I. J. An examination of the topography of the saccharide binding sites of concanavalin A and of the forces involved in complexation. Biochemistry. 1970 Jul 7;9(14):2890–2896. doi: 10.1021/bi00816a021. [DOI] [PubMed] [Google Scholar]
- Pusztai A., Watt W. B. Fractionation and characterization of glycoproteins containing hydroxyproline from the leaves of Vicia faba. Eur J Biochem. 1969 Oct;10(3):523–532. doi: 10.1111/j.1432-1033.1969.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld R. A., Börjeson J., Chessin L. N., Small P. A., Jr Isolation and characterization of a mitogen from pokeweek (Phytolacca americana). Proc Natl Acad Sci U S A. 1967 Nov;58(5):2020–2027. doi: 10.1073/pnas.58.5.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Sletten K., Aakesson I., Alvsaker J. O. Presence of ornithine in the urate-binding alpha-alpha2 globulin. Nat New Biol. 1971 May 26;231(21):118–119. doi: 10.1038/newbio231118a0. [DOI] [PubMed] [Google Scholar]
- WATKINS W. M., MORGAN W. T. Further observations on the inhibition of blood-group specific serological reactions by simple sugars of known structure. Vox Sang. 1962;7:129–150. doi: 10.1111/j.1423-0410.1962.tb03238.x. [DOI] [PubMed] [Google Scholar]


