Abstract
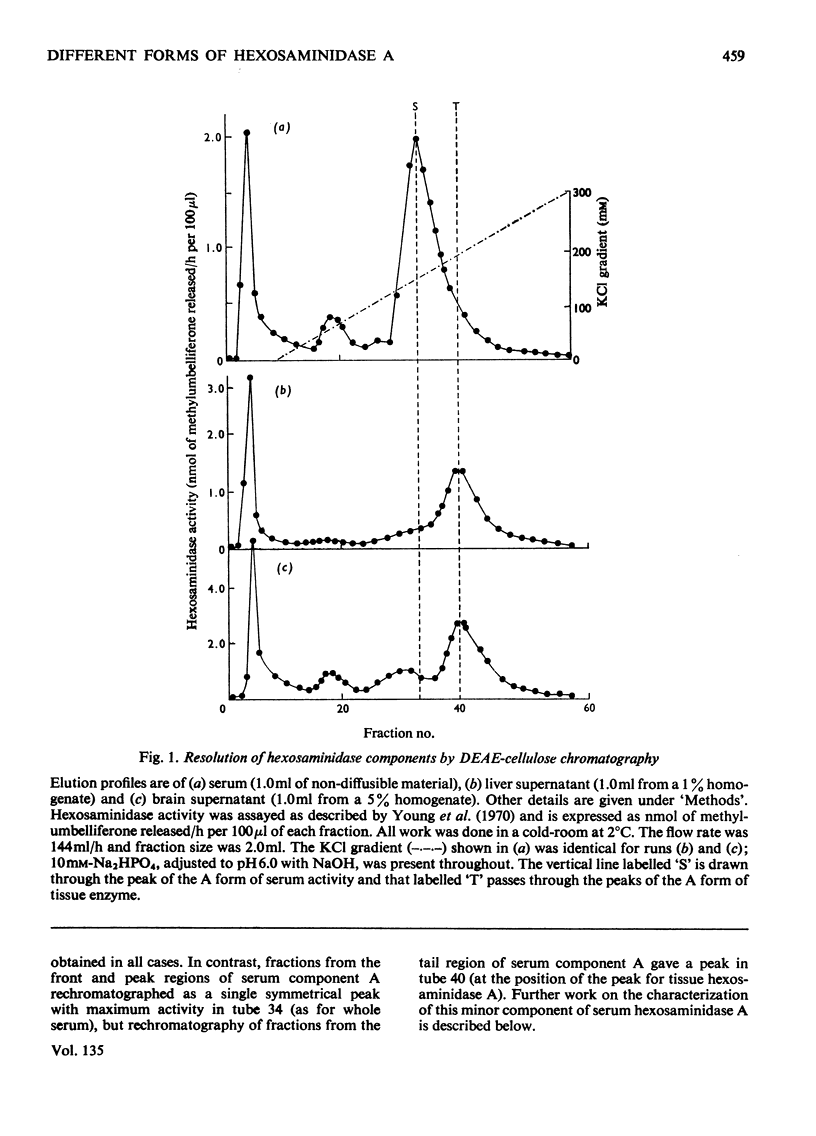
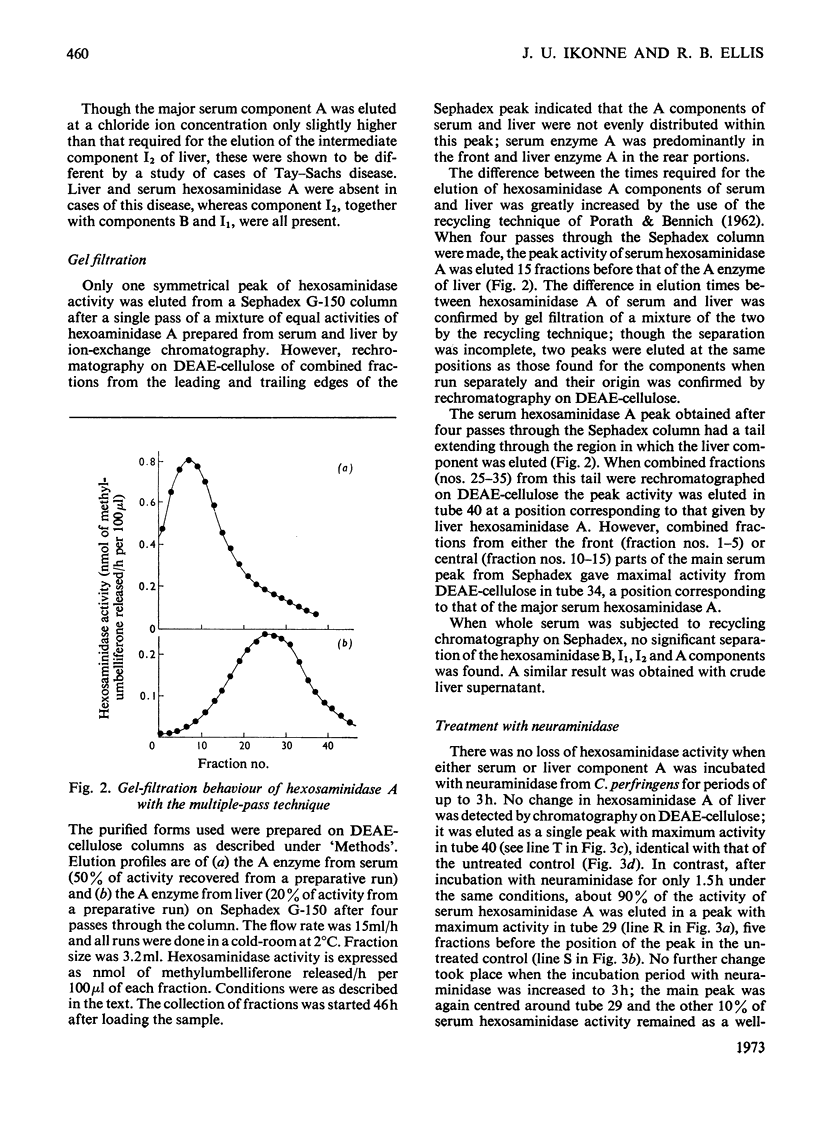
1. Hexosaminidase A of human serum was resolved into two components, a minor form with properties identical with those of the single hexosaminidase A component of human liver, and a major form with significantly different properties. 2. The major serum hexosaminidase A form was eluted from a DEAE-cellulose column at a lower salt concentration than that required to elute the liver form. 3. A multiple-pass technique was used to elute the major serum enzyme A from a Sephadex G-150 column before that of liver enzyme A. 4. Clostridium perfringens neuraminidase converted the major component of serum hexosaminidase A into a form that was held less tightly by DEAE-cellulose, but the minor component of the A enzyme of serum, and the A enzyme of liver were not affected. 5. The hexosaminidase A from tears was similar to the A enzyme from serum, whereas those from several human tissues and from urine and lymph were similar to the liver form. 6. The A enzyme from serum may be derived from the A enzyme from liver by glycosylation before secretion.
Full text
PDF

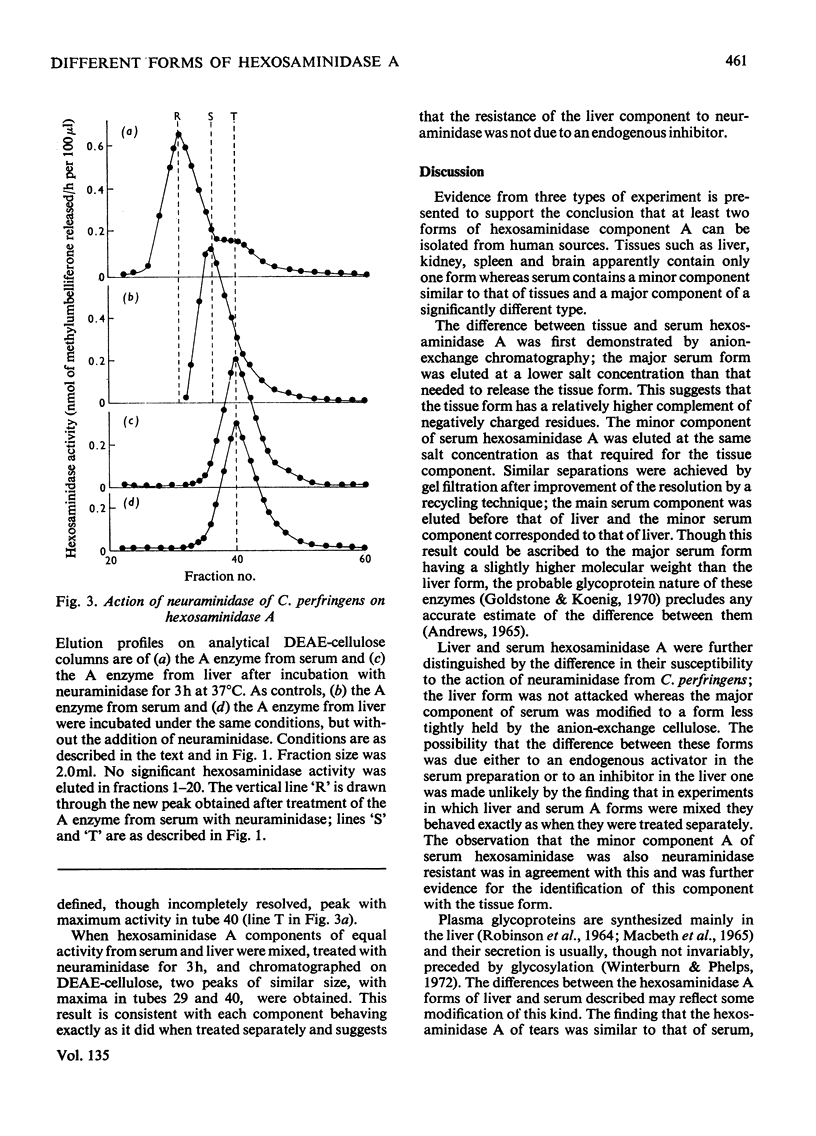

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M., Robinson D. Immunological properties of N-acetyl-beta-D-glucosaminidase of normal human liver and of GM2-gangliosidosis liver. Biochem J. 1973 Jan;131(1):91–96. doi: 10.1042/bj1310091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Lysosomal hydrolases as glycoproteins. Life Sci II. 1970 Dec 8;9(23):1341–1350. doi: 10.1016/0024-3205(70)90115-3. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Konecny P., Koenig H. Lysosomal hydrolases: Conversion of acidic to basic forms by neuraminidase. FEBS Lett. 1971 Feb 12;13(1):68–72. doi: 10.1016/0014-5793(71)80667-1. [DOI] [PubMed] [Google Scholar]
- Hultberg B. N-acetylhexosaminidase activities in Tay-Sachs disease. Lancet. 1969 Nov 29;2(7631):1195–1195. doi: 10.1016/s0140-6736(69)92520-3. [DOI] [PubMed] [Google Scholar]
- Kampine J. P., Brady R. O., Kanfer J. N., Feld M., Shapiro D. Diagnosis of gaucher's disease and niemann-pick disease with small samples of venous blood. Science. 1967 Jan 6;155(3758):86–88. doi: 10.1126/science.155.3758.86. [DOI] [PubMed] [Google Scholar]
- Macbeth R. A., Bekesi J. G., Sugden E., Bice S. The metabolism of plasma glycoproteins. I. Studies on the rate of incorporation of glucosamine-1-14C into protein-bound hexosamine and N-acetylneuraminic acid in the normal rat. J Biol Chem. 1965 Oct;240(10):3707–3713. [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- PORATH J., BENNICH H. Recycling chromatography. Arch Biochem Biophys. 1962 Sep;Suppl 1:152–156. [PubMed] [Google Scholar]
- Price R. G., Dance N. The demonstration of multiple heat stable forms of N-acetyl- -glucosaminidase in normal human serum. Biochim Biophys Acta. 1972 Jun 22;271(1):145–153. doi: 10.1016/0005-2795(72)90142-0. [DOI] [PubMed] [Google Scholar]
- ROBINSON G. B., MOLNAR J., WINZLER R. J. BIOSYNTHESIS OF GLYCOPROTEINS. I. INCORPORATION OF GLUCOSAMINE-14C INTO LIVER AND PLASMA PROTEINS OF THE RAT. J Biol Chem. 1964 Apr;239:1134–1141. [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K. Auftrennung der Säuger-N-Acetyl-beta-D-hexosaminidase in multiple Formen durch Elektrofokusserung. Hoppe Seylers Z Physiol Chem. 1968 Sep;349(9):1095–1098. [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. The significance of glycosylated proteins. Nature. 1972 Mar 24;236(5343):147–151. doi: 10.1038/236147a0. [DOI] [PubMed] [Google Scholar]
- Young E. P., Ellis R. B., Lake B. D., Patrick A. D. Tay-sachs disease and related disorders: Fractionation of brain N-acetyl-beta-hexosaminidase on DEAE-cellulose. FEBS Lett. 1970 Jul 15;9(1):1–4. doi: 10.1016/0014-5793(70)80295-2. [DOI] [PubMed] [Google Scholar]


