Abstract
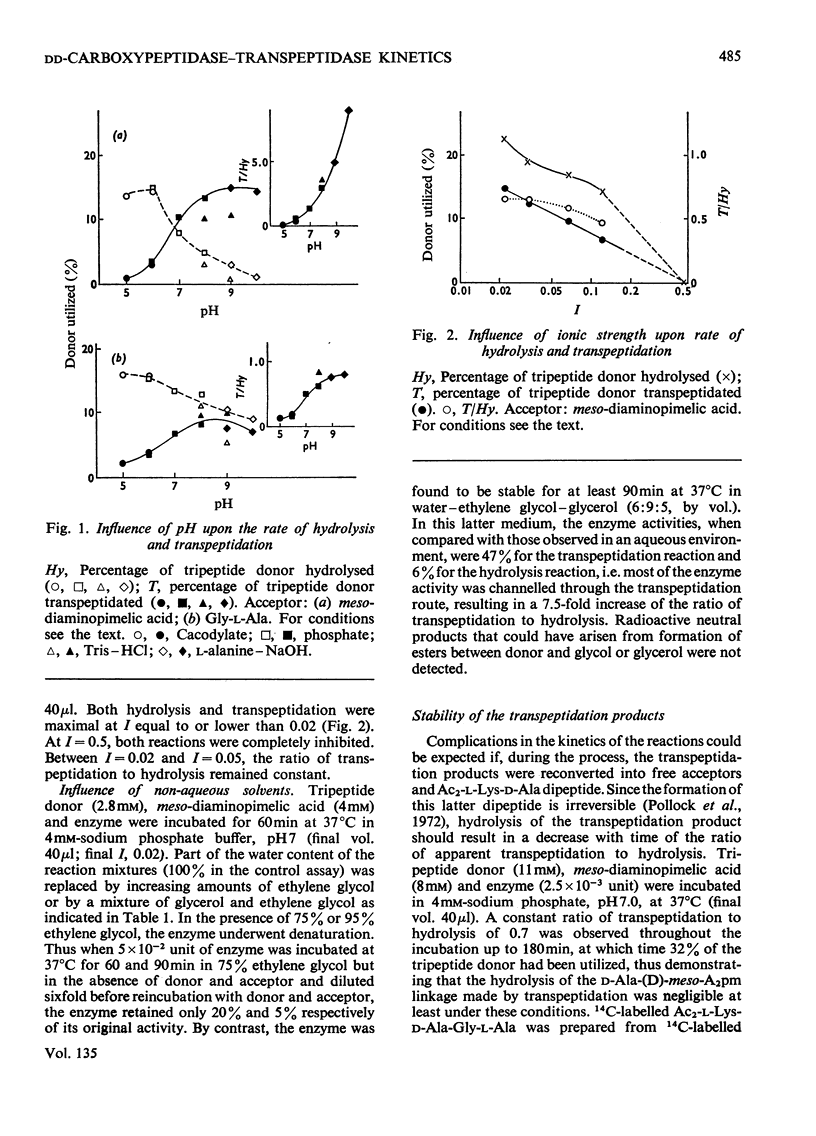
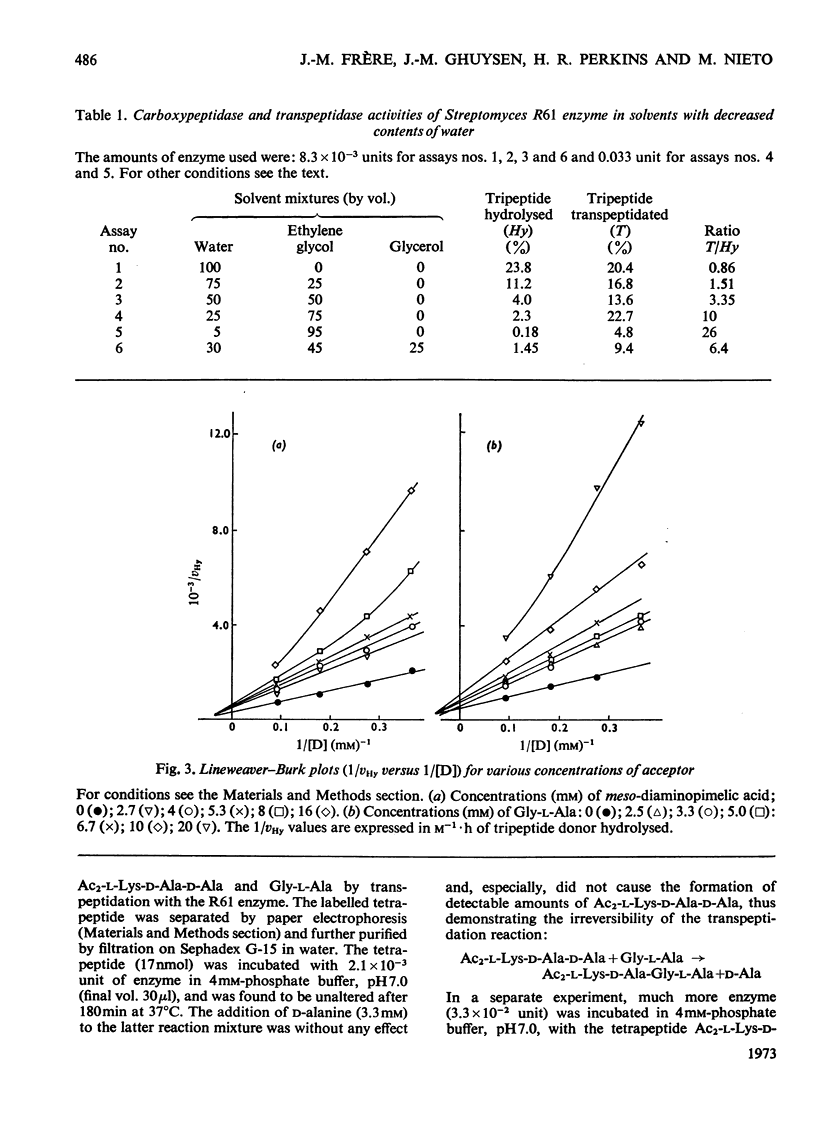
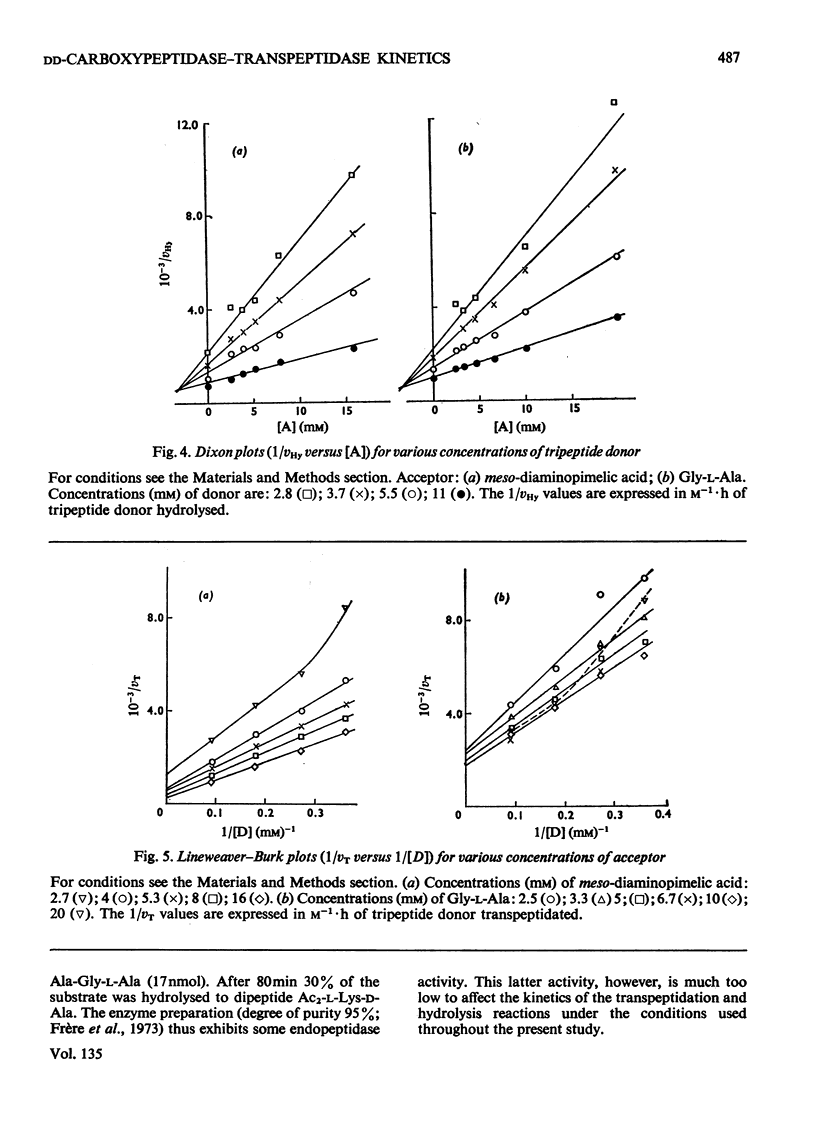
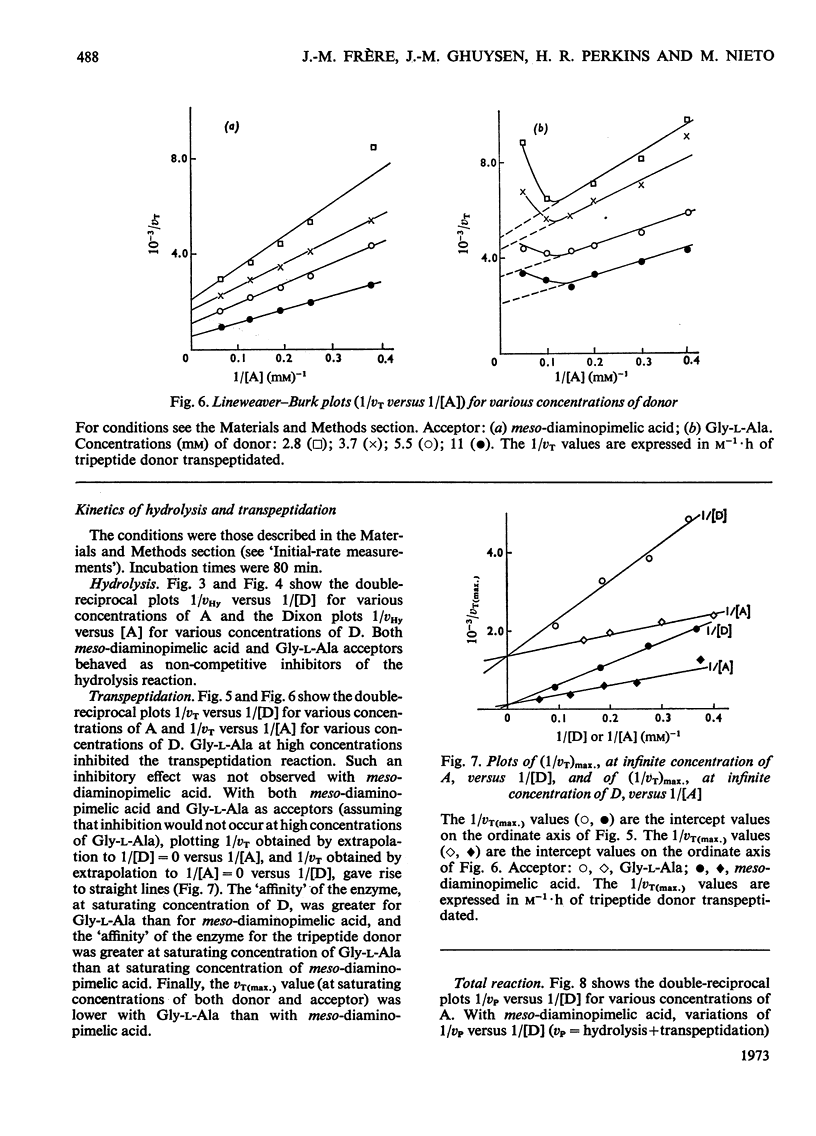
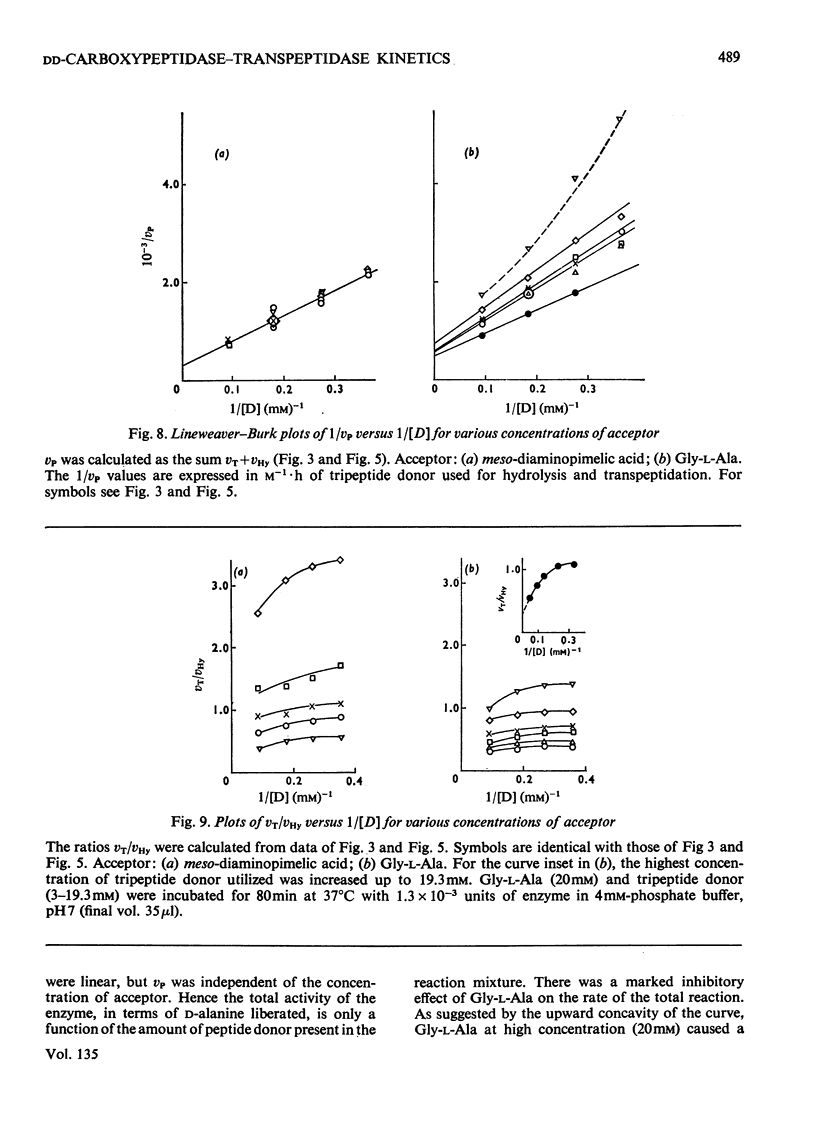
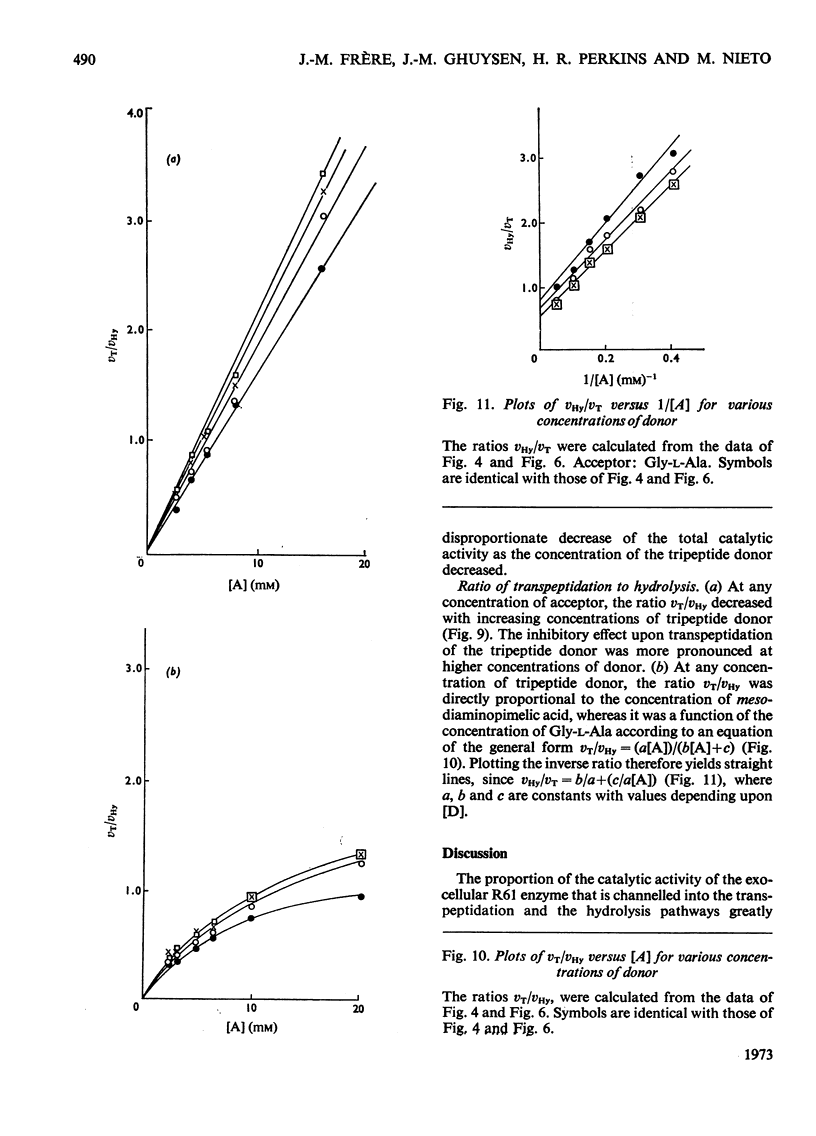
When Ac2-l-Lys-d-Ala-d-Ala and either meso-diaminopimelic acid or Gly-l-Ala are exposed to the exocellular dd-carboxypeptidase–transpeptidase of Streptomyces R61, transpeptidation reactions yielding Ac2-l-Lys-d-Ala-(d)-meso- diaminopimelic acid and Ac2-l-Lys-d-Ala-Gly-l-Ala occur concomitantly with the hydrolysis of the tripeptide into Ac2-l-Lys-d-Ala. The proportion of the enzyme activity which can be channelled in the transpeptidation and the hydrolysis pathways depends upon the pH and the polarity of the environment. Transpeptidation is favoured both by increasing the pH and by decreasing the water content of the reaction mixtures. Kinetics suggest that the reactions proceed through an ordered mechanism in which the acceptor molecule (meso-diaminopimelic acid or Gly-l-Ala) binds first to the enzyme. Both acceptors behave as non-competitive inhibitors of the hydrolysis pathway. Transpeptidation is inhibited by high concentrations of Gly-l-Ala but not by high concentrations of meso-diaminopimelic acid. The occurrence on the enzyme of an additional inhibitory binding site for Gly-l-Ala is suggested.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dusart J., Marquet A., Ghuysen J. M., Frère J. M., Moreno R., Leyh-Bouille M., Johnson K., Lucchi C., Perkins H. R., Nieto M. DD-carboxypeptidase-transpeptidase and killing site of beta-lactam antibiotics in Streptomyces strains R39, R61, and K11. Antimicrob Agents Chemother. 1973 Feb;3(2):181–187. doi: 10.1128/aac.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M. Enzymic mechanisms involving concomitant transfer and hydrolysis reactions. Biochem J. 1973 Nov;135(3):469–481. doi: 10.1042/bj1350469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Perkins H. R., Nieto M. Molecular weight and amino acid composition of the exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R61. Biochem J. 1973 Nov;135(3):463–468. doi: 10.1042/bj1350463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Leyh-Bouille M., Campbell J. N., Moreno R., Frére J. M., Duez C., Nieto M., Perkins H. R. Structure of the wall peptidoglycan of Streptomyces R39 and the specificity profile of its exocellular DD-carboxypeptidase--transpeptidase for peptide acceptors. Biochemistry. 1973 Mar 27;12(7):1243–1251. doi: 10.1021/bi00731a001. [DOI] [PubMed] [Google Scholar]
- Leyh-Bouille M., Coyette J., Ghuysen J. M., Idczak J., Perkins H. R., Nieto M. Penicillin-sensitive DD-carboxypeptidase from Streptomyces strain R 61. Biochemistry. 1971 May 25;10(11):2163–2170. doi: 10.1021/bi00787a032. [DOI] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R., Frère J. M., Ghuysen J. M. Fluorescence and circular dichroism studies on the Streptomyces R61 DD-carboxypeptidase-transpeptidase. Penicillin binding by the enzyme. Biochem J. 1973 Nov;135(3):493–505. doi: 10.1042/bj1350493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R., Leyh-Bouille M., Frère J. M., Ghuysen J. M. Peptide inhibitors of Streptomyces DD-carboxypeptidases. Biochem J. 1973 Jan;131(1):163–171. doi: 10.1042/bj1310163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M., Frére J. M., Leyh-Bouille M., Ghuysen J. M. Streptomyces DD-carboxypeptidases as transpeptidases. The specificity for amino compounds acting as carboxyl acceptors. Biochem J. 1973 Apr;131(4):707–718. doi: 10.1042/bj1310707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. J., Ghuysen J. M., Linder R., Salton M. R., Perkins H. R., Nieto M., Leyh-Bouille M., Frere J. M., Johnson K. Transpeptidase activity of Streptomyces D-alanyl-D carboxypeptidases. Proc Natl Acad Sci U S A. 1972 Mar;69(3):662–666. doi: 10.1073/pnas.69.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]


