Abstract
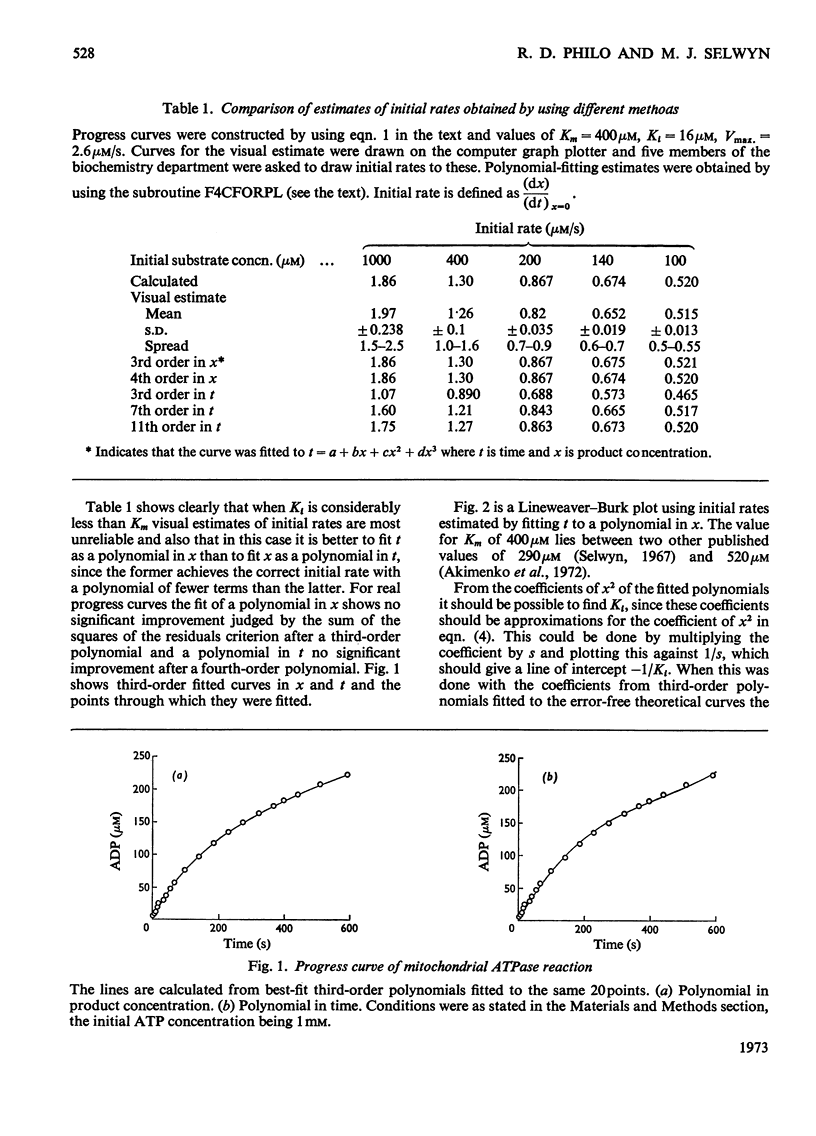
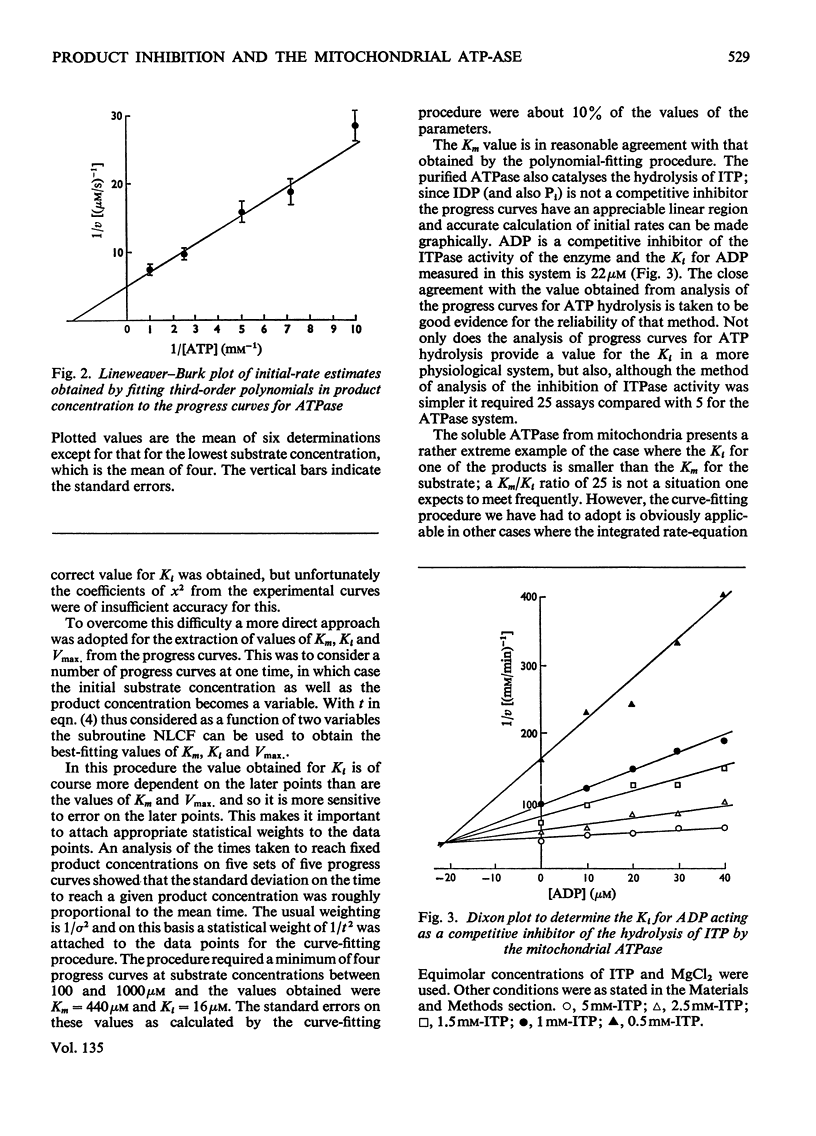
1. Several methods of analysing progress curves of enzyme-catalysed reactions are discussed briefly in relation to their usefulness in a situation where a reaction product has a Ki much lower than the Km for the substrate 2. A comparison is made of different methods of estimating initial rates in this situation. 3. The use of a computer curve-fitting routine capable of handling functions of more than one variable for the extraction of kinetic parameters from progress curves is described. 4. This method and that of fitting time as a polynomial in product concentration are applied to progress curves for the soluble mitochondrial adenosine triphosphatase and the results are compared with values obtained by more conventional methods.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimenko V. K., Minkov I. B., Bakeeva L. E., Vinogradov A. D. Vydelenie i svoistva rastvorimoi ATFazy mitokhondrii serdtsa byka. Biokhimiia. 1972 Mar-Apr;37(2):348–359. [PubMed] [Google Scholar]
- Foster R. J., Niemann C. The Evaluation of the Kinetic Constants of Enzyme Catalyzed Reactions. Proc Natl Acad Sci U S A. 1953 Oct;39(10):999–1003. doi: 10.1073/pnas.39.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesov A. A., Berezin I. V. Primenie integral'noi formy uravneniia skorosti dlia opredeleniia kineticheskikh konstant fermentativnykh reaktsii. Biokhimiia. 1972 Jan-Feb;37(1):170–183. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Schwert G. W. Use of integrated rate equations in estimating the kinetic constants of enzyme-catalyzed reactions. J Biol Chem. 1969 Mar 10;244(5):1278–1284. [PubMed] [Google Scholar]
- Selwyn M. J. A simple test for inactivation of an enzyme during assay. Biochim Biophys Acta. 1965 Jul 29;105(1):193–195. doi: 10.1016/s0926-6593(65)80190-4. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J. Preparation and general properties of a soluble adenosine triphosphatase from mitochondria. Biochem J. 1967 Oct;105(1):279–288. doi: 10.1042/bj1050279. [DOI] [PMC free article] [PubMed] [Google Scholar]


