Abstract
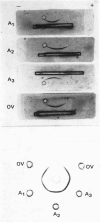
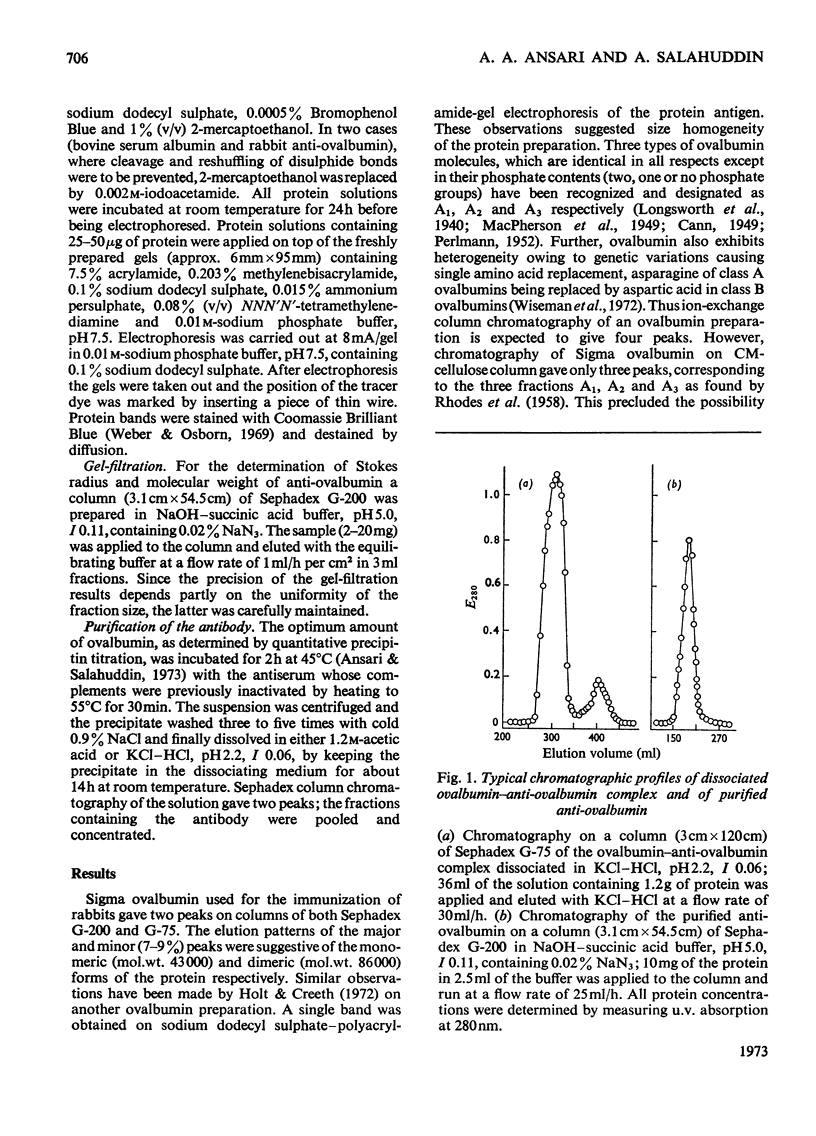
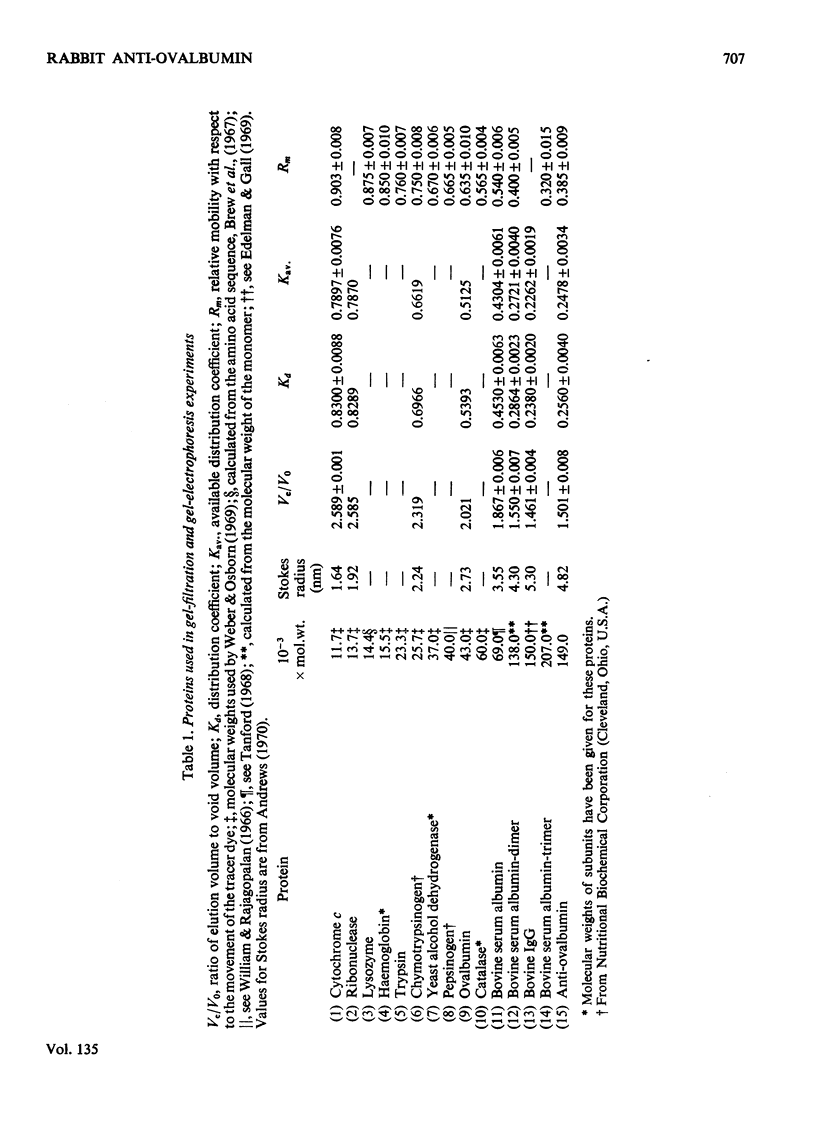
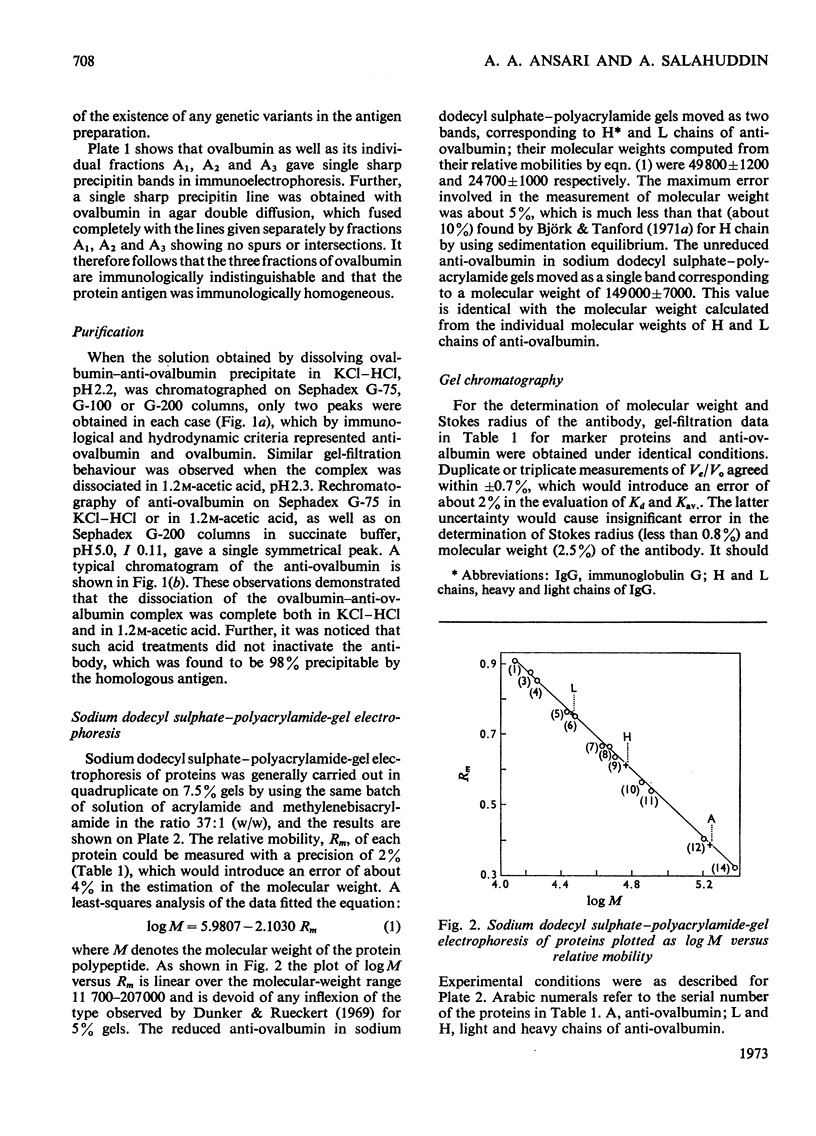
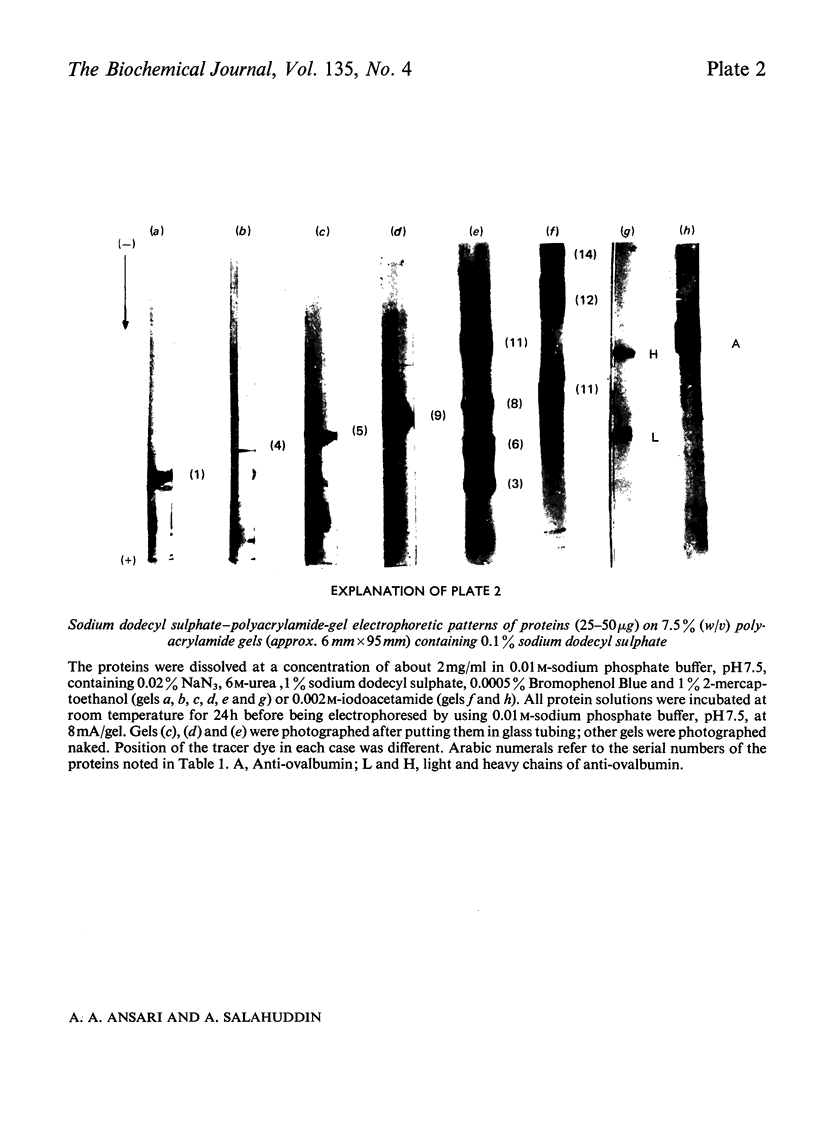
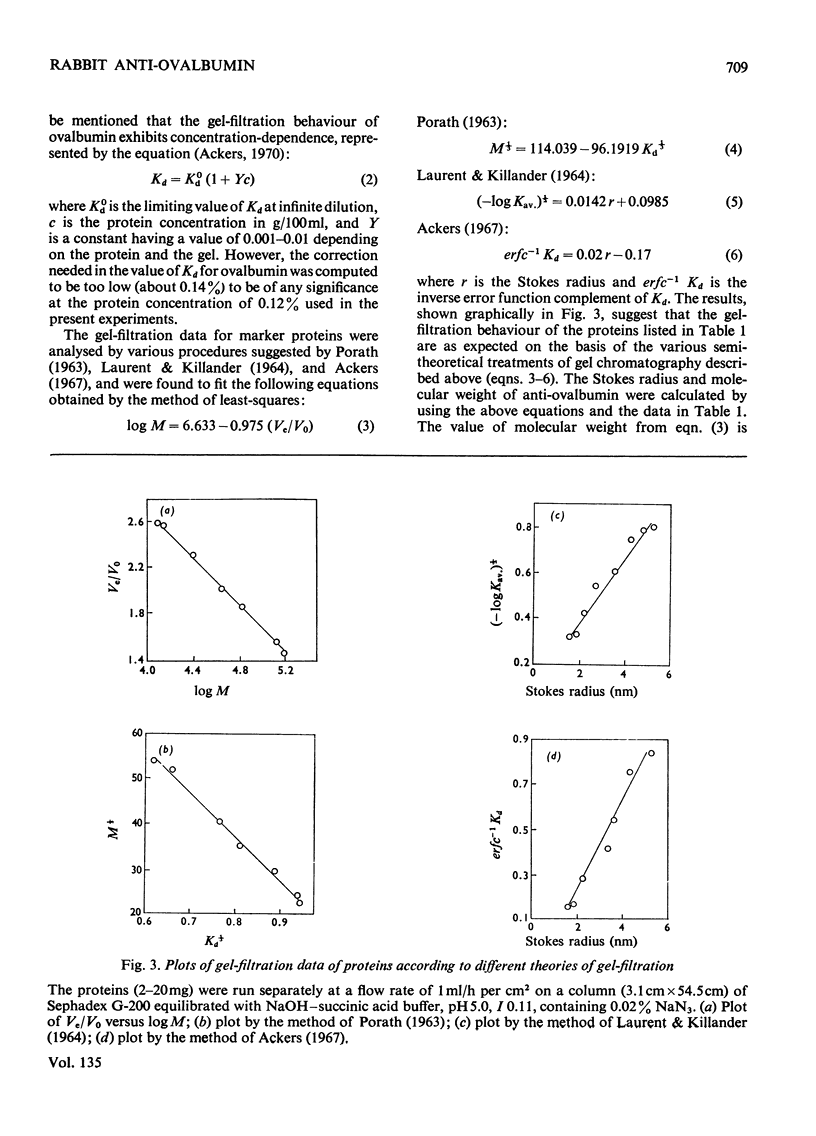
Unlike previous reports that the ovalbumin–anti-ovalbumin complex did not dissociate completely in acid media, our results showed complete dissociation of the complex both in 1.2m-acetic acid, pH2.3, and in KCl–HCl, pH2.2, I 0.06. Thus Sephadex chromatography of the solution obtained by dissolving the antigen–antibody precipitate in these media repeatedly gave two peaks corresponding to anti-ovalbumin and ovalbumin. Further, gel-diffusion and immunoelectrophoresis experiments showed that the phosphate groups of ovalbumin are not involved in the antigenic sites. The antibody thus purified was more easily precipitated than previous preparations. The molecular weight and Stokes radius of the antibody were calculated from its gel-filtration behaviour and were found to be 148000 and 4.8nm respectively. The molecular weight determined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis was essentially similar (about 0.7% lower).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K. Analytical gel chromatography of proteins. Adv Protein Chem. 1970;24:343–446. doi: 10.1016/s0065-3233(08)60245-4. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Björk I., Tanford C. Gross conformation of free polypeptide chains from rabbit immunoglobulin G. I. Heavy chain. Biochemistry. 1971 Apr 13;10(8):1271–1280. doi: 10.1021/bi00784a001. [DOI] [PubMed] [Google Scholar]
- Björk I., Tanford C. Gross conformation of free polypeptide chains from rabbit immunoglobulin G. II. Light chain. Biochemistry. 1971 Apr 13;10(8):1280–1288. doi: 10.1021/bi00784a002. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. Comparison of the amino acid sequence of bovine alpha-lactalbumin and hens egg white lysozyme. J Biol Chem. 1967 Aug 25;242(16):3747–3749. [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Edelman G. M., Gall W. E. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- GIVOL D., FUCHS S., SELA M. Isolation of antibodies to antigens of low molecular weight. Biochim Biophys Acta. 1962 Sep 10;63:222–224. doi: 10.1016/0006-3002(62)90362-1. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Creeth J. M. Studies of the denaturation and partial renaturation of ovalbumin. Biochem J. 1972 Sep;129(3):665–676. doi: 10.1042/bj1290665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Dandliker W. B. Purification of antiovalbumin from specific precipitates by gel filtration. Immunochemistry. 1968 Jan;5(1):75–77. doi: 10.1016/0019-2791(68)90226-7. [DOI] [PubMed] [Google Scholar]
- Kávai M., Jusupova S., Csaba B. Isolation of antibodies by gel-filtration. Acta Microbiol Acad Sci Hung. 1966;13(3):215–221. [PubMed] [Google Scholar]
- Lamm M. E., Small P. A., Jr Polypeptide chain structure of rabbit immunoglobulins. II. gamma-M-immunoglobulin. Biochemistry. 1966 Jan;5(1):267–276. doi: 10.1021/bi00865a035. [DOI] [PubMed] [Google Scholar]
- MARLER E., NELSON C. A., TANFORD C. THE POLYPEPTIDE CHAINS OF RABBIT GAMMA-GLOBULIN AND ITS PAPAIN-CLEAVED FRAGMENTS. Biochemistry. 1964 Feb;3:279–284. doi: 10.1021/bi00890a024. [DOI] [PubMed] [Google Scholar]
- PERLMANN G. E. Enzymatic dephosphorylation of ovalbumin and plakalbumin. J Gen Physiol. 1952 May;35(5):711–726. doi: 10.1085/jgp.35.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES M. B., AZARI P. R., FEENEY R. E. Analysis, fractionation, and purification of egg white proteins with cellulose-cation exchanger. J Biol Chem. 1958 Jan;230(1):399–408. [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams R. C., Jr, Rajagopalan T. G. Ultracentrifugal characterization of pepsin and pepsinogen. J Biol Chem. 1966 Nov 10;241(21):4951–4954. [PubMed] [Google Scholar]
- Wiseman R. L., Fothergill J. E., Fothergill L. A. Replacement of asparagine by aspartic acid in hen ovalbumin and a difference in immunochemical reactivity. Biochem J. 1972 May;127(5):775–780. doi: 10.1042/bj1270775. [DOI] [PMC free article] [PubMed] [Google Scholar]