Abstract
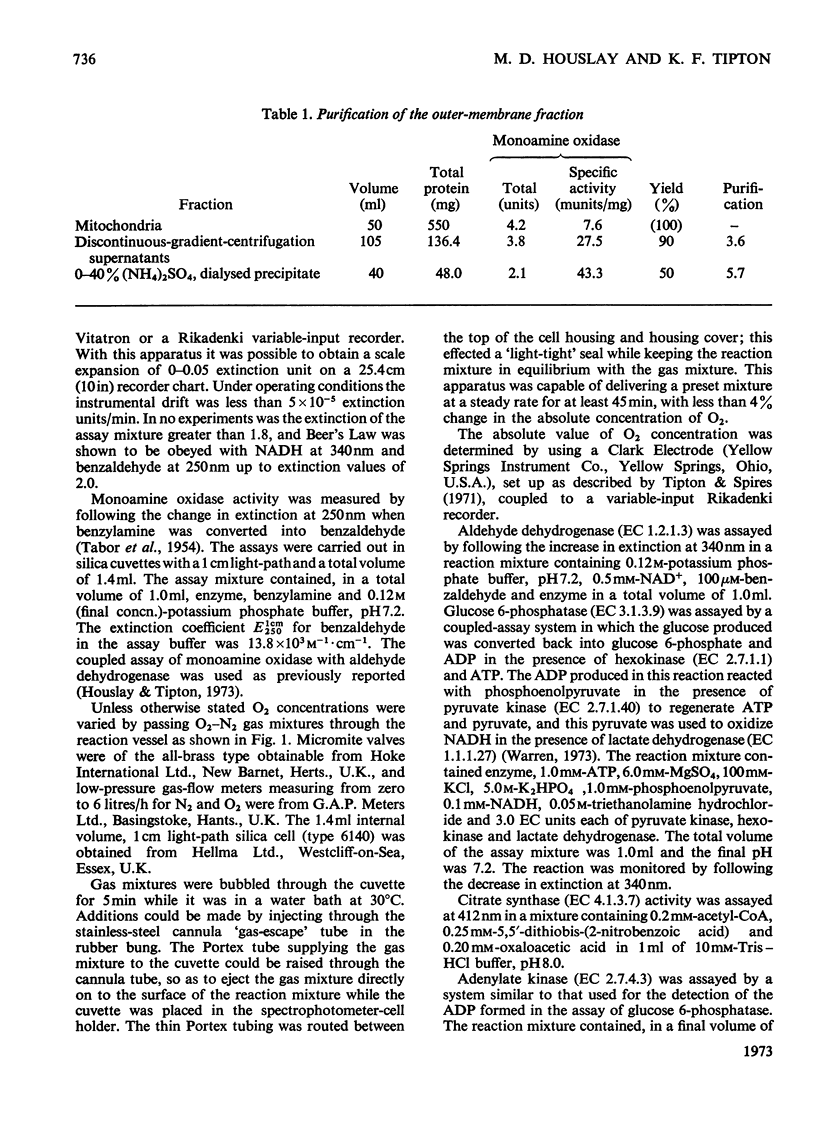
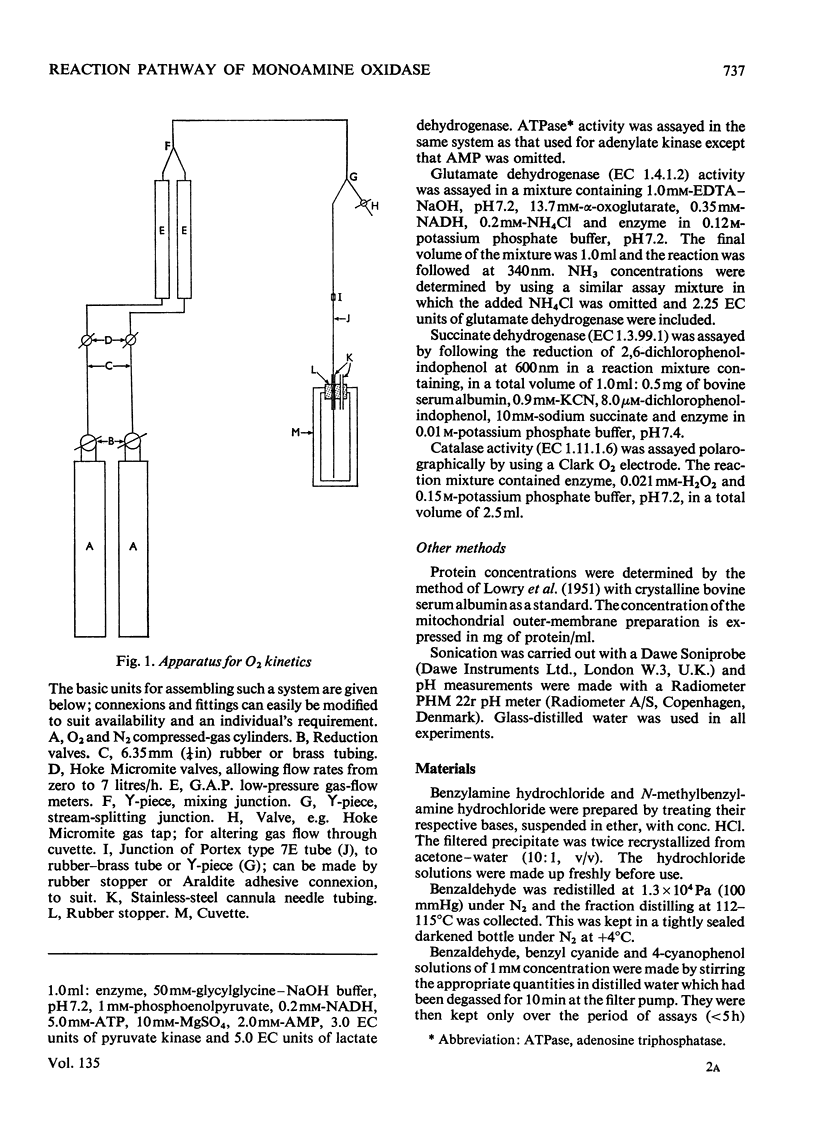
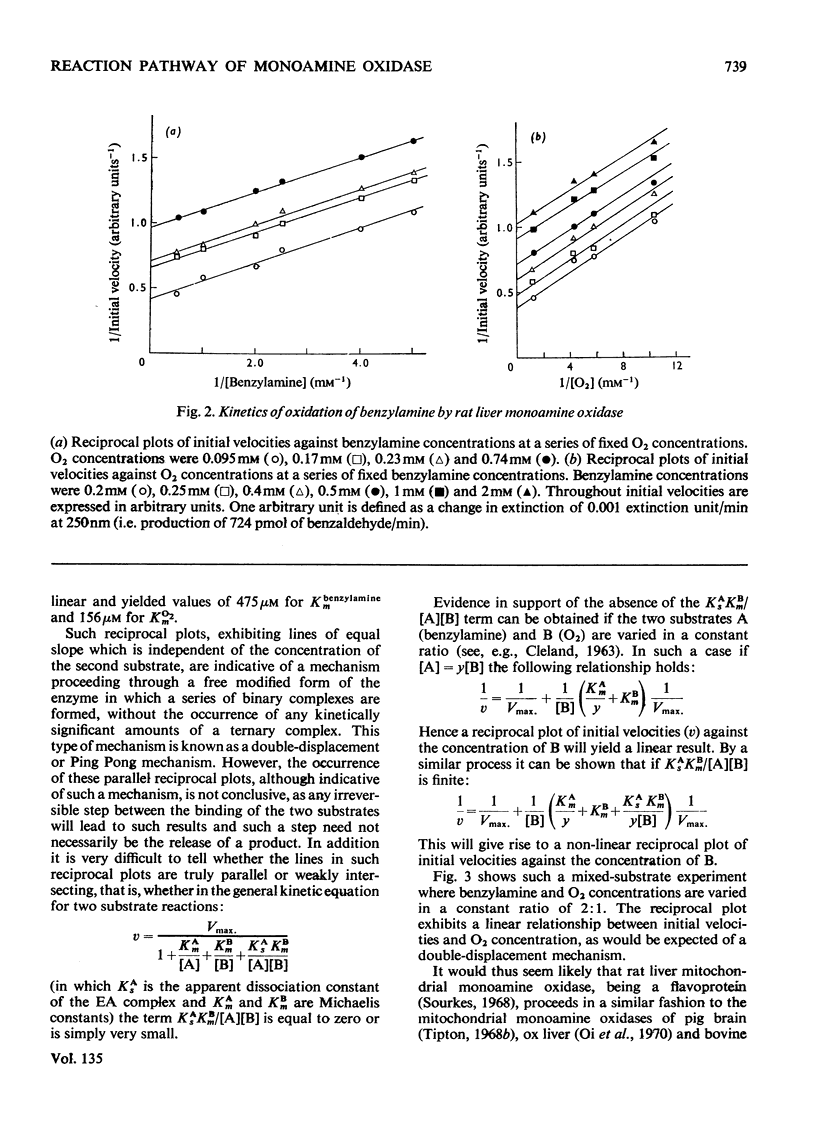
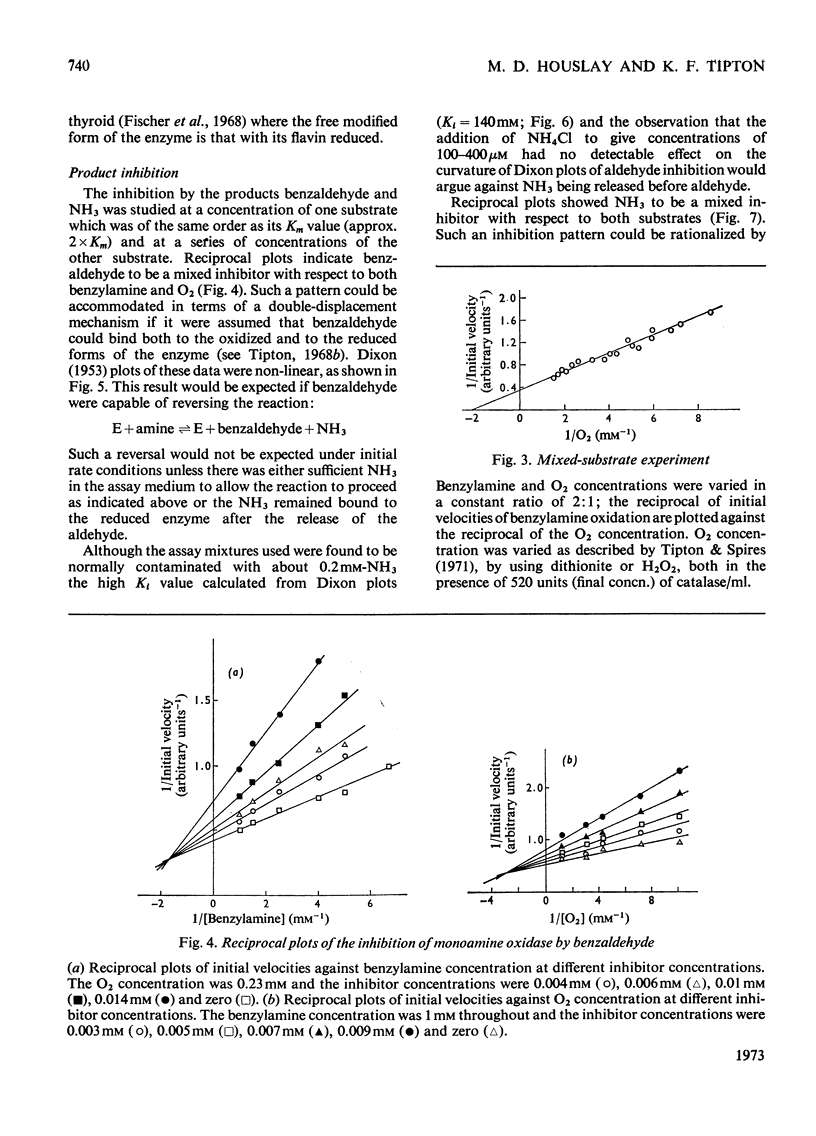
1. A preparation of a partly purified mitochondrial outer-membrane fraction suitable for kinetic investigations of monoamine oxidase is described. 2. An apparatus suitable for varying the O2 concentration in a spectrophotometer cuvette is described. 3. The reaction catalysed by the membrane-bound enzyme is shown to proceed by a double-displacement (Ping Pong) mechanism, and a formal mechanism is proposed. 4. KCN, NaN3, benzyl cyanide and 4-cyanophenol are shown to be reversible inhibitors of the enzyme. 5. The non-linear reciprocal plot obtained with impure preparations of benzylamine, which is typical of high substrate inhibition, is shown to be due to aldehyde contamination of the substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALIVISATOS S. G., MOURKIDES G. A., JIBRIL A. Non-enzymic reactions of indoles with coenzyme I. Nature. 1960 May 28;186:718–719. doi: 10.1038/186718a0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
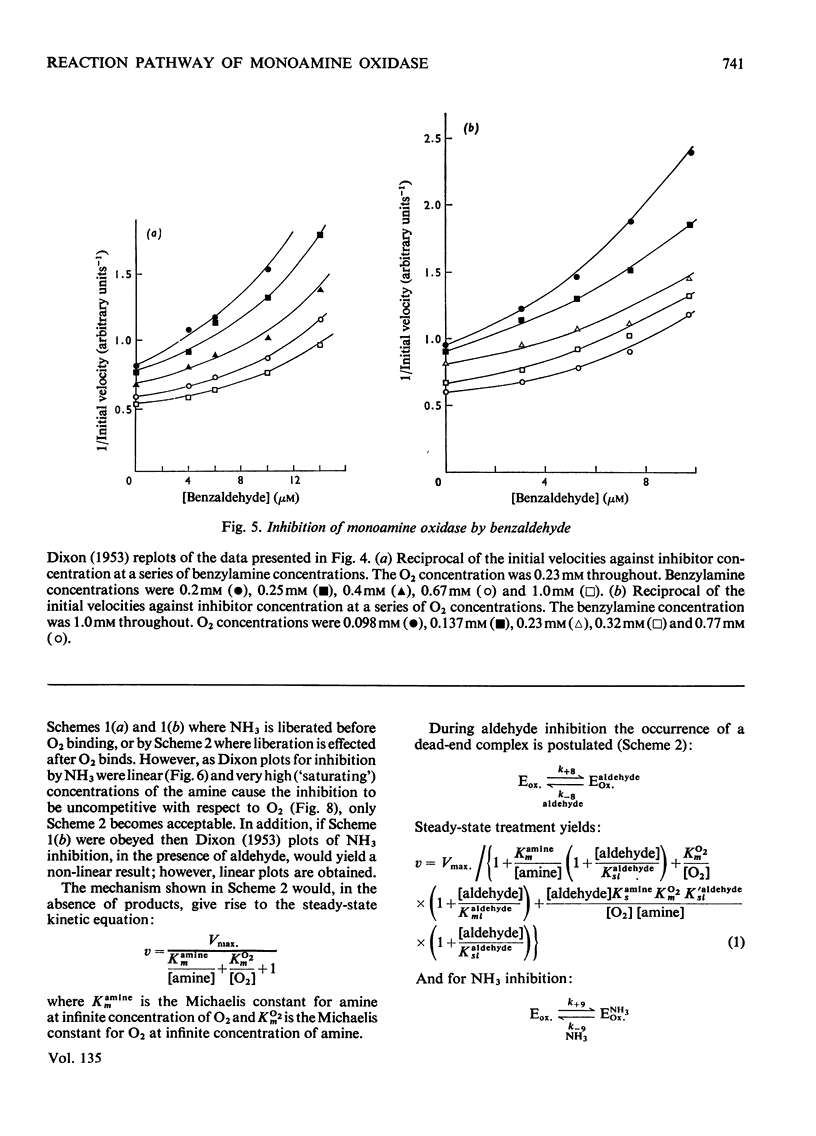
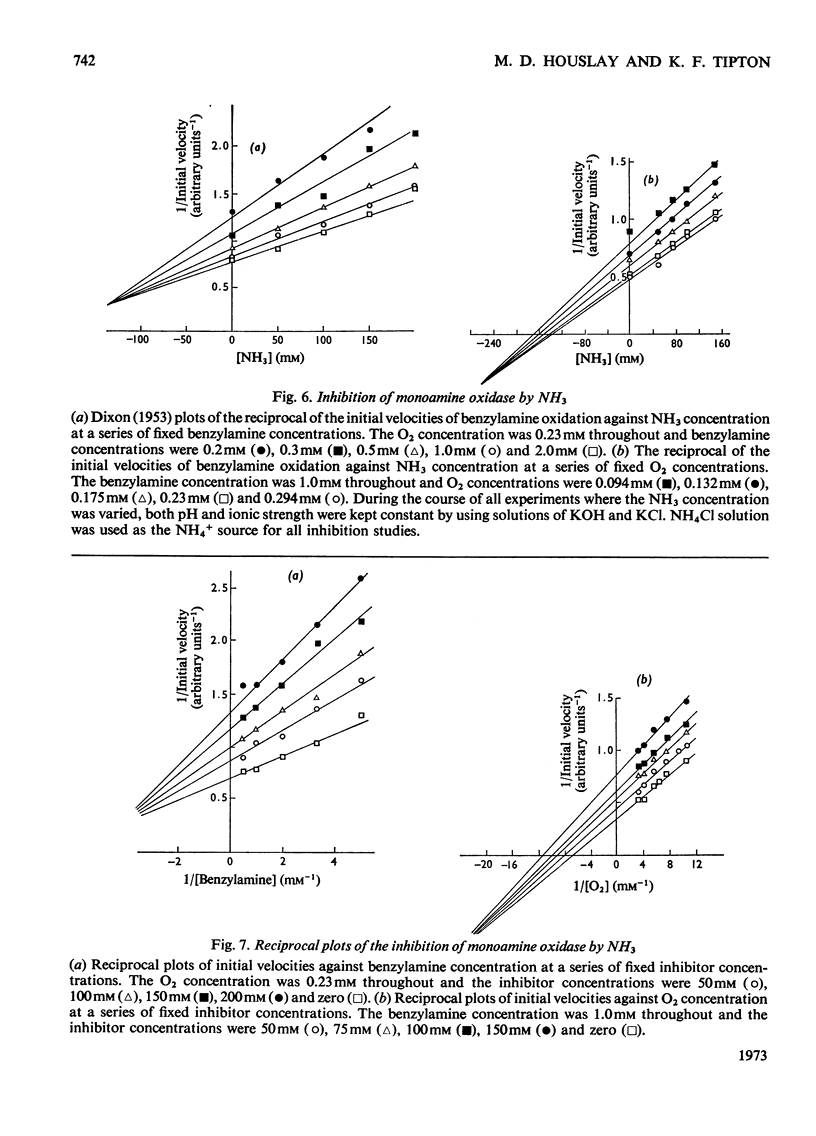
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
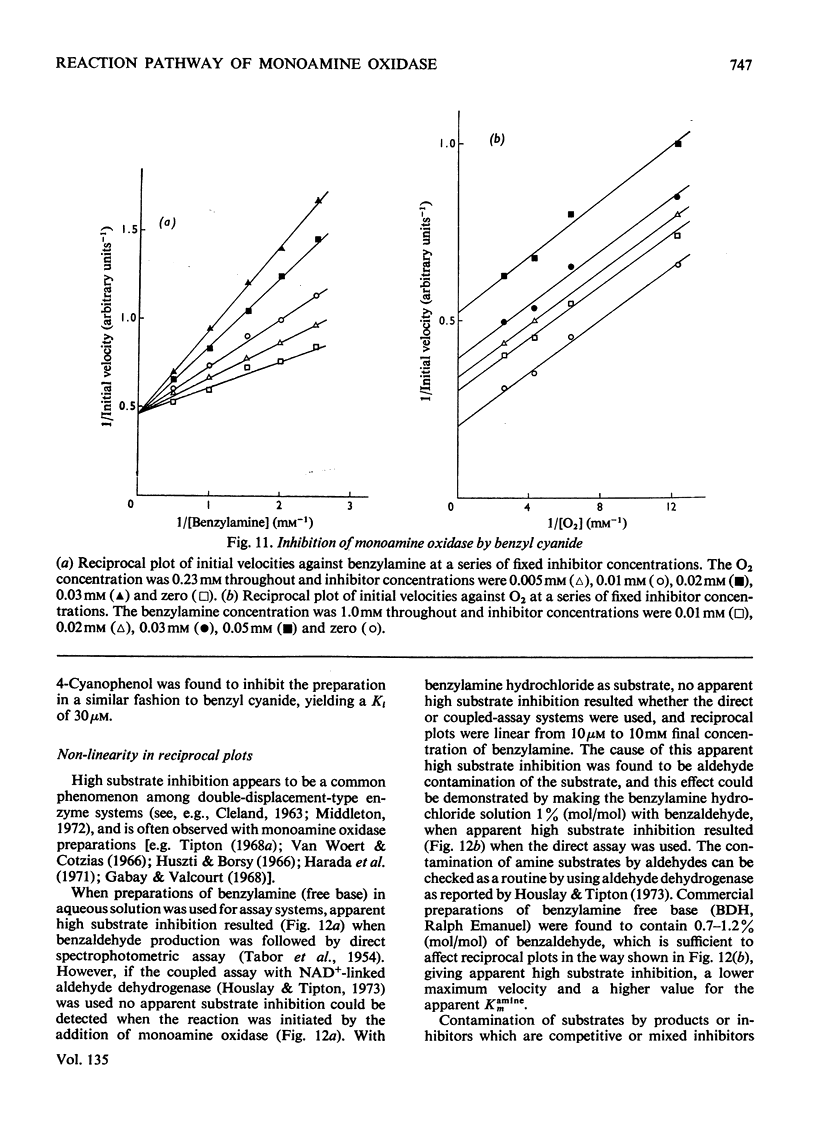
- Duncan R. J., Tipton K. F. The purification and properties of the NAD-linked aldehyde dehydrogenase from pig brain. Eur J Biochem. 1971 Sep 24;22(2):257–262. doi: 10.1111/j.1432-1033.1971.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Feldman R. I., Weiner H. Horse liver aldehyde dehydrogenase. I. Purification and characterization. J Biol Chem. 1972 Jan 10;247(1):260–266. [PubMed] [Google Scholar]
- Feldman R. I., Weiner H. Horse liver aldehyde dehydrogenase. I. Purification and characterization. J Biol Chem. 1972 Jan 10;247(1):260–266. [PubMed] [Google Scholar]
- Fischer A. G., Schulz A. R., Oliner L. Thyroidal biosynthesis of iodothyronines. II. General charactero acteristics and purification of mitochondrial monoamine oxidase. Biochim Biophys Acta. 1968 Jul 9;159(3):460–471. doi: 10.1016/0005-2744(68)90130-7. [DOI] [PubMed] [Google Scholar]
- Gabay S., Valcourt A. J. Studies of monoamine oxidases. I. Purification and properties of the rabbit liver mitochondrial enzyme. Biochim Biophys Acta. 1968 Jul 9;159(3):440–450. doi: 10.1016/0005-2744(68)90128-9. [DOI] [PubMed] [Google Scholar]
- Gomes B., Igaue I., Kloepper H. G., Yasunobu K. T. Amine oxidase. XIV. Isolation and characterization of the multiple beef liver amine oxidase components. Arch Biochem Biophys. 1969 Jun;132(1):16–27. doi: 10.1016/0003-9861(69)90334-8. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W. Localization of monoamine oxidase in rat liver mitochondria. Adv Biochem Psychopharmacol. 1972;5:207–226. [PubMed] [Google Scholar]
- Harada M., Mizutani K., Nagatsu T. Purification and properties of mitochondrial monoamine oxidase in beef brain. J Neurochem. 1971 Apr;18(4):559–569. doi: 10.1111/j.1471-4159.1971.tb11986.x. [DOI] [PubMed] [Google Scholar]
- Hare M. L. Tyramine oxidase: A new enzyme system in liver. Biochem J. 1928;22(4):968–979. doi: 10.1042/bj0220968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. The nature of the electrophoretically separable multiple forms of rat liver monoamine oxidase. Biochem J. 1973 Sep;135(1):173–186. doi: 10.1042/bj1350173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszti Z., Borsy J. Differences between amine oxidases deaminating mescaline and the structurally related 3,4-dimethoxyphenylethyl amine. Biochem Pharmacol. 1966 Apr;15(4):475–480. doi: 10.1016/0006-2952(66)90257-7. [DOI] [PubMed] [Google Scholar]
- Jarrott B. Occurrence and properties of monoamine oxidase in adrenergic neurons. J Neurochem. 1971 Jan;18(1):7–16. doi: 10.1111/j.1471-4159.1971.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Johnston J. P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968 Jul;17(7):1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- Kohn H. I. Tyramine oxidase. Biochem J. 1937 Oct;31(10):1693–1704. doi: 10.1042/bj0311693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McEwen C. M., Jr, Sasaki G., Jones D. C. Human liver mitochondrial monoamine oxidase. II. Determinants of substrate and inhibitor specificities. Biochemistry. 1969 Oct;8(10):3952–3962. doi: 10.1021/bi00838a011. [DOI] [PubMed] [Google Scholar]
- Middleton B. The kinetic mechanism of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from baker's yeast. Biochem J. 1972 Jan;126(1):35–47. doi: 10.1042/bj1260035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSWALD E. O., STRITTMATTER C. F. COMPARATIVE STUDIES IN THE CHARACTERIZATION OF MONOAMINE OXIDASES. Proc Soc Exp Biol Med. 1963 Dec;114:668–673. doi: 10.3181/00379727-114-28765. [DOI] [PubMed] [Google Scholar]
- Oi S., Shimada K., Inamasu M., Yasunobu K. T. Mechanistic studies of beef liver mitochondrial amine oxidase. 18. Amine oxidase. Arch Biochem Biophys. 1970 Jul;139(1):28–37. doi: 10.1016/0003-9861(70)90041-x. [DOI] [PubMed] [Google Scholar]
- Oi S., Yasunobu K. T., Westley J. The effect of pH on the kinetic parameters and mechanism of beef liver monoamine oxidase. Arch Biochem Biophys. 1971 Aug;145(2):557–564. doi: 10.1016/s0003-9861(71)80015-2. [DOI] [PubMed] [Google Scholar]
- Philpot F. J. Some observations on the oxidation of tyramine in the liver. Biochem J. 1937 Jun;31(6):856–861. doi: 10.1042/bj0310856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. S., Lovenberg W., Keiser H., Sjoerdsma A. Effects of drugs on human blood platelet and plasma amine oxidase activity in vitro and in vivo. Biochem Pharmacol. 1968 Jan;17(1):109–119. doi: 10.1016/0006-2952(68)90163-9. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
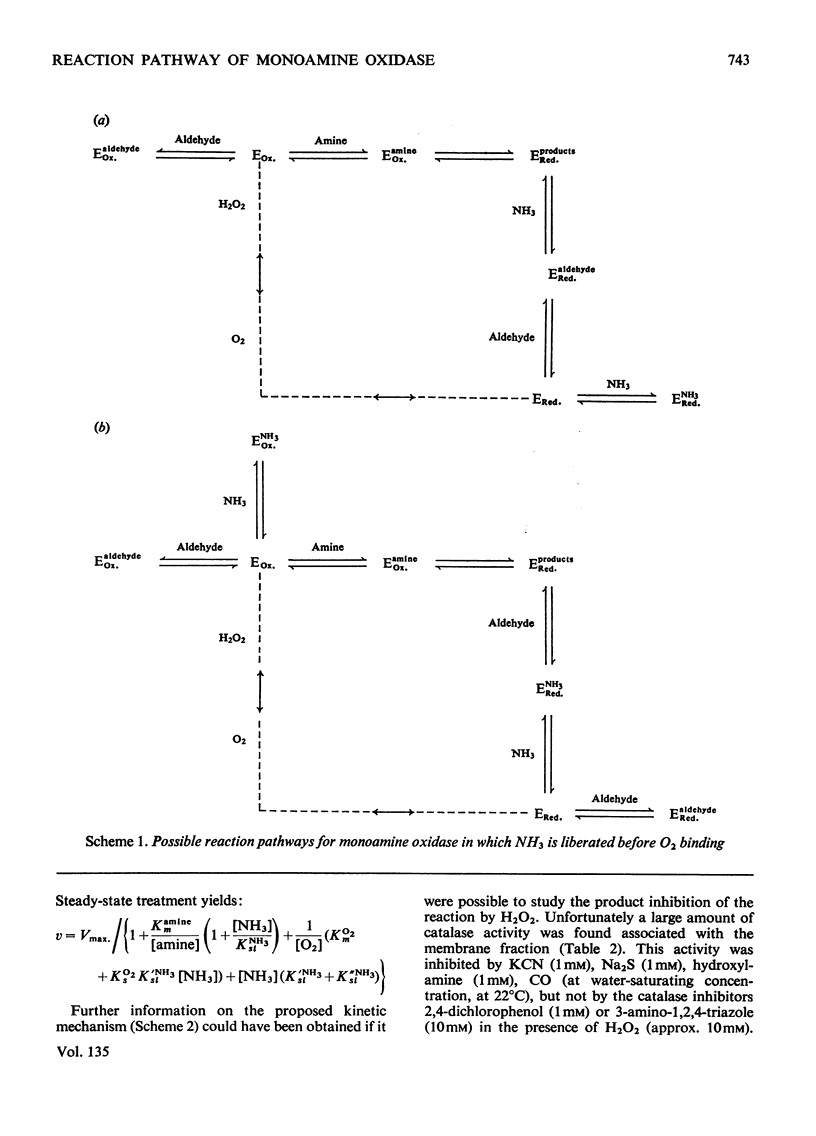
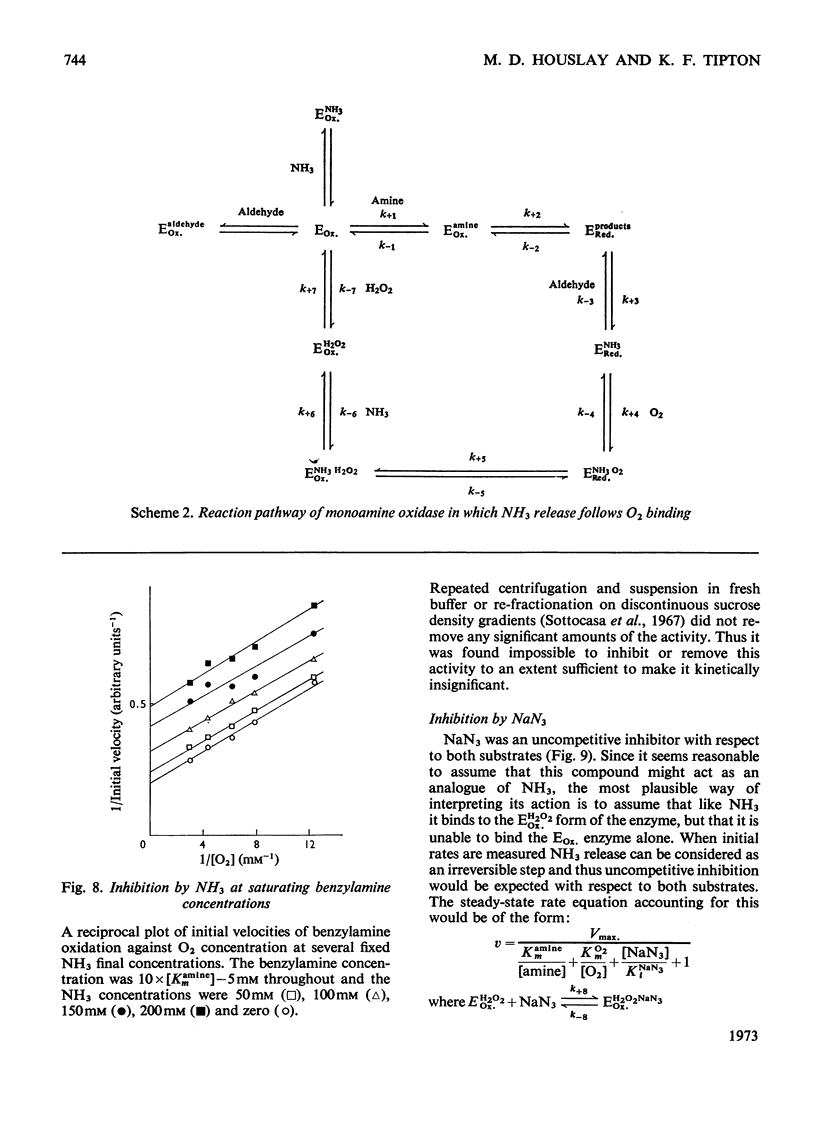
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourkes T. L. Properties of the monoamine oxidase of rat liver mitochondria. Adv Pharmacol. 1968;6(Pt A):61–69. doi: 10.1016/s1054-3589(08)61156-4. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Lassen J. B. Some pharmacological and biochemical properties of gamma-morpholino-butyrophenone (NSD 2023), a new monoamine oxidase inhibitor. Biochem Pharmacol. 1968 Mar;17(3):369–384. doi: 10.1016/0006-2952(68)90247-5. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- TUBBS P. K. Effects of inhibitors on mitochondrial D-alpha-hydroxy acid dehydrogenase. Biochem J. 1962 Jan;82:36–42. doi: 10.1042/bj0820036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton K. F., Spires I. P. Oxidation of 2-phenylethylhydrazine by monoamine oxidase. Biochem Pharmacol. 1972 Jan 15;21(2):268–270. doi: 10.1016/0006-2952(72)90278-x. [DOI] [PubMed] [Google Scholar]
- Tipton K. F., Spires I. P. The kinetics of phenethylhydrazine oxidation by monoamine oxidase. Biochem J. 1971 Nov;125(2):521–524. doi: 10.1042/bj1250521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton K. F. The prosthetic groups of pig brain mitochondrial monoamine oxidase. Biochim Biophys Acta. 1968 Jul 9;159(3):451–459. doi: 10.1016/0005-2744(68)90129-0. [DOI] [PubMed] [Google Scholar]
- Tipton K. F. The purification of pig brain mitochondrial monoamine oxidase. Eur J Biochem. 1968 Mar;4(1):103–107. doi: 10.1111/j.1432-1033.1968.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Tipton K. F. The reaction pathway of pig brain mitochondrial monoamine oxidase. Eur J Biochem. 1968 Aug;5(3):316–320. doi: 10.1111/j.1432-1033.1968.tb00372.x. [DOI] [PubMed] [Google Scholar]
- Tipton K. F. The sub-mitochondrial localization of monoamine oxidase in rat liver and brain. Biochim Biophys Acta. 1967;135(5):910–920. doi: 10.1016/0005-2736(67)90060-0. [DOI] [PubMed] [Google Scholar]
- Van Woert M. H., Cotzias G. C. Anion inhibition of monoamine oxidase. Biochem Pharmacol. 1966 Mar;15(3):275–285. doi: 10.1016/0006-2952(66)90299-1. [DOI] [PubMed] [Google Scholar]
- Veryovkina I. V., Samed M. M., Gorkin V. Z. Mitochondrial monoamine oxidase of rat liver: reversible qualitative alterations in catalytic properties. Biochim Biophys Acta. 1972 Jan 20;258(1):56–70. doi: 10.1016/0005-2744(72)90966-7. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Goridis C., Neff N. H. Properties of monoamine oxidases in sympathetic nerve and pineal gland. J Neurochem. 1972 May;19(5):1241–1250. doi: 10.1111/j.1471-4159.1972.tb01450.x. [DOI] [PubMed] [Google Scholar]


