Abstract
Cannabis sativa L., one of the oldest cultivated crops, has a complex domestication history due to its diverse uses for fibre, seed, oil, and drugs, and its wide geographic distribution. This review explores how human selection has shaped the biology of hemp and drug-type Cannabis, focusing on acquisition and utilization of nitrogen and phosphorus, and how resulting changes in source–sink relations shape their contrasting phenology. Hemp has been optimized for rapid, slender growth and nutrient efficiency, whereas drug-type cultivars have been selected for compact growth with large phytocannabinoid-producing female inflorescences. Understanding these nutrient use and ontogenetic differences will enhance our general understanding of resource allocation in plants. Knowledge gained in comparison with other model species, such as tomato, rice, or Arabidopsis can help inform crop improvement and sustainability in the cannabis industry.
Keywords: Cannabis sativa L., domestication, fibre, flowering, hemp, medicinal, nitrogen, nutrient use, phosphorus, sink, source
Cannabis sativa L. with its highly divergent usage types represents an interesting case study to compare nutrient acquisition and translocation strategies for high vegetative versus reproductive biomass yield.
Introduction
Cannabis sativa L. (hereafter Cannabis) has a long domestication history dating back at least 12 000 years (Clarke and Merlin, 2016; Ren et al., 2021). A unique feature of Cannabis plants is their production of secondary metabolites called cannabinoids within the glandular trichomes of their female flowers, with the major ones being cannabidiolic acid (CBDA) and ∆9-tetrahydrocannabinolic acid (THCA). Decarboxylation during the processing of flowers leads to their pharmacologically active forms, with tetrahydrocannabinol (THC) having psychoactive and intoxicating properties, while cannabidiol (CBD) has pharmaceutical uses as an anti-convulsant and anti-inflammatory drug (Lu and Mackie, 2016). In living plant tissues, the decarboxylated THC or CBD usually represents less than 2% the total (carboxylated plus decarboxylated) pools (Happyana et al., 2013).
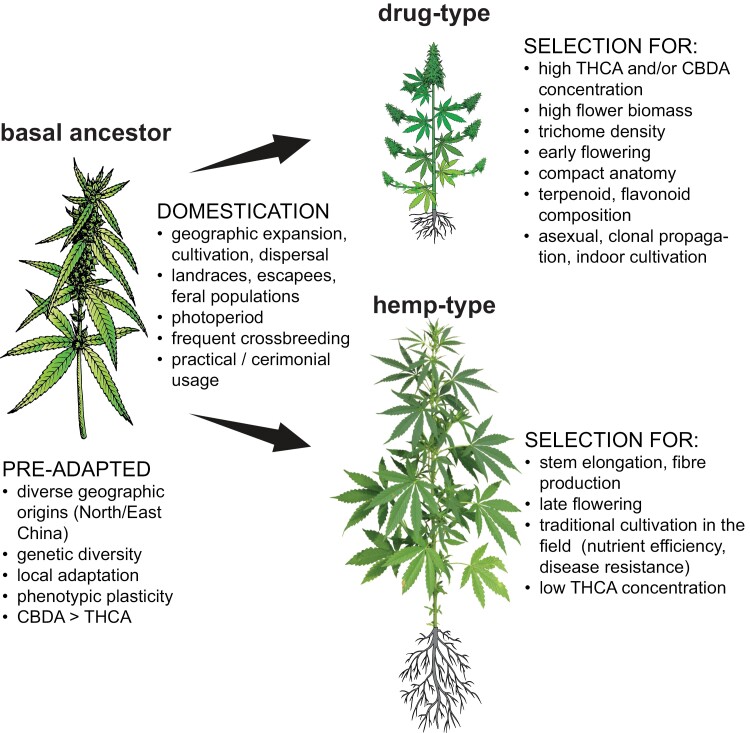
The wide geographic distribution of Cannabis led to genetic and phenotypic variation in local varieties and its subsequent domestication throughout most of Eurasia. Complex genetic diversity within the species is likely attributed to a successive blend of human selection, escapees, and outcrossing leading to unique feral landraces, and their subsequent reintroduction into domesticated germplasm (Clarke and Merlin, 2016; Barcaccia et al., 2020; Ren et al., 2021). Extensive targeted selection produced two divergent usage types with distinct genetics and contrasting plant architecture: hemp-type (aka hemp or industrial hemp for fibre and seed) and drug-type Cannabis (aka marijuana or weed for female flowers and cannabinoids) (Fig. 1) (Small, 2015; Clarke and Merlin, 2016). The tall-growing hemp type was selected for fibre production and contains high concentrations of CBDA and very little THCA. By contrast, drug-type Cannabis was selected for recreational and ceremonial purposes due to high amounts of the acid precursor of hallucinogenic THC and prolific production of female flowers. Recent heightened interest in CBDA accumulating drug-type chemovars for pharmaceuticals has instigated re-introgression of hemp- into drug-type cultivars (Grassa et al., 2021). The term ‘chemovar’ refers to Cannabis germplasm categorized either as THCA dominant, or CBDA dominant, or balanced (approximately equal levels of THCA and CBDA) with further differentiation according to terpenoid profiles (Hazekamp et al., 2016; Reimann-Philipp et al., 2020). It is often used instead of the more rigorous botanical term of ‘cultivar’, which implies that the progeny of those plants retains traits of interest—which due to the heterogeneous nature of Cannabis is often not the case for seed stock.
Fig. 1.
Divergent positive trait selection in Cannabis led to two distinct usage types. Illustrated are processes accompanying Cannabis domestication and the subsequent split into the two main usage types (drug- and hemp type). Major selected traits are cannabinoid content, plant architecture (tall versus compact stature, compound highly branched female inflorescence), biomass allocation (flower versus stem/fibre), flowering (early/late) as well as cultivation (indoors/field). See text for details. Abbreviations: CBDA, cannabidiolic acid; THCA, ∆9-tetrahydrocannabinolic acid.
The difference in specific usage of hemp- and drug-type Cannabis is reflected in their cultivation. Hemp types are grown in field conditions across a divergent range of environments and regions, often on marginal lands, and following traditional agricultural practise in broad acre systems with moderate inputs of fertilizer (Struik et al., 2000; Tang et al., 2017; Landi et al., 2019; Wylie et al., 2020; Blandinières and Amaducci, 2022). By contrast, drug types are largely grown in high-input, cost-intensive protected cropping or indoor environments due to their substantial value and quality standards imposed on pharmaceutical production (Madhusoodanan, 2019; Wartenberg et al., 2021; Velechovsky et al., 2024). Hence, growth regimes vary greatly for both Cannabis usage types, with human mitigation efforts being strongly influenced by abiotic and biotic stresses inherent to each type (Small, 2015; Clarke and Merlin, 2016; Wylie et al., 2020; Blandinières and Amaducci, 2022; Llewellyn et al., 2023). Inherent differences in nutrient requirements pose a challenge towards optimizing fertilizer input, which is an important factor for plant biomass production and product yield in commercial settings as well as for reducing the detrimental impact on local environments (Tilman et al., 2002).
In this respect, Cannabis presents an interesting case study to investigate how human selection and natural genetic diversity influence each other, and how these, advertently or inadvertently, influence nutrient use and its effects on plant development. While recent advances in next-generation sequencing have led to increased understanding of Cannabis genetic diversity to enable functional genomics (Kovalchuk et al., 2020; Grassa et al., 2021; Hurgobin et al., 2021; Lynch et al., 2024, Preprint), building on these resources to gain a better insight into the physiology of Cannabis plants is still lacking. Our review will focus on current knowledge of nutrient use, that is, the acquisition and utilization of the two macronutrients nitrogen (N) and phosphorus (P) and how these may influence growth and development in Cannabis. We will also highlight knowledge gaps that need to be addressed to optimize fertilizer application for Cannabis cultivation.
The domestication history of Cannabis is reflected in current cultivation practices
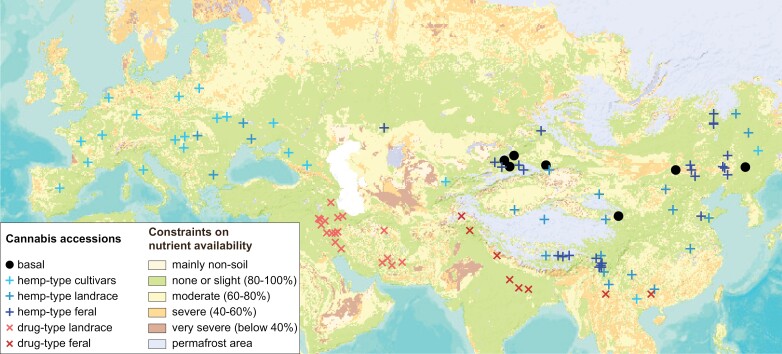
Although the true ancestor will likely remain unknown, recent genomic studies suggest that current Cannabis cultivars originate from a small number of domestication events (Kovalchuk et al., 2020; Ren et al., 2021). Their origins can be traced back to a few locations across northeast China with a subsequent geographic expansion and scattered distribution of genetically diverse landraces, escapees, and feral populations (Fig. 2). These early diverse geographic origins ensured development of trait combinations within subpopulations that made Cannabis ‘preadapted’ for human cultivation in divergent locations with contrasting temperature zones, photoperiods, water availabilities, and/or soil types with distinct nutrient availabilities (Clarke and Merlin, 2016). This allowed a wide distribution of Cannabis throughout Eurasia driven by the close co-evolutionary relationship of Cannabis and humans. Early selection led to cultivars with specialized purposes with frequent outcrossing and re-introduction enhancing genetic diversity, while recent hybridization led to the modern commercial cultivars (Clarke and Merlin, 2016). These processes resulted in Cannabis cultivars with greatly varying architectures, morphology, and biochemistry, but the underlying molecular processes, trait associations or genes driving these variations are not well understood (Ren et al., 2021). Analysis is further complicated because certain trait combinations might have been unknowingly selected for or may have been lost over time, while some genetic features neutral in certain growth conditions or locations became beneficial or detrimental in others. In addition, traits might have purposefully or inadvertently been re-introduced from wild populations at various stages over thousands of years of domestication.
Fig. 2.
Cannabis accessions originate from diverse regions with contrasting soil nutrient availabilities. Geographic distribution of accessions included in the determination of Cannabis domestication history was overlaid with soil nutrient availability constraints. Although the exact origin of Cannabis is unknown, accessions genetically close to the progenitor (basal accessions) originate from regions of northern China with constraints on nutrient availability ranging from none/slight to severe (percentages indicate ratings of growth potential). This wide ancestral adaptation to various soil types enabled subsequent worldwide domestication and local selection of hemp- and drug-type landraces with diverse trait combinations at least partially linked to agronomic constraints of their ancestral origin. Data for Cannabis accessions are based on field or seed bank collections (Zhang et al., 2018; Ren et al., 2021; Mostafaei Dehnavi et al., 2022). Soil nutrient availability data were downloaded from the FAO Global Soil Partnership website (https://www.fao.org/global-soil-partnership) and visualized using Data Basin (https://databasin.org).
About 12 000 years ago, the two usage types of Cannabis diverged from the basal ancestors by human targeted selection, and from these a limited number of domesticated accessions expanded to the current hemp- and drug-type Cannabis cultivars about 4000 years ago (Clarke and Merlin, 2016; Kovalchuk et al., 2020; Ren et al., 2021). Hemp was selected for bast fibre production and constituted the main and most wide-spread usage type of Cannabis for millennia. Selection of hemp types favoured late flowering to maximize vegetative growth, and for not well-understood reasons this also led to a preferential biosynthesis of CBDA over THCA. Decreasing concentrations of THCA in feral drug types suggest its accumulation has no adaptive significance in wild populations, and in hemp-type cultivars extensive flower development and high THCA concentrations were not desired (Clarke and Merlin, 2016). More recently, strict requirements by regulatory bodies for low total THC concentrations have resulted in hemp types almost devoid of any THCA.
By contrast, for drug-type Cannabis the aim of obtaining high THCA concentrations and flower biomass led to narrow, targeted selection, which started about 1000 years ago in Afghanistan and India due to more widespread ceremonial usage (Small, 2015). This included preference for a short photoperiod to induce flower production, short stature, and quick flower maturation. Introduction of Afghan cultivars into existing North American and European drug-type chemovars over the last decades produced the modern asexually propagated ‘sensimilla’ or seedless/unpollinated female accessions (Small, 2015; Clarke and Merlin, 2016). This further facilitated extensive indoor cultivation, initially largely in concealed growth spaces due to their illicit status. However, narrow selection under high-input, protected cropping conditions and inbreeding between already significantly related individuals for extremely high THC-yielding drug-type chemovars led to ‘spoiled’ cultivars with limited genetic diversity. These founder effects created genetic bottlenecks that constrained subsequent breeding efforts. They also resulted in the loss of beneficial traits such as pathogen resistance or nutrient efficiency that have become key determinants for modern cost-efficient, environmentally sustainable, and large-scale horticulture (McKernan et al., 2020, Preprint). For example, the dense, racemose flower structure introduced from Afghan cultivars is highly beneficial for commercial production but also increases susceptibility to fungal infections (Clarke and Merlin, 2016).
In contrast to hemp, very little is known about the nutrient efficiency of drug-type Cannabis and differences between cultivars or accessions. While drug-type Cannabis is often described as a neutrophile (Small, 2015), it is not clear if this reflects requirement or tolerance of high nitrogen supply. Overlaying the geographic origin with data on nutrient availability indicates some ancestral Cannabis progenitors originated on soils constraining growth by as much as 60% or more, while others evolved under non-limiting conditions (Fig. 2). This implies that the genetic diversity for nutrient-related traits was extensive even before domestication began. The contrasting selection of hemp- and drug-type Cannabis suggests varying degrees of conservation of the ancestral genetics. Traditional cultivation of hemp on diverse soil types across a wide geographical range suggests that traits related to the root uptake and organ distribution of important nutrients such as N and P were retained. In addition, common cultivation of hemp on marginal soils and its use in phytoremediation demonstrate its ability to grow with limited fertilizer inputs and its efficient heavy metal absorption and tolerance (Struik et al., 2000; Blandinières and Amaducci, 2022). For drug-type Cannabis, preferential growth in protected cropping environments with a ‘more is better’ attitude to increasing product yield together with a general (mis)interpretation of generic stress symptoms as nutrient limitation has likely led to an overestimation of the optimal fertilizer range (Westmoreland and Bugbee, 2022). Inadvertently, this may have also led to selection of drug-type cultivars able to tolerate, or even require, elevated nutrient input for biomass production. In this respect, drug-type Cannabis might be considered an extreme example for the genetic erosion observed in other crop species during the course of their domestication (Khoury et al., 2022).
Differences in nutrient response between Cannabis usage types: current knowledge
Macronutrients N and P are examples for factors constraining establishment of a more sustainable crop production. While increasing N and P fertilizer use in agricultural soils accompanying the ‘green revolution’ has resulted in increasing crop yields over the last century (Khush, 1999), a recent push to decrease fertilizer inputs to ensure sustainability and reduce environmental impact is hampered by limited genetic diversity in high-yielding varieties and their stagnating N and P use efficiencies (Hirel et al., 2007; Han et al., 2015). Thus, understanding the differences in the usage of these two nutrients between hemp and drug type might not only improve cultivation outcomes for Cannabis, but also provide valuable clues for the sustainability of other crops. As discussed in the previous section, the selection of basal Cannabis led to distinct usage types with divergent traits and resulted in a high degree of heterogeneity in phenotypic and genotypic representation. The nutrient requirements for optimal growth for each usage type, and indeed across cultivars within respective types, will hence be different (Bernstein et al., 2019a; Cockson et al., 2020; Yep et al., 2020; Bevan et al., 2021; Dilena et al., 2023).
Understanding the physiological traits that are responsive to nutrient supply, as well as the molecular mechanisms underlying traits associated with nutrient use, is key for the selection of nutrient-efficient genotypes in any crop breeding program (Ferrante et al., 2017). Complementing this approach with molecular tools to determine the genetic basis of these traits allows for the identification and/or development of nutrient-efficient cultivars in other crop species (van de Wiel et al., 2015; Tiwari et al., 2017; Goel et al., 2018). For Cannabis, this approach has so far been limited to morphological and physiological traits within a small number of cultivars that are not representing the full and divergent phenotypic range in the existing germplasm (Cockson et al., 2020; Anderson et al., 2021; Bevan et al., 2021; Saloner and Bernstein, 2021, 2022, 2023; Shiponi and Bernstein, 2021a; Dilena et al., 2023; Farnisa et al., 2023; Massuela et al., 2023). It is therefore necessary to evaluate our current knowledge base to identify gaps and how to address them. Given the long-standing illicit legal status of drug-type Cannabis, the underground ‘hobbyist’ nature of its cultivation has led to many myths penetrating commercial growing practices which go against the horticultural science behind the cultivation of other commercial crops such as tomato, cabbage, or lettuce (Rengel et al., 2022). Building a mechanistic understanding of the regulation of nutrient acquisition and utilization in Cannabis will thus be critical for improving its yield potential and product quality. Here, we will focus on current knowledge on the response to supply of two macronutrients, nitrogen (N) and phosphorus (P), which are of high importance for the sustainability of Cannabis cultivation, and will highlight the potential of knowledge transfer from other crops.
Usage types differ in their nitrogen acquisition and utilization
N is critical for plant growth and development as an integral component of DNA, RNA, proteins, and free amino acids. Leaf biomass is tightly linked to available N in growth substrates, as N is also a structural component of chlorophyll and hence critical for carbon fixation through photosynthesis (Rengel et al., 2022). In angiosperms, N is present at an average 6% of total dry matter (n=62 species) and 18% of total protein (n=74) (Yeoh and Wee, 1994; Broadley et al., 2004). While it can be taken up as mineral N (nitrate and ammonium), oxides of nitrogen (NOx), as well as amino acids and peptides, N acquisition through nitrate (NO3−) is typically the preferred pathway for most plants and tightly coupled to photosynthetic activity (Lejay et al., 2008; O’Brien et al., 2016). Due to the high energetic cost of nitrate reduction, a mixture of nitrate and ammonium is considered beneficial in plant cultivation as compared with either source of mineral N alone (Hachiya and Sakakibara, 2017). A ratio of 1 part ammonium to 3 or 4 parts nitrate is considered optimal for most plants with some benefits of higher ammonium levels reported for secondary metabolites such as vitamin C, carotenoids, flavonoids, and phenolic compounds (Shilpha et al., 2023). Cannabis appears to be quite sensitive to ammonium with a nitrate-to-ammonium supply ratio of more than 3 to 1 at 15 mM total N supply considered optimal to balance flower biomass and cannabinoid concentrations in one drug-type Cannabis cultivar (Saloner and Bernstein, 2022). However, some species like rice, onion, and blueberry are more tolerant to ammonium (Britto and Kronzucker, 2002). This is linked to the environment these plants have evolved in, that is, the presence of biological nitrogen fixation and whether soil N, P, or both are limiting plant productivity (Britto and Kronzucker, 2013; Prodhan et al., 2019; Shilpha et al., 2023).
Most research suggests that whilst human selection has driven cannabinoid concentrations in female inflorescences of drug-type Cannabis to near maximum metabolic capacity, flower architecture and overall biomass are key targets for further increasing yield potential (Saloner and Bernstein, 2021; Shiponi and Bernstein, 2021a; Massuela et al., 2023; Stack et al., 2023; Wei et al., 2023). This highlights the importance of understanding sink–source relationships and their response to nutrient supply for yield improvement (Smith et al., 2018; Tegeder and Masclaux-Daubresse, 2018; Burnett, 2019). In rice and tomato, modern hybrids already feature increased spikelet/fruit number and higher spikelet/fruit to leaf biomass ratios (Li et al., 2015; Li et al., 2023). Having a larger sink size, rice hybrids also have higher leaf N content, pre-flowering biomass, and efficient resource translocation during grain filling (Li et al., 2023). However, these properties are highly dependent on soil fertility with source–sink relations in need of optimization for specific cultivation conditions, in particularly for more sustainable low nutrient input systems. In tomato, sink–source relationships are responsive to nutrient supply (de Groot et al., 2003; Kanai et al., 2007) as well as environmental stress factors (drought, light intensity, CO2 levels, salt) (Matsuda et al., 2011; Osorio et al., 2014; Ji et al., 2020) and vary between cultivars (Matsuda et al., 2011; Li et al., 2015). Genetic manipulation may be needed given that modern crop varieties rarely surpass the trait boundaries of their wild progenitors. The latter were domesticated precisely because they already featured desired traits as adaptations to environmental conditions in their natural habitat (Gómez-Fernández et al., 2024).
The diverse response of hemp- and drug-type Cannabis to N supply can be viewed as a case in point. Industrial hemp is bred for vegetative (fibre) biomass—and to some degree seed production—and features relatively weak sink strength in female flowers. In general, flower biomass of industrial/fibre hemp makes up less than 10% of total plant dry matter (Tang et al., 2018; Wei et al., 2023). By contrast, drug types are selected based on cannabinoid content, flower architecture, and flower biomass and grown in the presence of excess nutrients (Llewellyn et al., 2023). Female flowers of drug types as well as many ‘floral’ hemp types are strong sinks and make up between 30% and 50% of total plant biomass (Bernstein et al., 2019b; Anderson et al., 2021; Rodriguez-Morrison et al., 2021; Farnisa et al., 2023).
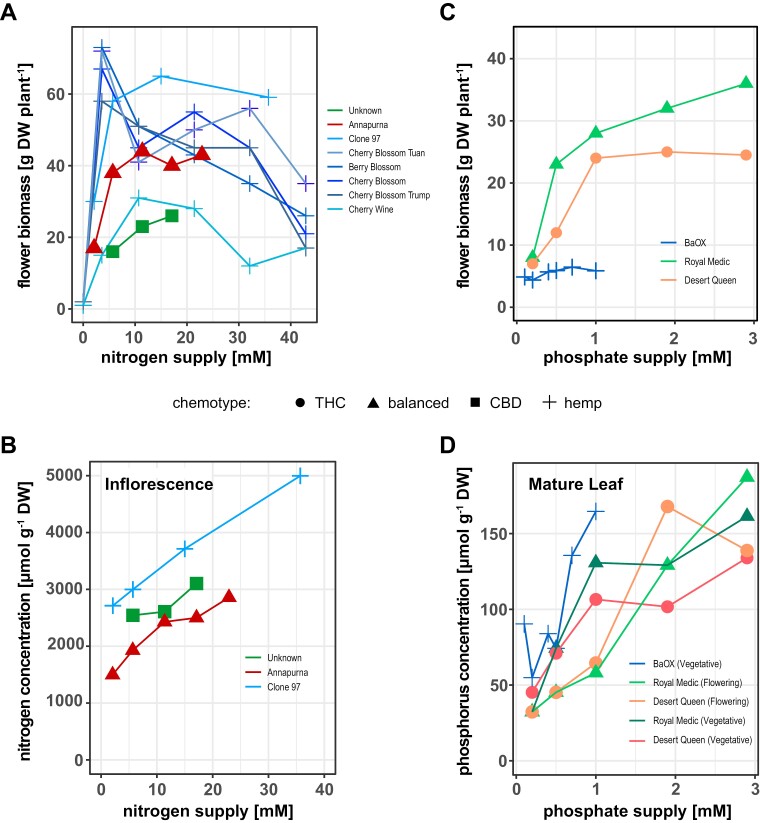
Comparing inflorescence biomass in response to increasing N supply across both usage types grown in controlled environments (Fig. 3A) highlights their different nutrient requirements for optimal growth, as well as differences in what constitutes limiting levels of N supply. While it is difficult to compare the absolute values of flower yield with N supply due to differences in growing conditions between the studies in terms of light quality/intensity and growth environment (e.g. open versus closed greenhouses), the trends observed suggest differences in nutrient use and flower yield across usage types and cultivars. In hemp, flower biomass initially increases with higher N supply, reaching a cultivar-dependent optimum, after which increased N supply becomes detrimental (Fig. 3A). In comparison with drug-type Cannabis, this optimum is reached at lower N supply. There also appears to be a higher tolerance in drug types, whereby what would be a toxic N supply and detrimental to growth in hemp or other species is instead accepted as luxury supply without growth benefit (e.g. accumulation of N without increasing flower yield). However, drug types are sensitive to N limitation of net photosynthesis as leaf chlorophyll concentration starts to decline at a total N supply of less than 11 mM (Saloner and Bernstein, 2021). By contrast, despite substantial variations in biomass, there are no significant differences in leaf chlorophyll concentrations of hemp cultivars grown at between 4 mM and 32 mM total N (Anderson et al., 2021). Regulatory differences in N acquisition and translocation between drug- and hemp-type Cannabis are also apparent (Fig. 3B). In hemp, N concentration in inflorescences increases with N supply despite overall reduction in flower biomass due to toxicity (Fig. 3A) and is higher than that in drug types at similar levels of N supply. Together, these data confirm a higher N use efficiency of hemp (Tang et al., 2018; Landi et al., 2019).
Fig. 3.
Response of hemp- and drug-type cultivars to variation in N or P supply. (A, C) In hemp types, flower biomass increases with higher N or Pi supply, reaching a cultivar-dependent optimum, after which additional supply becomes detrimental. By contrast, drug-type Cannabis tolerates higher N and Pi supplies without impacting flower biomass. (B) Total nitrogen concentration in flowers continuously increases with increasing N supply in hemp, while it tends to level off in flowers of drug-type cultivars. (D) At any given level of supply during vegetative growth, total P concentration of fan leaves is higher in hemp- than drug-type cultivars, and accumulation in response to supply is exponential in the former. At flowering stage, cultivar specific responses are observed in the latter as P accumulation is either up- or down-regulated with Pi supply. Data points shown are replotted with averages (n=4–10) from Anderson et al. (2021), Cockson et al. (2020), Massuela et al. (2023), Saloner and Bernstein (2020, 2021), and Shiponi and Bernstein (2021b). For Cockson et al. (2020), fresh weights were converted to approximate dry weights assuming 85% moisture content. Abbreviations: CBD, cannabidiol; DW, dry weight; THC, tetrahydrocannabinol.
In higher plants, there is a strong effect of nutrient supply on secondary metabolism (Amtmann and Armengaud, 2009). In tomato, nitrogen and phosphorus supply impact sink strength and fruit quality—especially in terms of carbohydrate, carotenoid, amino acid, and polyamine composition (Sung et al., 2015; Vallarino et al., 2020; Weinert et al., 2021). Excess N supply often has a negative or no impact on the metabolite profile of tomato fruit (Truffault et al., 2019; Schmidt and Zinkernagel, 2021). In Cannabis, the impact of nutrient supply on the synthesis of cannabinoids and terpenes in floral organs is of key concern. Consistent with impacts on secondary metabolite profiles in tomato, increasing N supply decreases cannabinoid concentrations in inflorescences across usage types: total content of CBD and its precursor cannabigerol decrease with increasing N supply in five hemp cultivars, and a relatively low N supply of about 4 mM was considered optimal for both cannabinoid concentration and total yield (Anderson et al., 2021). Similar trends were obtained for another hemp cultivar that showed a broader optimal N supply range of between 2 and 15 mM (Dilena et al., 2023). In a balanced drug-type chemovar, however, there was no effect of N supply on total CBD and THC content in dried and cured female inflorescences (Saloner and Bernstein, 2021). In a later study using the same chemovar, total cannabinoid content determined in fresh-frozen inflorescence material decreased with increasing N supply while terpenoid concentration remained unchanged (Song et al., 2023). N-containing compounds such as chlorophylls and amino acids increased, while hexose and pentose sugars (fructose, glucose, and xylose), phenols, and flavonoids as well as total carbon content decreased. A decrease of phosphate and hexose phosphates furthermore indicates that with increasing N supply, P becomes limiting, which may explain the increase in nicotinic acid, proline, ornithine, lysine, and polyamines such as putrescine (Morcuende et al., 2007; Huang et al., 2008; Aleksza et al., 2017).
In summary, while industrial and floral hemp seem to be more N efficient with high growth rates at low levels of N supply, meta-analysis of different studies indicates that they have a lower threshold for N toxicity as compared with drug types. In contrast to hemp and tomato, many drug types require high N inputs of >11 mM total N to reach maximum photosynthetic rates and flower biomass. Drug-type Cannabis seems to also tolerate higher N supply with no discernible effects on either performance or metabolic profile (Saloner and Bernstein, 2021; Song et al., 2023). The complex breeding history involving frequent introgression of hemp- into drug-type Cannabis means that the N response of each cultivar or genotype needs to be assessed for optimal cannabinoid yield. It remains to be seen whether a more sustainable N use is achievable through selective introgression of desired hemp traits into drug-type Cannabis without affecting yield.
Phosphorus requirements vary between hemp and drug-types
In most rhizosphere environments, P is one of the least plant-available nutrients, with inorganic phosphate (Pi) often tightly adsorbed onto metal-based complexes (Hinsinger et al., 2005). Therefore, field crops are heavily dependent not only on external fertilizer applications but also root exudation of Pi-releasing enzymes such as nucleases and phosphatases as well as of organic (carboxylic) acids such as malate, oxalate, and citrate, which induce localized soil acidification to encourage Pi mobilization (Vance et al., 2003; Richardson et al., 2011; Gerke, 2015). Further root adaptations include auxin-dependent lateral root and root hair formation and increasing Pi uptake and xylem loading (Peret et al., 2011; Zhang et al., 2014). All these adaptations are critical as P itself is a major macronutrient required for essential plant processes such as photosynthesis, respiration, protein activity regulation, and the synthesis of nucleic acids and membrane lipids (Veneklaas et al., 2012). In mature legume and cereal seed, the main P storage compound, phytate, accounts for 1–5% by weight, representing 65–85% of total P (Oo et al., 2023). Pi is also tightly linked to both sucrose and starch synthesis through the Calvin–Benson cycle, an integral process for plant development that requires triose phosphates and releases Pi in a tightly regulated process (McClain and Sharkey, 2019). Due to its strong impact on carbon flows and feedback regulation of N assimilation, cytosolic levels of Pi are tightly controlled in most crop and model plants, and once plant P status is sufficient, transporters involved in Pi uptake and translocation are rapidly suppressed on both transcriptional and post-translational levels (Aung et al., 2006; Bari et al., 2006; Franco-Zorrilla et al., 2007). During development, plastid Pi translocators are critically important for the translocation of photoassimilates from source to sink tissues, and trehalose-6-phosphate is an important signal for coordination of carbon and nitrogen assimilation to provide the building blocks for organ growth (Fluegge, 1995; Hammond and White, 2008; Griffiths et al., 2016). Pi supply is therefore critical for plant metabolism and growth, with strong links to flower development and reproduction.
In Cannabis, despite the effect of P on plant development, there are even fewer studies on the impact of P than that of N supply for both usage types. An impact of Pi on cannabinoid biosynthesis might be expected given cannabigerolic acid and cannabigerovarinic acid, the common precursors of phytocannabinoids, require geranyl pyrophosphate to be synthesized (Gülck and Møller, 2020). Geranyl pyrophosphate is in turn synthesized by the plastidial methylerythritol phosphate pathway, of which Pi is a key component and regulator through triose phosphate utilization in the Calvin–Benson cycle (McClain and Sharkey, 2019). Early studies point towards cannabinoid content being positively correlated with soil P content in drug types of Afghan origin (Coffman and Gentner, 1977). In a recent study of a balanced and a THC-dominant drug type, the highest cannabinoid concentrations were reported under the lowest Pi supply of 0.16 mM, which severely limited plant growth and total flower biomass, while there were no changes in total cannabinoid concentrations with Pi supply levels greater than 0.5 mM (Shiponi and Bernstein, 2021a). In a hemp cultivar, cannabinoid concentrations, predominantly total CBD, increased up to 0.36 mM Pi supply, after which concentrations remained relatively unchanged (Cockson et al., 2020). The CBD-dominant hemp-type cultivar BaOx reached an optimal level of Pi supply for flower production much earlier than the two drug types (Fig. 3C). Higher Pi supply had a negative impact on flower biomass, while there was no response in plant height, total above-ground biomass or root-to-shoot ratio (Cockson et al., 2020). In drug types, Pi supply above the hemp optimum did not affect flower or total plant dry matter, which was fairly constant between 1 mM and 3 mM Pi supply, with female flowers also strong P sinks accumulating about 80% of total organ P (Fig. 3C) (Shiponi and Bernstein, 2021a). Within drug types, there are cultivar-specific differences in their Pi response: the THC-dominant chemovar was slower growing, and as a consequence, had a more compact stature with reduced branching, while, due to stronger vegetative growth that was evident at the two lowest Pi supply levels, leaves and stems of the THC/CBD-balanced chemovar became N-limited at Pi supplies of 1 mM and above. This was accompanied by increased translocation of N to reduced leaves in the inflorescence helping to maintain sink strength (Shiponi and Bernstein, 2021a). N-limited growth in the balanced chemovar may also explain lower net photosynthetic rates compared with the THC-dominant chemovar across treatments. Total P accumulation in flowers reached a maximum of about 400 µmol g−1 dry weight at the high Pi supplies across chemovars. Total P content also plateaued in roots of the balanced chemovar, while P content kept increasing in roots of the THC-dominant chemovar, which resulted in higher P acquisition efficiency with increasing Pi supply. Despite its higher P acquisition efficiency, the THC-dominant chemovar had a lower root-to-shoot biomass ratio (Shiponi and Bernstein, 2021a).
These data may indicate repression of Pi uptake in the balanced chemovar because of N-limitation at higher Pi supplies. It is of note that at limiting Pi supply, the balanced chemovar experienced only an approximate 15% flower yield loss while the THC-dominant chemovar incurred nearly 50%, relative to the proposed ‘optimal’ 1 mM Pi supply (Fig. 3C) (Shiponi and Bernstein, 2021a). The higher yield under P limiting conditions in the balanced chemovar could be due to its higher root-to-shoot ratio most likely in combination with molecular traits such as increased expression of high-affinity phosphate transporters in roots, as well as more efficient remobilization of Pi from source to sink tissues (Akhtar et al., 2008; Julia et al., 2016). Interestingly, the studied hemp cultivar had a 3-fold higher root-to-shoot biomass ratio at the lowest Pi supply in the vegetative phase and it did not change its biomass allocation with increasing Pi supply (Cockson et al., 2020). For hemp, uninhibited P accumulation in leaves would suggest a constitutive phosphate starvation response with sustained Pi uptake as observed in plant species adapted to P limited environments (Shane et al., 2004). At higher Pi supply to hemp in the reproductive phase, reductions in N and microelement pools in source leaves together with a lack of increase in flower biomass, premature senescence, and browning of mature fan leaves indicate that plants are becoming increasingly P toxic and/or N limited (Shukla et al., 2017; Cockson et al., 2020). P accumulation in hemp leaves is much stronger than in the two drug types, as a critical toxicity level of 160 µmol total P g−1 dry weight is already reached at an external Pi supply of 1 mM, while such levels are only reached at between 2 and 3 mM Pi in drug types (Fig. 3D) (Shane et al., 2004). This again supports the notion that hemp types are more nutrient efficient than drug-type Cannabis, with higher yield at relatively lower levels of fertilizer input (Tang et al., 2018; Stack et al., 2023).
Integrating molecular approaches for improvements in understanding nutrient regulation in Cannabis
The available data suggest that drug types have a higher tolerance for excessive or luxurious nutrient supply than hemp-type Cannabis. Within usage types, cultivar specific differences in nutrient response and sink strength are also evident (Wylie et al., 2020). Recent introgression of hemp (Grassa et al., 2021), coupling a functional CANNABIDIOLIC ACID SYNTHASE gene with the capacity of modern drug-type cultivars to produce high cannabinoid concentrations and flower biomass, has resulted in progeny with high allelic diversity, especially since drug-type parental lines are not homozygous to begin with (Barcaccia et al., 2020). These more recent introgressions led to complex segregation of associated nutrient efficiency traits that were relatively fixed in hemp- and drug-type Cannabis with restricted gene flow between domesticated populations (Small, 2015; Ren et al., 2021).
However, these progenies also provide a rich genetic resource for the analysis and interaction of such traits. Assessing the genetic basis of selected traits of interest will rely on both traditional and molecular techniques to uncover quantitative trait loci and/or molecular markers (Platten et al., 2019; Li et al., 2022). Such approaches are advantageous as conventional breeding through crossing and selection is highly impractical due to the dioecious nature of Cannabis and the unclear lineage and heterozygosity of drug types (Sawler et al., 2015; Ingvardsen and Brinch-Pedersen, 2023). As proof-of-concept, desirable domestication traits relating to plant anatomy, fruit shape, fruit size, fruit number, and nutritional quality were transferred to wild tomato by CRISPR–Cas9 genome editing to improve agronomical value (Zsögön et al., 2018). Extensive knowledge of carbon and nitrogen fluxes across different plant model species has allowed an increase in fruit yield in tomato through multi-gene metabolic engineering (Vallarino et al., 2020). In Cannabis, marker-assisted methods have been employed successfully for cannabinoid profiling (Weiblen et al., 2015; Laverty et al., 2019; Jin et al., 2021; Welling et al., 2022), sex determination (Toth et al., 2020), and usage type distinction (Cascini et al., 2019; Barbaric and Bezbradica, 2023). However, the fidelity of these markers is reliant on high quality and continuous improvement of well-annotated reference genomes (Barcaccia et al., 2020; Ingvardsen and Brinch-Pedersen, 2023). The creation of a Cannabis pangenome in combination with phased genomes is likely to help bridge the high heterogeneity present in current germplasm, facilitating breeding via identification of elite and stable markers conserved across the variable Cannabis genomes (Hurgobin et al., 2021).
Optimizing for nutrient efficiency and sink strength
Nutrient-efficient Cannabis cultivars are key to meeting industry sustainability goals (Landi et al., 2019; Velechovsky et al., 2024). The commercialization of Cannabis of both usage types into pharmaceutical products also requires good manufacturing practices to be consistently met by growers to ensure product quality standards (Craven et al., 2019; Montoya et al., 2020; Jameson et al., 2022). Hemp is well known for its phytoremediation potential due to its high uptake capacity for heavy metals and other soil contaminants (Testa et al., 2023). These traits may become detrimental if cultivation conditions are not well defined and constantly monitored. For example, arsenic is taken up by high-affinity phosphate transporters (Navarro et al., 2021), and thus selecting lines with high Pi acquisition efficiency may lead to higher tissue accumulation of heavy metals in field-grown produce (Blandinières and Amaducci, 2022). The nutritional value of some hemp products could also be compromised by the hyperaccumulation of phosphate in flowers and phytate in seed—given that phosphate toxicity is an issue for both human and animal health (Razzaque, 2011; Lei et al., 2013; Alexander et al., 2022). Nutrient management, and by extension how nutrient status is regulated across different cultivars and usage types, is necessary for uniform plant growth, secondary metabolite synthesis, and flower development, as well as maintaining plant health through decreasing susceptibility towards opportunistic pathogens detrimental to harvest quality (Dordas, 2008; Punja, 2021). For these purposes, cannabinoid production at an industrial scale is largely indoors under controlled environmental conditions, which also maximizes the number of crop cycles per year (Chandra et al., 2020).
Several studies have emphasized the importance of flower biomass for overall cannabinoid yield in both hemp- and drug-type Cannabis (Naim-Feil et al., 2021; Wei et al., 2023). By contrast, cannabinoid concentrations are relatively stable across moderate changes in nutrient supply and/or composition (Saloner and Bernstein, 2021; Shiponi and Bernstein, 2021a; Massuela et al., 2023; Stack et al., 2023; Wei et al., 2023). This recapitulates findings in tomato that emphasize the importance of source–sink interactions for fruit quality and yield (Osorio et al., 2014; Smith et al., 2018). A high N-to-P ratio is known to promote vegetative growth with a negative impact on sink strength and thus fruit harvest index and fruit quality (Schauer et al., 2006; Tegeder and Masclaux-Daubresse, 2018). High N levels suppress circadian clock and flowering genes in Arabidopsis whilst low N supply accelerates flowering either through promoting the expression of genes for transcriptional regulators CONSTANS (CO) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), altering the phosphorylation state of transcription factor FLOWERING BHLH4, and/or inhibiting expression of transcription factors GATA, NITRATE-INDUCIBLE, CARBON-METABOLISM INVOLVED (GNC), and GNC-LIKE (Cho et al., 2017; Lin and Tsay, 2017; Olas et al., 2019; Y. Zhang et al., 2023). By contrast, high Pi supply promotes flower initiation and development, whilst P limitation delays flowering (Nord and Lynch, 2008; Y. Zhang et al., 2023). This is mediated through altered expression of flowering genes such as CO and SOC1; however, effects seen for mutants with altered shoot P status support a metabolic control over trehalose-6-phosphate, an important flowering signal (Kant et al., 2011; Wahl et al., 2013). In Cannabis, orthologues for these key flowering genes have been identified pending further functional characterization (Steel et al., 2023; Dowling et al., 2024). The emergence of inositol pyrophosphates as important signals of plant nutrient status and associated sensor proteins that coordinate nitrate and phosphate acquisition as well as developmental programs will provide new targets for metabolic engineering (Poza-Carrion and Paz-Ares, 2019; Z. Zhang et al., 2023). These sensors and their regulatory networks are yet to be explored in plant species that differ in their nutrient efficiencies and breeding history. Hemp- and drug-type Cannabis provide an excellent opportunity to study these. Significant differences in sink strength within the two usage types provides valuable insight into the impact of N and/or P redistribution on C balance during the reproductive stage (Veneklaas et al., 2012). Vegetative plants largely rely on Pi uptake by roots during early growth stages, with a shift to P remobilization from shaded, older to young, growing leaves occurring in the later stages (Han et al., 2022). After the shift to flowering, plants depend much more heavily on Pi translocation from source organs—and drug-type Cannabis plants allocate a large proportion of dry mass to female unpollinated flowers (see above). It remains to be seen how increasing P use efficiency in drug types grown indoors will affect cannabinoid and terpene concentrations and their profiles given the importance of P for photosynthesis, carbon flux, and source–sink interactions in field grown crops (Wissuwa et al., 2005).
Understanding the molecular regulation of plant nutrient status and source–sink interactions will be crucial towards the development of nutrient-efficient cultivars and sustainable crop management and will tie into optimizing crop quality and yield whilst reducing agrochemical inputs and therefore costs and environmental pollution (Zheng et al., 2021).
Towards community standards in Cannabis nutrient research
In Cannabis, trends in growth responses to various environmental factors such as nutrient supply, daylength, light intensity, or light quality are emerging from recent published data (Shukla et al., 2017; Magagnini et al., 2018; Saloner et al., 2019; Anderson et al., 2021; Bevan et al., 2021; Rodriguez-Morrison et al., 2021; Shiponi and Bernstein, 2021a; Llewellyn et al., 2022, 2023; Reichel et al., 2022; Saloner and Bernstein, 2022; Westmoreland and Bugbee, 2022; Dilena et al., 2023; Massuela et al., 2023; Peterswald et al., 2023; Song et al., 2023). However, a lack of community standards presently impedes straight-forward comparison of results and their interpretation. Currently, hemp- and drug-type Cannabis cultivars are often independently tested under different ranges of P or N supply (Fig. 3), making it difficult to determine where actual limitations to their performance lie across the entire genetic range. Determination of the mass balance between nutrients supplied, nutrients taken up by the plant, and their partitioning into downstream metabolites requires accurate description of tissue concentrations, nutrient quantities supplied, and frequency of application, and sensible information on nutrient formulations (Lambers and Barrow, 2021). For comparative analyses, a greater number of genetically well-defined hemp- and drug-type Cannabis grown in parallel under standardized cultivation conditions is needed to identify traits of interest and obtain a functional understanding. Furthermore, accurate identifiers and terms are needed to describe morphological and physiological features, especially around flowering time and flower maturity, for both hemp- and drug-type Cannabis (Mediavilla et al., 1998; Brym et al., 2023). This will help to detect differences in the timing of developmental transitions caused by changes in nutrient supply ratios and resource allocation. It is also important to study organs that respond quickly to environmental conditions, given that in many species individual reproductive organs are protected against fluctuations in nutrient status whilst photosynthetically active source leaves along the main stem quickly display signs of nutrient stress (Veneklaas et al., 2012).
Systematic analysis of the metabolomic and transcriptomic response to nutrient limitation or excess will help to define key metabolites and marker genes for cultivar selection (Watanabe et al., 2010; Cobb et al., 2013; Vallarino et al., 2020; Cuyas et al., 2023). In this respect, determination of cannabinoid profiles and concentrations often lacks standardization of methodology and preparation of the plant materials before analysis (Welling et al., 2019; Kim et al., 2022). The current practice of trimming reduced leaves from flowers and drying harvested materials over long periods of time (up to several weeks) at temperatures ranging from 15 °C to 60 °C introduces artifacts and reduces reproducibility between studies with information on residual water content often lacking. Shock-freezing plant materials on-site and storage at below −70 °C until further processing, tissue homogenization under liquid N2 or on dry ice, and the use of internal standards improve cannabinoid preservation to reflect the actual profile and concentrations of individual cannabinoids in planta at harvest.
While the common practice of clonal propagation is eliminating some of the phenotypic variation, comparison of experimental results between growth facilities is still difficult, even if the same seed source is used. Highly heterozygous plants and the lack of true Cannabis F1 hybrids in most breeding programs lead to a high degree of genetic diversity, even in ‘all-female’ or sinsemilla cultivars (Barcaccia et al., 2020). For research purposes, it would be desirable to generate suitable representations of hemp- and drug-type Cannabis that are genetically fixed as much as possible and create near-homozygous parental clones that serve as stable reference populations.
Optimizing nutrient use is highly dependent on cultivation practices. Soil-grown crops are often nutrient or water limited for at least some part of their growth cycle, so that boosting soil health through increasing organic matter and fostering the soil microbiome have dramatic effect on yields (Qiao et al., 2022; Suman et al., 2022). Plants grown in protected cropping systems, on the other hand, often benefit from inorganic fertilizer formulations without competition from microbes (Sanjuan-Delmás et al., 2020; Chavan et al., 2022). Breeding programs therefore must generate a diverse range of cultivars that cater for these very different growth environments.
Concluding remarks and future perspectives
Cannabis sativa L. is a monotypic genus with highly polymorphic, heterogeneous accessions, varieties, and chemovars. Human selection for divergent purposes has resulted in two very distinct usage types—hemp-type (fibre or industrial hemp) and psychoactive compound-producing drug-type (marijuana) Cannabis. In contrast to other crop plants, very little research has gone into exploring the genetic basis for the very strong contrasts in growth habit, flowering control, and nutrient use between the two main usage types. This review has highlighted differences in nutrient use based on the analysis of relevant Cannabis literature, as well as the knowledge gaps that still exist between observed physiological responses to nutrient supply and the underlying genetic factors. There is great potential to increase our understanding of source–sink interactions as Cannabis displays stronger contrasts in developmental programs, nutrient use, and metabolic fluxes than many current model plants such as Brassicaceae, cereals, and tomato. While progress in this area is encouraging, employing available genetic resources and ‘omics’ tools towards understanding the complex breeding history and biology of Cannabis will further boost genetic improvement. As has been the case for tomato breeding, scientific research and methodological advances for Cannabis will generate more resilient germplasm for different purposes and growth environments. Preserving a diverse gene pool that includes wild accessions and landraces will furthermore provide genetic resources for future demands on pharmaceuticals, food products, clothing, biofuels, and building materials that this highly adaptable species can no doubt cater for.
Acknowledgements
The authors would like to thank colleagues and industry partners within the Australian Research Council’s Industrial Transformation Hub for Medicinal Agriculture for useful discussions and feedback. We apologize to colleagues whose work is not cited here due to space constraints.
Contributor Information
Benjamin Wee Y, ARC Research Hub for Medicinal Agriculture, Department of Animal, Plant and Soil Sciences, School of Agriculture, Biomedicine and Environment, La Trobe University, Bundoora VIC 3086, Australia; La Trobe Institute for Sustainable Agriculture & Food, La Trobe University, Bundoora VIC 3086, Australia.
Oliver Berkowitz, ARC Research Hub for Medicinal Agriculture, Department of Animal, Plant and Soil Sciences, School of Agriculture, Biomedicine and Environment, La Trobe University, Bundoora VIC 3086, Australia; La Trobe Institute for Sustainable Agriculture & Food, La Trobe University, Bundoora VIC 3086, Australia.
James Whelan, ARC Research Hub for Medicinal Agriculture, Department of Animal, Plant and Soil Sciences, School of Agriculture, Biomedicine and Environment, La Trobe University, Bundoora VIC 3086, Australia; La Trobe Institute for Sustainable Agriculture & Food, La Trobe University, Bundoora VIC 3086, Australia; Present Address: College of Life Science, Zhejiang University, Hangzhou, Zhejiang, 310058, P.R. China.
Ricarda Jost, ARC Research Hub for Medicinal Agriculture, Department of Animal, Plant and Soil Sciences, School of Agriculture, Biomedicine and Environment, La Trobe University, Bundoora VIC 3086, Australia; La Trobe Institute for Sustainable Agriculture & Food, La Trobe University, Bundoora VIC 3086, Australia.
Susanne Schilling, University College Dublin, Ireland.
Conflict of interest
The authors declare no conflicting interests.
Funding
This work was funded by the Australian Research Council's Industrial Transformation Hub for Medicinal Agriculture (grant ID IH180100006).
References
- Akhtar MS, Oki Y, Adachi T.. 2008. Intraspecific variations of phosphorus absorption and remobilization, P forms, and their internal buffering in Brassica cultivars exposed to a P-stressed environment. Journal of Integrative Plant Biology 50, 703–716. [DOI] [PubMed] [Google Scholar]
- Aleksza D, Horvath GV, Sandor G, Szabados L.. 2017. Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiology 175, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander R, Debiec N, Razzaque MS, He P.. 2022. Inorganic phosphate-induced cytotoxicity. IUBMB Life 74, 117–124. [DOI] [PubMed] [Google Scholar]
- Amtmann A, Armengaud P.. 2009. Effects of N, P, K and S on metabolism: new knowledge gained from multi-level analysis. Current Opinion in Plant Biology 12, 275–283. [DOI] [PubMed] [Google Scholar]
- Anderson SL 2nd, Pearson B, Kjelgren R, Brym Z.. 2021. Response of essential oil hemp (Cannabis sativa L.) growth, biomass, and cannabinoid profiles to varying fertigation rates. PLoS One 16, e0252985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ.. 2006. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiology 141, 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaric L, Bezbradica SC.. 2023. A forensic application of genetic markers for distinction between drug-type and fiber-type Cannabis sativa L. Forensic Science International 353, 111853. [DOI] [PubMed] [Google Scholar]
- Barcaccia G, Palumbo F, Scariolo F, Vannozzi A, Borin M, Bona S.. 2020. Potentials and challenges of genomics for breeding Cannabis cultivars. Frontiers in Plant Science 11, e573299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Pant BD, Stitt M, Scheible WR.. 2006. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiology 141, 988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N, Gorelick J, Koch S.. 2019a. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.). Industrial Crops and Products 129, 185–194. [Google Scholar]
- Bernstein N, Gorelick J, Zerahia R, Koch S.. 2019b. Impact of N, P, K, and humic acid supplementation on the chemical profile of medical cannabis (Cannabis sativa L.). Frontiers in Plant Science 10, e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan L, Jones M, Zheng Y.. 2021. Optimisation of nitrogen, phosphorus, and potassium for soilless production of Cannabis sativa in the flowering stage using response surface analysis. Frontiers in Plant Science 12, e764103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandinières H, Amaducci S.. 2022. Adapting the cultivation of industrial hemp (Cannabis sativa L.) to marginal lands: a review. GCB Bioenergy 14, 1004–1022. [Google Scholar]
- Britto DT, Kronzucker HJ.. 2002. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology 159, 567–584. [Google Scholar]
- Britto DT, Kronzucker HJ.. 2013. Ecological significance and complexity of N-source preference in plants. Annals of Botany 112, 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley MR, Bowen HC, Cotterill HL, Hammond JP, Meacham MC, Mead A, White PJ.. 2004. Phylogenetic variation in the shoot mineral concentration of angiosperms. Journal of Experimental Botany 55, 321–336. [DOI] [PubMed] [Google Scholar]
- Brym ZT, Philpott SC, Rheay H, et al. 2023. Hemp morphology and physiology standards for research and industry applications. HortScience 58, 756–760. [Google Scholar]
- Burnett AC. 2019. Source–sink relationships. Encyclopedia of Life Sciences. https://doi.org/10.1002/9780470015902.a0001304.pub2 [Google Scholar]
- Cascini F, Farcomeni A, Migliorini D, Baldassarri L, Boschi I, Martello S, Amaducci S, Lucini L, Bernardi J.. 2019. Highly predictive genetic markers distinguish drug-type from fiber-type Cannabis sativa L. Plants 8, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Lata H, ElSohly MA.. 2020. Propagation of cannabis for clinical research: an approach towards a modern herbal medicinal products development. Frontiers in Plant Science 11, e958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan SG, Chen Z-H, Ghannoum O, Cazzonelli CI, Tissue DT.. 2022. Current technologies and target crops: a review on Australian protected cropping. Crops 2, 172–185. [Google Scholar]
- Cho LH, Yoon J, An G.. 2017. The control of flowering time by environmental factors. The Plant Journal 90, 708–719. [DOI] [PubMed] [Google Scholar]
- Clarke RC, Merlin MD.. 2016. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. Critical Reviews in Plant Sciences 35, 293–327. [Google Scholar]
- Cobb JN, Declerck G, Greenberg A, Clark R, McCouch S.. 2013. Next-generation phenotyping: requirements and strategies for enhancing our understanding of genotype-phenotype relationships and its relevance to crop improvement. Theoretical and Applied Genetics 126, 867–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockson P, Schroeder-Moreno M, Veazie P, Barajas G, Logan D, Davis M, Whipker BE.. 2020. Impact of phosphorus on Cannabis sativa reproduction, cannabinoids, and terpenes. Applied Sciences 10, 7875–7818. [Google Scholar]
- Coffman CB, Gentner WA.. 1977. Responses of greenhouse-grown Cannabis sativa L. to nitrogen, phosphorus, and potassium. Agronomy Journal 69, 832–836. [Google Scholar]
- Craven CB, Wawryk N, Jiang P, Liu Z, Li XF.. 2019. Pesticides and trace elements in cannabis: analytical and environmental challenges and opportunities. Journal of Environmental Sciences 85, 82–93. [DOI] [PubMed] [Google Scholar]
- Cuyas L, David P, de Craieye D, et al. 2023. Identification and interest of molecular markers to monitor plant Pi status. BMC Plant Biology 23, e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot CC, Marcelis LFM, van den Boogaard R, Kaiser WM, Lambers H.. 2003. Interaction of nitrogen and phosphorus nutrition in determining growth. Plant and Soil 248, 257–268. [Google Scholar]
- Dilena E, Close DC, Hunt I, Garland SM.. 2023. Investigating how nitrogen nutrition and pruning impacts on CBD and THC concentration and plant biomass of Cannabis sativa. Scientific Reports 13, e19533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordas C. 2008. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agronomy for Sustainable Development 28, 33–46. [Google Scholar]
- Dowling CA, Shi J, Toth JA, Quade MA, Smart LB, McCabe PF, Schilling S, Melzer R.. 2024. A FLOWERING LOCUS T ortholog is associated with photoperiod-insensitive flowering in hemp (Cannabis sativa L.). The Plant Journal 119, 383–403. [DOI] [PubMed] [Google Scholar]
- Farnisa MM, Miller GC, Solomon JKQ, Barrios-Masias FH.. 2023. Floral hemp (Cannabis sativa L.) responses to nitrogen fertilization under field conditions in the high desert. PLoS One 18, e0284537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A, Nocito FF, Morgutti S, Sacchi GA.. 2017. Plant breeding for improving nutrient uptake and utilization efficiency. In: Tei F, Nicola S, Benincasa P, eds. Advances in research on fertilization management of vegetable crops. Cham: Springer International Publishing, 221–246. [Google Scholar]
- Fluegge UI. 1995. Phosphate translocation in the regulation of photosynthesis. Journal of Experimental Botany 46, 1317–1323. [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, Garcia JA, Paz-Ares J.. 2007. Target mimicry provides a new mechanism for regulation of microRNA activity. Nature Genetics 39, 1033–1037. [DOI] [PubMed] [Google Scholar]
- Gerke J. 2015. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. Journal of Plant Nutrition and Soil Science 178, 351–364. [Google Scholar]
- Goel P, Sharma NK, Bhuria M, et al. 2018. Transcriptome and co-expression network analyses identify key genes regulating nitrogen use efficiency in Brassica juncea L. Scientific Reports 8, e7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Fernández A, Aranda I, Milla R.. 2024. Early human selection of crops’ wild progenitors explains the acquisitive physiology of modern cultivars. Nature Plants 10, 25–36. [DOI] [PubMed] [Google Scholar]
- Grassa CJ, Weiblen GD, Wenger JP, Dabney C, Poplawski SG, Timothy Motley S, Michael TP, Schwartz CJ.. 2021. A new Cannabis genome assembly associates elevated cannabidiol (CBD) with hemp introgressed into marijuana. New Phytologist 230, 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CA, Paul MJ, Foyer CH.. 2016. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochimica et Biophysica Acta 1857, 1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülck T, Møller BL.. 2020. Phytocannabinoids: origins and biosynthesis. Trends in Plant Science 25, 985–1004. [DOI] [PubMed] [Google Scholar]
- Hachiya T, Sakakibara H.. 2017. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. Journal of Experimental Botany 68, 2501–2512. [DOI] [PubMed] [Google Scholar]
- Hammond JP, White PJ.. 2008. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany 59, 93–109. [DOI] [PubMed] [Google Scholar]
- Han M, Okamoto M, Beatty PH, Rothstein SJ, Good AG.. 2015. The genetics of nitrogen use efficiency in crop plants. Annual Review of Genetics 49, 269–289. [DOI] [PubMed] [Google Scholar]
- Han Y, White PJ, Cheng L.. 2022. Mechanisms for improving phosphorus utilization efficiency in plants. Annals of Botany 129, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happyana N, Agnolet S, Muntendam R, Van Dam A, Schneider B, Kayser O.. 2013. Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 87, 51–59. [DOI] [PubMed] [Google Scholar]
- Hazekamp A, Tejkalová K, Papadimitriou S.. 2016. Cannabis: from cultivar to chemovar II—A metabolomics approach to cannabis classification. Cannabis and Cannabinoid Research 1, 202–215. [Google Scholar]
- Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW.. 2005. Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytologist 168, 293–303. [DOI] [PubMed] [Google Scholar]
- Hirel B, Le Gouis J, Ney B, Gallais A.. 2007. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. Journal of Experimental Botany 58, 2369–2387. [DOI] [PubMed] [Google Scholar]
- Huang CY, Roessner U, Eickmeier I, Genc Y, Callahan DL, Shirley N, Langridge P, Bacic A.. 2008. Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficient barley plants (Hordeum vulgare L.). Plant and Cell Physiology 49, 691–703. [DOI] [PubMed] [Google Scholar]
- Hurgobin B, Tamiru-Oli M, Welling MT, Doblin MS, Bacic A, Whelan J, Lewsey MG.. 2021. Recent advances in Cannabis sativa genomics research. New Phytologist 230, 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvardsen CR, Brinch-Pedersen H.. 2023. Challenges and potentials of new breeding techniques in Cannabis sativa. Frontiers in Plant Science 14, e1154332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson LE, Conrow KD, Pinkhasova DV, et al. 2022. Comparison of state-level regulations for cannabis contaminants and implications for public health. Environmental Health Perspectives 130, e97001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Nunez Ocana D, Choe D, Larsen DH, Marcelis LFM, Heuvelink E.. 2020. Far-red radiation stimulates dry mass partitioning to fruits by increasing fruit sink strength in tomato. New Phytologist 228, 1914–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Henry P, Shan J, Chen J.. 2021. Identification of chemotypic markers in three chemotype categories of cannabis using secondary metabolites profiled in inflorescences, leaves, stem bark, and roots. Frontiers in Plant Science 12, e699530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julia C, Wissuwa M, Kretzschmar T, Jeong K, Rose T.. 2016. Phosphorus uptake, partitioning and redistribution during grain filling in rice. Annals of Botany 118, 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai S, Ohkura K, Adu-Gyamfi JJ, Mohapatra PK, Nguyen NT, Saneoka H, Fujita K.. 2007. Depression of sink activity precedes the inhibition of biomass production in tomato plants subjected to potassium deficiency stress. Journal of Experimental Botany 58, 2917–2928. [DOI] [PubMed] [Google Scholar]
- Kant S, Peng MS, Rothstein SJ.. 2011. Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genetics 7, e1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury CK, Brush S, Costich DE, et al. 2022. Crop genetic erosion: understanding and responding to loss of crop diversity. New Phytologist 233, 84–118. [DOI] [PubMed] [Google Scholar]
- Khush GS. 1999. Green revolution: preparing for the 21st century. Genome 42, 646–655. [PubMed] [Google Scholar]
- Kim AL, Yun YJ, Choi HW, Hong CH, Shim HJ, Lee JH, Kim YC.. 2022. Profiling cannabinoid contents and expression levels of corresponding biosynthetic genes in commercial cannabis (Cannabis sativa L.) cultivars. Plants 11, 3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk I, Pellino M, Rigault P, et al. 2020. The genomics of Cannabis and its close relatives. Annual Review of Plant Biology 71, 713–739. [DOI] [PubMed] [Google Scholar]
- Lambers H, Barrow NJ.. 2021. The pervasive use of P2O5, K2O, CaO, MgO and other molecules that do not exist in soil or fertiliser bags. New Phytologist 232, 1901–1903. [DOI] [PubMed] [Google Scholar]
- Landi S, Berni R, Capasso G, Hausman JF, Guerriero G, Esposito S.. 2019. Impact of nitrogen nutrition on Cannabis sativa: an update on the current knowledge and future prospects. International Journal of Molecular Sciences 20, 5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty KU, Stout JM, Sullivan MJ, et al. 2019. A physical and genetic map of Cannabis sativa identifies extensive rearrangements at the THC/CBD acid synthase loci. Genome Research 29, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei XG, Weaver JD, Mullaney E, Ullah AH, Azain MJ.. 2013. Phytase, a new life for an ‘old’ enzyme. Annual Review of Animal Biosciences 1, 283–309. [DOI] [PubMed] [Google Scholar]
- Lejay L, Wirth J, Pervent M, Cross JMF, Tillard P, Gojon A.. 2008. Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiology 146, 2036–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ikram M, Xia Y, Li R, Yuan Q, Zhao W, Siddique KHM, Guo P.. 2022. Genome-wide identification and development of InDel markers in tobacco (Nicotiana tabacum L.) using RAD-seq. Physiology and Molecular Biology of Plants 28, 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Heuvelink E, Marcelis LF.. 2015. Quantifying the source-sink balance and carbohydrate content in three tomato cultivars. Frontiers in Plant Science 6, e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhou Y, Shuai P, Wang X, Peng S, Wang F.. 2023. Source–sink balance optimization depends on soil nitrogen condition so as to increase rice yield and N use efficiency. Agronomy 13, 907. [Google Scholar]
- Lin YL, Tsay YF.. 2017. Influence of differing nitrate and nitrogen availability on flowering control in Arabidopsis. Journal of Experimental Botany 68, 2603–2609. [DOI] [PubMed] [Google Scholar]
- Llewellyn D, Golem S, Foley E, Dinka S, Jones AMP, Zheng Y.. 2022. Indoor grown cannabis yield increased proportionally with light intensity, but ultraviolet radiation did not affect yield or cannabinoid content. Frontiers in Plant Science 13, e974018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn D, Golem S, Jones AMP, Zheng Y.. 2023. Foliar symptomology, nutrient content, yield, and secondary metabolite variability of cannabis grown hydroponically with different single-element nutrient deficiencies. Plants 12, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Mackie K.. 2016. An introduction to the endogenous cannabinoid system. Biological Psychiatry 79, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RC, Padgitt-Cobb LK, Garfinkel AR, et al. 2024. Domesticated cannabinoid synthases amid a wild mosaic cannabis pangenome. bioRxiv, 2024.05.21.595196 [Preprint]. [Google Scholar]
- Madhusoodanan J. 2019. Can cannabis go green? Nature 572, S8–S9. [DOI] [PubMed] [Google Scholar]
- Magagnini G, Grassi G, Kotiranta S.. 2018. The effect of light spectrum on the morphology and cannabinoid content of Cannabis sativa L. Medical Cannabis and Cannabinoids 1, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massuela DC, Munz S, Hartung J, Nkebiwe PM, Graeff-Honninger S.. 2023. Cannabis hunger games: nutrient stress induction in flowering stage – impact of organic and mineral fertilizer levels on biomass, cannabidiol (CBD) yield and nutrient use efficiency. Frontiers in Plant Science 14, e1233232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda R, Nakano A, Ahn D-H, Suzuki K, Yasuba K-I, Takaichi M.. 2011. Growth characteristic and sink strength of fruit at different CO2 concentrations in a Japanese and a Dutch tomato cultivar. Scientia Horticulturae 127, 528–534. [Google Scholar]
- McClain AM, Sharkey TD.. 2019. Triose phosphate utilization and beyond: from photosynthesis to end product synthesis. Journal of Experimental Botany 70, 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan KJ, Helbert Y, Kane LT, et al. 2020. Sequence and annotation of 42 Cannabis genomes reveals extensive copy number variation in cannabinoid synthesis and pathogen resistance genes. bioRxiv, 2020.01.03.894428 [Preprint]. [Google Scholar]
- Mediavilla V, Jonquera M, Schmid-Slembrouck I, Soldati A.. 1998. Decimal code for growth stages of hemp (Cannabis sativa L.). Journal of the International Hemp Association 5, 68–74. [Google Scholar]
- Montoya Z, Conroy M, Vanden Heuvel BD, Pauli CS, Park SH.. 2020. Cannabis contaminants limit pharmacological use of cannabidiol. Frontiers in Pharmacology 11, e571832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende R, Bari R, Gibon Y, et al. 2007. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant, Cell and Environment 30, 85–112. [DOI] [PubMed] [Google Scholar]
- Mostafaei Dehnavi M, Ebadi A, Peirovi A, Taylor G, Salami SA.. 2022. THC and CBD fingerprinting of an elite cannabis collection from Iran: quantifying diversity to underpin future cannabis breeding. Plants 11, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim-Feil E, Pembleton LW, Spooner LE, Malthouse AL, Miner A, Quinn M, Polotnianka RM, Baillie RC, Spangenberg GC, Cogan NOI.. 2021. The characterization of key physiological traits of medicinal cannabis (Cannabis sativa L.) as a tool for precision breeding. BMC Plant Biology 21, e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C, Mateo-Elizalde C, Mohan TC, et al. 2021. Arsenite provides a selective signal that coordinates arsenate uptake and detoxification through the regulation of PHR1 stability in Arabidopsis. Molecular Plant 14, 1489–1507. [DOI] [PubMed] [Google Scholar]
- Nord EA, Lynch JP.. 2008. Delayed reproduction in Arabidopsis thaliana improves fitness in soil with suboptimal phosphorus availability. Plant, Cell and Environment 31, 1432–1441. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Vega A, Bouguyon E, Krouk G, Gojon A, Coruzzi G, Gutierrez RA.. 2016. Nitrate transport, sensing and responses in plants. Molecular Plant 9, 837–856. [DOI] [PubMed] [Google Scholar]
- Olas JJ, Van Dingenen J, Abel C, Dzialo MA, Feil R, Krapp A, Schlereth A, Wahl V.. 2019. Nitrate acts at the Arabidopsis thaliana shoot apical meristem to regulate flowering time. New Phytologist 223, 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo AZ, Asai H, Win KT, Marui J, Saito H.. 2023. Seed phytic acid concentration affects rice seedling vigor irrespective of soil phosphorus bioavailability. Physiologia Plantarum 175, e13913. [DOI] [PubMed] [Google Scholar]
- Osorio S, Ruan YL, Fernie AR.. 2014. An update on source-to-sink carbon partitioning in tomato. Frontiers in Plant Science 5, e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peret B, Clement M, Nussaume L, Desnos T.. 2011. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science 16, 442–450. [DOI] [PubMed] [Google Scholar]
- Peterswald TJ, Mieog JC, Azman Halimi R, Magner NJ, Trebilco A, Kretzschmar T, Purdy SJ.. 2023. Moving away from 12:12; the effect of different photoperiods on biomass yield and cannabinoids in medicinal cannabis. Plants 12, 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten JD, Cobb JN, Zantua RE.. 2019. Criteria for evaluating molecular markers: Comprehensive quality metrics to improve marker-assisted selection. PLoS One 14, e0210529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poza-Carrion C, Paz-Ares J.. 2019. When nitrate and phosphate sensors meet. Nature Plants 5, 339–340. [DOI] [PubMed] [Google Scholar]
- Prodhan MA, Finnegan PM, Lambers H.. 2019. How does evolution in phosphorus-impoverished landscapes impact plant nitrogen and sulfur assimilation? Trends in Plant Science 24, 69–82. [DOI] [PubMed] [Google Scholar]
- Punja ZK. 2021. Emerging diseases of Cannabis sativa and sustainable management. Pest Management Science 77, 3857–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Wang X, Smith P, et al. 2022. Soil quality both increases crop production and improves resilience to climate change. Nature Climate Change 12, 574–580. [Google Scholar]
- Razzaque MS. 2011. Phosphate toxicity: new insights into an old problem. Clinical Science 120, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel P, Munz S, Hartung J, Kotiranta S, Graeff-Honninger S.. 2022. Impacts of different light spectra on CBD, CBDA and terpene concentrations in relation to the flower positions of different Cannabis sativa L. strains. Plants 11, 2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann-Philipp U, Speck M, Orser C, Johnson S, Hilyard A, Turner H, Stokes AJ, Small-Howard AL.. 2020. Cannabis chemovar nomenclature misrepresents chemical and genetic diversity; survey of variations in chemical profiles and genetic markers in Nevada medical cannabis samples. Cannabis and Cannabinoid Research 5, 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Zhang X, Li Y, et al. 2021. Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa. Science Advances 7, eabg2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z, Cakmak I, White PJ (eds). 2022. Marschner’s mineral nutrition of plants. Amsterdam: Academic Press. [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, et al. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil 349, 121–156. [Google Scholar]
- Rodriguez-Morrison V, Llewellyn D, Zheng Y.. 2021. Cannabis yield, potency, and leaf photosynthesis respond differently to increasing light levels in an indoor environment. Frontiers in Plant Science 12, e646020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner A, Bernstein N.. 2020. Response of medical cannabis (Cannabis sativa L.) to nitrogen supply under long photoperiod. Frontiers in Plant Science 11, e572293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner A, Bernstein N.. 2021. Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Industrial Crops and Products 167, 113516. [Google Scholar]
- Saloner A, Bernstein N.. 2022. Nitrogen source matters: High NH4/NO3 ratio reduces cannabinoids, terpenoids, and yield in medical cannabis. Frontiers in Plant Science 13, e830224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner A, Bernstein N.. 2023. Dynamics of mineral uptake and plant function during development of drug-type medical cannabis plants. Agronomy 13, 2865. [Google Scholar]
- Saloner A, Sacks MM, Bernstein N.. 2019. Response of medical cannabis (Cannabis sativa L.) genotypes to K supply under long photoperiod. Frontiers in Plant Science 10, e1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan-Delmás D, Josa A, Muñoz P, Gassó S, Rieradevall J, Gabarrell X.. 2020. Applying nutrient dynamics to adjust the nutrient-water balance in hydroponic crops. A case study with open hydroponic tomato crops from Barcelona. Scientia Horticulturae 261, 108908. [Google Scholar]
- Sawler J, Stout JM, Gardner KM, Hudson D, Vidmar J, Butler L, Page JE, Myles S.. 2015. The genetic structure of marijuana and hemp. PLoS One 10, e0133292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N, Semel Y, Roessner U, et al. 2006. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nature Biotechnology 24, 447–454. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Zinkernagel J.. 2021. Opportunities of reduced nitrogen supply for productivity, taste, valuable compounds and storage life of cocktail tomato. Horticulturae 7, 48. [Google Scholar]
- Shane MW, Szota C, Lambers H.. 2004. A root trait accounting for the extreme phosphorus sensitivity of Hakea prostrata (Proteaceae). Plant, Cell and Environment 27, 991–1004. [Google Scholar]
- Shilpha J, Song J, Jeong BR.. 2023. Ammonium phytotoxicity and tolerance: An insight into ammonium nutrition to improve crop productivity. Agronomy 13, 1487. [Google Scholar]
- Shiponi S, Bernstein N.. 2021a. The highs and lows of P supply in medical cannabis: Effects on cannabinoids, the ionome, and morpho-physiology. Frontiers in Plant Science 12, e657323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiponi S, Bernstein N.. 2021b. Response of medical cannabis (Cannabis sativa L.) genotypes to P supply under long photoperiod: functional phenotyping and the ionome. Industrial Crops and Products 161, 113154. [Google Scholar]
- Shukla D, Rinehart CA, Sahi SV.. 2017. Comprehensive study of excess phosphate response reveals ethylene mediated signaling that negatively regulates plant growth and development. Scientific Reports 7, e3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E. 2015. Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. The Botanical Review 81, 189–294. [Google Scholar]
- Smith MR, Rao IM, Merchant A.. 2018. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Frontiers in Plant Science 9, e1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Saloner A, Fait A, Bernstein N.. 2023. Nitrogen deficiency stimulates cannabinoid biosynthesis in medical cannabis plants by inducing a metabolic shift towards production of low-N metabolites. Industrial Crops and Products 202, 116969. [Google Scholar]
- Stack GM, Carlson CH, Toth JA, Philippe G, Crawford JL, Hansen JL, Viands DR, Rose JKC, Smart LB.. 2023. Correlations among morphological and biochemical traits in high-cannabidiol hemp (Cannabis sativa L.). Plant Direct 7, e503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel L, Welling M, Ristevski N, Johnson K, Gendall A.. 2023. Comparative genomics of flowering behavior in Cannabis sativa. Frontiers in Plant Science 14, e1227898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struik PC, Amaducci S, Bullard MJ, Stutterheim NC, Venturi G, Cromack HTH.. 2000. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Industrial Crops and Products 11, 107–118. [Google Scholar]
- Suman J, Rakshit A, Ogireddy SD, Singh S, Gupta C, Chandrakala J.. 2022. Microbiome as a key player in sustainable agriculture and human health. Frontiers in Soil Science 2, e821589. [Google Scholar]
- Sung J, Lee S, Lee Y, Ha S, Song B, Kim T, Waters BM, Krishnan HB.. 2015. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Science 241, 55–64. [DOI] [PubMed] [Google Scholar]
- Tang K, Fracasso A, Struik PC, Yin X, Amaducci S.. 2018. Water- and nitrogen-use efficiencies of hemp (Cannabis sativa L.) based on whole-canopy measurements and modeling. Frontiers in Plant Science 9, e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Struik PC, Amaducci S, Stomph T-J, Yin X.. 2017. Hemp (Cannabis sativa L.) leaf photosynthesis in relation to nitrogen content and temperature: implications for hemp as a bio-economically sustainable crop. GCB Bioenergy 9, 1573–1587. [Google Scholar]
- Tegeder M, Masclaux-Daubresse C.. 2018. Source and sink mechanisms of nitrogen transport and use. New Phytologist 217, 35–53. [DOI] [PubMed] [Google Scholar]
- Testa G, Corinzia SA, Cosentino SL, Ciaramella BR.. 2023. Phytoremediation of cadmium-, lead-, and nickel-polluted soils by industrial hemp. Agronomy 13, 995. [Google Scholar]
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S.. 2002. Agricultural sustainability and intensive production practices. Nature 418, 671–677. [DOI] [PubMed] [Google Scholar]
- Tiwari JK, Devi S, Ali N, Buckseth T, Moudgil V, Singh RK, Chakrabarti SK, Dua VK, Kumar D, Kumar M.. 2017. Genomics approaches for improving nitrogen use efficiency in potato. In: Kumar Chakrabarti S, Xie C, Kumar Tiwari J, eds. The potato genome. Compendium of plant genomes. Cham: Springer, 171–193.
- Toth JA, Stack GM, Cala AR, et al. 2020. Development and validation of genetic markers for sex and cannabinoid chemotype in Cannabis sativa L. GCB Bioenergy 12, 213–222. [Google Scholar]
- Truffault V, Marlene R, Brajeul E, Vercambre G, Gautier H.. 2019. To stop nitrogen overdose in soilless tomato crop: a way to promote fruit quality without affecting fruit yield. Agronomy 9, e80. [Google Scholar]
- Vallarino JG, Kubiszewski-Jakubiak S, Ruf S, et al. 2020. Multi-gene metabolic engineering of tomato plants results in increased fruit yield up to 23%. Scientific Reports 10, e17219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL.. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157, 423–447. [DOI] [PubMed] [Google Scholar]
- van de Wiel CCM, van der Linden CG, Scholten OE.. 2015. Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica 207, 1–22. [Google Scholar]
- Velechovsky J, Malik M, Senkyrik JB, Tlustos P.. 2024. Effect of augmented nutrient composition and fertigation system on biomass yield and cannabinoid content of medicinal cannabis (Cannabis sativa L.) cultivation. Frontiers in Plant Science 15, e1322824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneklaas EJ, Lambers H, Bragg J, et al. 2012. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytologist 195, 306–320. [DOI] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M.. 2013. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339, 704–707. [DOI] [PubMed] [Google Scholar]
- Wartenberg AC, Holden PA, Bodwitch H, et al. 2021. Cannabis and the environment: what science tells us and what we still need to know. Environmental Science & Technology Letters 8, 98–107. [Google Scholar]
- Watanabe M, Hubberten H-M, Saito K, Hoefgen R.. 2010. General regulatory patterns of plant mineral nutrient depletion as revealed by serat quadruple mutants disturbed in cysteine synthesis. Molecular Plant 3, 438–466. [DOI] [PubMed] [Google Scholar]
- Wei X, Zhou W, Long S, Guo Y, Qiu C, Zhao X, Wang Y.. 2023. Effects of different N, P, and K rates on the growth and cannabinoid content of industrial hemp. Journal of Natural Fibers 20, e2159605. [Google Scholar]
- Weiblen GD, Wenger JP, Craft KJ, ElSohly MA, Mehmedic Z, Treiber EL, Marks MD.. 2015. Gene duplication and divergence affecting drug content in Cannabis sativa. New Phytologist 208, 1241–1250. [DOI] [PubMed] [Google Scholar]
- Weinert CH, Sonntag F, Egert B, Pawelzik E, Kulling SE, Smit I.. 2021. The effect of potassium fertilization on the metabolite profile of tomato fruit (Solanum lycopersicum L.). Plant Physiology and Biochemistry 159, 89–99. [DOI] [PubMed] [Google Scholar]
- Welling MT, Deseo MA, Bacic A, Doblin MS.. 2022. Biosynthetic origins of unusual cannabimimetic phytocannabinoids in Cannabis sativa L: a review. Phytochemistry 201, 113282. [DOI] [PubMed] [Google Scholar]
- Welling MT, Liu L, Hazekamp A, Dowell A, King GJ.. 2019. Developing robust standardised analytical procedures for cannabinoid quantification: Laying the foundations for an emerging Cannabis-based pharmaceutical industry. Medical Cannabis and Cannabinoids 2, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland FM, Bugbee B.. 2022. Sustainable Cannabis nutrition: Elevated root-zone phosphorus significantly increases leachate P and does not improve yield or quality. Frontiers in Plant Science 13, e1015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissuwa M, Gamat G, Ismail AM.. 2005. Is root growth under phosphorus deficiency affected by source or sink limitations? Journal of Experimental Botany 56, 1943–1950. [DOI] [PubMed] [Google Scholar]
- Wylie SE, Ristvey AG, Fiorellino NM.. 2020. Fertility management for industrial hemp production: Current knowledge and future research needs. GCB Bioenergy 13, 517–524. [Google Scholar]
- Yeoh H-H, Wee Y-C.. 1994. Leaf protein contents and nitrogen-to-protein conversion factors for 90 plant species. Food Chemistry 49, 245–250. [Google Scholar]
- Yep B, Gale NV, Zheng Y.. 2020. Aquaponic and hydroponic solutions modulate NaCl-induced stress in drug-type Cannabis sativa L. Frontiers in Plant Science 11, e1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chen X, Guo H, Trindade LM, Salentijn EMJ, Guo R, Guo M, Xu Y, Yang M.. 2018. Latitudinal adaptation and genetic insights into the origins of Cannabis sativa L. Frontiers in Plant Science 9, e1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu B, Kong F, Chen L.. 2023. Nutrient-mediated modulation of flowering time. Frontiers in Plant Science 14, e1101611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liao H, Lucas WJ.. 2014. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. Journal of Integrative Plant Biology 56, 192–220. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhong Z, Xiong Y.. 2023. Sailing in complex nutrient signaling networks: where I am, where to go, and how to go? Molecular Plant 16, 1635–1660. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Fiddes K, Yang L.. 2021. A narrative review on environmental impacts of cannabis cultivation. Journal of Cannabis Research 3, e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsögön A, Cermak T, Naves ER, Notini MM, Edel KH, Weinl S, Freschi L, Voytas DF, Kudla J, Peres LEP.. 2018. De novo domestication of wild tomato using genome editing. Nature Biotechnology 36, 1211–1216. [DOI] [PubMed] [Google Scholar]