Abstract
Medicinal plants are integral to traditional medicine systems worldwide, being pivotal for human health. Harvesting plant material from natural environments, however, has led to species scarcity, prompting action to develop cultivation solutions that also aid conservation efforts. Biotechnological tools, specifically plant tissue culture and genetic transformation, offer solutions for sustainable, large-scale production and enhanced yield of valuable biomolecules. While these techniques are instrumental to the development of the medicinal plant industry, the challenge of inherent regeneration recalcitrance in some species to in vitro cultivation hampers these efforts. This review examines the strategies for overcoming recalcitrance in medicinal plants using a holistic approach, emphasizing the meticulous choice of explants (e.g. embryonic/meristematic tissues), plant growth regulators (e.g. synthetic cytokinins), and use of novel regeneration-enabling methods to deliver morphogenic genes (e.g. GRF/GIF chimeras and nanoparticles), which have been shown to contribute to overcoming recalcitrance barriers in agriculture crops. Furthermore, it highlights the benefit of cost-effective genomic technologies that enable precise genome editing and the value of integrating data-driven models to address genotype-specific challenges in medicinal plant research. These advances mark a progressive step towards a future where medicinal plant cultivation is not only more efficient and predictable but also inherently sustainable, ensuring the continued availability and exploitation of these important plants for current and future generations.
Keywords: Explants, medicinal plants, morphogenic genes, nanoparticles, plant growth regulators, recalcitrance, regeneration, transformation
We review strategies to overcome transformation and regeneration recalcitrance in medicinal plants through explant choice, plant growth regulators, and use of novel techniques such as application of morphogenic genes and nanoparticles.
Introduction
Among the breadth and diversity of plant species, medicinal plants have held a significant place in human health and culture since ancient times. The World Health Organization estimates that two-thirds of the global population relies on plant medicines for primary healthcare. A quarter of newly developed drugs sold worldwide are based on molecules derived from plants (Calixto, 2019). With >35 000 identified medicinal plant species, the repertoire of biomolecules of benefit to humankind is yet to be mined to its full potential. The global herbal medicine market compound annual growth rate (CAGR) is estimated to be ~11% over the 2022–2030 period and is expected to be worth ~US$348 billion by 2030 (www.databridgemarketresearch.com). By 2050, the market value is projected to reach US$5 trillion, with China and India to dominate the herb trading market (Booker et al., 2012). Such demand in countries where self-medication through sourcing from the natural environment is current practice has put preservation of native medicinal plant species under significant pressure. As the universal interest in plant-based medicines continues to expand, there is a growing need to generate sufficient supply of medicinal plants and preserve their native populations.
Increasingly, biotechnological approaches are being utilized to satisfy this growing demand. Since the 1990s, plant tissue culture and genetic transformation have been the enabling technologies for crop improvement, and promise to fulfil the same role in medicinal species (Canter et al., 2005). In vitro cultivation enables large-scale multiplication of plant tissue and thus yield of desirable biomolecules. Growth in a controlled environment, encompassing both the medium and external conditions, delivers a more consistent product per cropping cycle, improving market value, and provides a platform for germplasm preservation. Tissue culture (TC) medium normally contains plant growth regulators (PGRs) that, when dosed, can induce plants to generate high numbers of multiplication units. Under controlled conditions, micropropagation enables growth at scale. Unfortunately, not all species can acclimate to in vitro culture conditions, and this inability to grow and be propagated in tissue culture is called TC recalcitrance (Benson, 2000).
In most species, TC methods are also essential to the success of genetic transformation, the process whereby a beneficial segment of DNA is transferred into the plant’s genome (Yildiz, 2012). Genetic transformation offers an ability to amplify biomolecule yields to much higher levels that are well beyond what can be achieved through traditional breeding methods (Pandey et al., 2010). After DNA integration, transformed cells require cues to initiate morphological reprogramming to produce an entire plantlet. Typically, PGRs in the medium stimulate this process. Cellular receptivity to transformation and regeneration is often species or even genotype specific. The inability of a plant to incorporate foreign DNA into its genome is termed transformation recalcitrance, and the failure to form tissue, typically shoots or embryos, post-transformation is defined as regeneration recalcitrance (Fig. 1). Discovery and characterization of the genes and pathways involved in the process of plant morphogenesis, their interplay with phytohormones, and an understanding of the events following a wound response have paved the way towards providing a set of molecular tools to circumvent plant recalcitrance. Multiple recalcitrant crop species have benefited from the controlled expression of morphogenic genes (MGs) to stimulate the regeneration of transgenic plants. Although still in its infancy, the use of MGs to combat regeneration recalcitrance in medicinal species has shown the potential to be broadly applicable (Zhang et al., 2021). In this review, we describe the benefits to TC and transformation and the three main approaches that have been used to overcome regeneration recalcitrance in medicinal plants to date: (i) selection of explants with innate ability to regenerate; (ii) addition of PGRs in TC media; and (iii) the use of enabling technologies involving MGs.
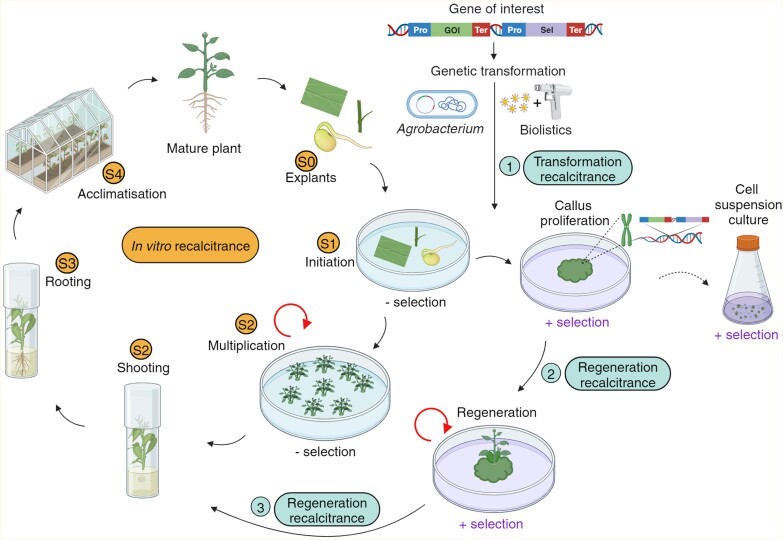
Fig. 1.
A schematic of the plant tissue culture and transformation process. Left: a typical plant tissue culture/micropropagation cycle is represented with its different stages: collection of explants from a mature donor plant (Stage 0, S0), sterilization and initiation of explants on shoot proliferation medium (Stage 1, S1), repeated shoot multiplication and elongation (Stage 2-S2), rooting (Stage 3, S3), and acclimatization to ex vitro growing conditions (Stage 4, S4). The circular arrow at the multiplication stage represents the iteration of in vitro multiplication cycles for large-scale production of TC plantlets. In vitro recalcitrance can occur at any stage (S1–S4). Right: genetic transformation of a plant species involves transferring a piece of DNA, such as a gene(s) of interest (GOI), with a selectable marker (Sel), into cells within the explants either through co-cultivation with Agrobacterium cells carrying the transformation vector or directly through biolistics. The transformed explants are proliferated on a callus initiation medium with selection pressure (+selection) to select only those cells which have the GOI integrated into their genome. The proliferated calli are then transferred to regeneration medium for embryo development and shoot regeneration with continued selection pressure. The regenerated plantlets enter the usual micropropagation stages (S2–S4). The circular arrow at the regeneration stage represents the continued multiplication of transformed plants. Alternatively, transformed calli can be used to initiate sterile cell suspension cultures, bypassing the need to generate a transgenic plant. In vitro recalcitrance can be encountered during genetic transformation (1) or through the process of transgenic plant regeneration at callus proliferation, embryo development, and/or plantlet growth stages (2, 3).
Plant tissue culture: an important biotechnological tool
Plant tissues can be preserved aseptically in vitro through TC practices. Although TC requires specialized facilities, equipment, and trained labour, its major advantages over traditional propagation methods include unparalleled scalability free of seasonal constraints within a smaller physical and environmental (e.g. water, fertilizer) footprint, pest-free plants upon release to the glasshouse or field, and is economical in terms of daily maintenance costs. Another benefit is that elite germplasm from heterogenous and outcrossing species can be maintained without the need to pass through fertilization and a seed stage (Kenta et al., 2016). The same advantage is applicable to annuals and tree species alike. For the latter, TC provides maintenance conditions without the development of secondary growth, often considered detrimental to in vitro life. In some species and genotypes, the constant exposure to TC conditions can lead to somaclonal variations, genetic and/or epigenetic, that are perpetuated in the culture and can lead to loss of valuable traits in TC-maintained lines (Kenta et al., 2016). However, in some species such as strawberry, somaclonal variation is a deliberate strategy to gain novel traits that can provide tools for crop improvement (Krishna et al., 2016).
In contrast to well-established TC protocols for agricultural crops, medicinal plants often lack standardized procedures, as is the case for Frangula purshiana, Arctostaphylos uva-ursi, Physostigma venenosum, Strychnos nux-vomica, and Ochrosia elliptica, all dicotyledonous species (Chaturvedi et al., 2007). The wide range of species diversity, genetic variability, complex secondary metabolite mixtures which influence growth and development, the limited research and resources, and in some cases regulatory challenges often hinder the TC progress in medicinal plants. The stages of in vitro plant culture are summarized in Fig. 1. Establishing an in vitro culture requires that plant tissue(s) are sterilized and placed in media within enclosed vessels. Ideally, TC explants free of embryonic/meristematic cells can produce entire plants under the right media and growth conditions. This remarkable developmental plasticity, which naturally facilitates a species’ survival and reproduction success under various natural biotic and abiotic pressures, has enabled the fundamental elements of TC to be developed. This potential of a cell to change its cellular identity into any other cell type has been termed cellular totipotency (Condic, 2014). Under the right PGR cues, differentiated somatic cells can re-enact embryonic developmental pathways, a process termed somatic embryogenesis (SE) (Fig. 2). This regeneration capacity of plant species has long been exploited for vegetative plant propagation and biotechnology endeavours (Fehér, 2019). However, the ease of establishment of a plant species in TC is inexplicably variable, with the majority of medicinal plant species lying towards the recalcitrant end of the spectrum, as opposed to being highly regenerative.
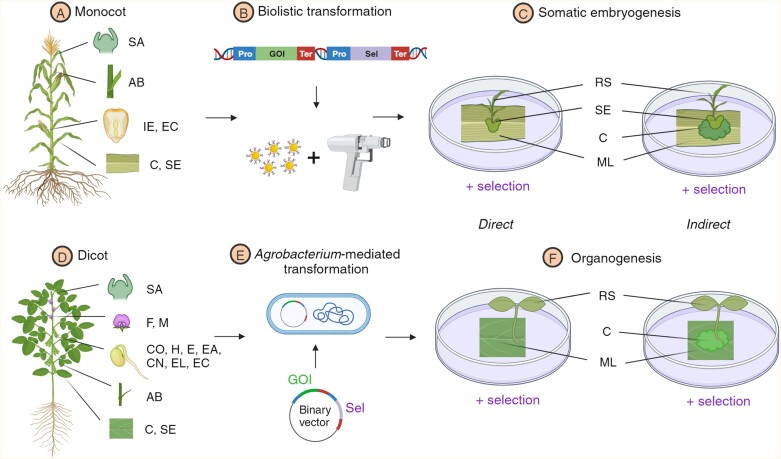
Fig. 2.
Tissue culture and genetic transformation differences between monocots and dicots. (A) Preferred choice of explants in monocots are: shoot apex (SA), axillary bud (AB), immature embryo (IE), embryogenic callus (EC), callus (C), and somatic embryo (SE). (B) Biolistic transformation is more frequently used to transform monocot species where a piece of linearized DNA containing a gene(s) of interest (GOI) and one of a limited set of selectable markers (Sel) are coated onto microparticles that are delivered into the explant through high velocity bombardment. (C) Direct or indirect somatic embryogenesis is the predominant regeneration pathway in monocot post-transformation. (D) Preferred choice of explants in dicots are: SA, flowers (F), microspores (M), cotyledons (CO), hypocotyls (H), epicotyls (E), embryonic axis (EA), cotyledonary nodes (CN), embryonic leaflets (EL), EC, AB, C, and SE. (E) Agrobacterium-mediated transformation of GOI with one of a broader range of selectable markers in a transformation vector. (F) Dicots exhibit regenerable callus formation from many types of explants due to direct or indirect organogenesis, the products of which can be readily regenerated into entire plants. Regenerated shoot (RS), somatic embryo (SE), callus (C), and mature leaf (ML).
Genetic transformation: enabling plant improvement
While TC practices and techniques facilitate the generation of high volumes of genotype-specific clones, they also provide tissue for genetic modification purposes. To deliver the desired piece of DNA into plant cells, traditional transformation methodologies use either physical means (particle bombardment or biolistics) or Agrobacterium sp., a bacterium which naturally transfers a DNA segment (transfer or T-DNA) across the plant cell membrane (Fig. 1). Under a selective agent(s) (antibiotic or herbicide), non-transgenic cells are eliminated and, with the appropriate external stimuli in the media, the genetically modified cells can regenerate (Fig. 1). The ability to genetically modify medicinal plant species is not only important to introduce novel traits or modify existing ones, but is also a scientific tool for the purposes of dissecting the molecular basis for the production and regulation of specific biomolecules, for example knowledge which can refine subsequent efforts to enhance their yield. Whilst tissue culture and transformation protocols have been successfully developed in several agriculturally important crops, efforts in medicinal species have been limited (Gómez-Galera et al., 2007). For example, Aloe vera, Ginkgo biloba, and Garcinia indica with well-defined regeneration systems do not have a transformation protocol or may become less regenerative after genetic transformation, as is the case with Plumbago zeylanica and Euphorbia nivulia (Pandey et al., 2010). Regeneration-recalcitrant species have limitations with respect to the bioengineering applications that can be implemented for the improvement of agromorphological traits and the alteration of their beneficial biomolecule profiles.
In contrast to crop species that have benefited from the concerted knowledge of many years of breeding, medicinal plants generally have a highly heterozygous genome often exacerbated by being obligate outcrossing species. Until the recent application of MG expression, Coker was the sole Gossypium hirsutum (cotton) cultivar amenable to transformation (Juturu et al., 2015). Similarly, in Cannabis sativa (cannabis), regeneration from calli has been shown to be highly cultivar dependent (Zhang et al., 2021). In this study, transgenic plants were produced in only one cultivar of 100 tested, a result achieved with the combined use of MGs and explants with high potential for totipotency stimulated with exogenous application of potent synthetic phytohormones (Zhang et al., 2021). These examples demonstrate the complexities encountered in recalcitrant species and the diverse approaches required to enable genetic modification and regenerability.
Modern molecular technologies that have been widely applied to agriculture crops are yet to be routinely used in most medicinal plants. These species would benefit from leveraging large-scale sequencing methodologies that have seen dramatic cost reductions in the last decade to provide (pan)genome information, spatiotemporal tissue transcriptome datasets, ideally at cellular resolution, together with an understanding of the epigenome. This information will significantly facilitate bioengineering of medicinal plants, offering, for example, markers for trait selection via traditional breeding approaches and the ability to use gene editing tools such as CRISPR (clustered regularly interspaced short palindromic repeats) effectively by enabling accurate design of guide RNAs (gRNAs) that are target specific and avoid off-target edits (Yang et al., 2021).
Choice of explants
Successful standardization of in vitro regeneration protocols depends on the health and accessibility of tissues from a plant donor (S0, Fig. 1). Exploring TC capabilities in a medicinal plant demands consideration of the specific requirements of the taxonomic group to which the species belongs during explant selection and regeneration. For example, gymnosperm medicinal tree species often exhibit varied responses to TC due to a ‘phase change’ (shift from juvenile to adult state) that results in a significant loss of vegetative propagation capacity, reducing the ability of tissues to regenerate in vitro (Pereira et al., 2021). In such cases, production of SEs using juvenile embryo organs has emerged as a preferred regeneration method, maintaining explant juvenility and ensuring a high regeneration rate. Similarly, distinct morphological and developmental differences between monocots and dicots significantly influences their TC responses, the favoured transformation method, and ultimately regeneration efficiency. Monocots provide a limited range of explant types that commonly regenerate through either direct or indirect SE (Fig. 2). In contrast, regeneration in dicots can occur through direct or indirect organogenesis in a wider range of explant types which are readily regenerated into plants. Many medicinal dicots are amenable to TC, and a diverse array of regeneration approaches have been successfully applied compared with monocots, as exemplified by the examples listed in Table 1.
Table 1.
Selected examples showing the different factors affecting the regeneration recalcitrance of medicinal and other plant species
| Plant species | Explant type(s), (age), and regeneration pathway | Media+PGRsa | Additivesb | Outcome | Reference | |
|---|---|---|---|---|---|---|
| Silver compound | Others | |||||
|
Eurycoma longifolia
(Tongkat ali) |
Cotyledonary node (14 d) Direct organogenesis |
MS+1 mg l–1 BAP | – | 3% sucrose +0.25% gelrite |
Highest frequency of multiple shoot induction (76.7%), max. shoot no. (4.87 ± 0.70) per explant observed in BAP cf. KIN or TDZ | Alttaher et al. (2020) |
|
Gossypium hirsutum
(cotton) |
Cotyledonary node (14 d) Direct organogenesis |
MS+B5 vitamins+2.5 mg l–1 BAP | 2 mg l–1 AgNO3 | 3% sucrose +0.8% agar |
Max. no. of shoots (22.2) per explant achieved with AgNO3 alone without any PGRs | Prem Kumar et al. (2016) |
|
Lallemantia iberica
(Dragon’s head plant) |
Cotyledonary node, cotyledons, and hypocotyl (21 d) Direct organogenesis |
MS+1 mg l–1 BAP+0.05 mg l–1 NAA | – | – | Plant regeneration from all explants with a maximum no. (23 ± 3.60) observed from the cotyledonary nodes cf. other explants | Ebrahimzadegan and Maroufi (2022) |
|
Mucuna pruriens
(velvet bean) |
Cotyledonary node (7 d) Direct organogenesis |
MS+2.5 µM BAP | – | 3% sucrose+ 0.8% agar+10.0 µM putrescine +3% PVP |
Max. no. of shoots (22.8) and shoot length (6.0 cm) per explant observed in BAP+putrescine cf. other polyamines, e.g. spermine, spermidine | Alam et al. (2023) |
|
Ocimum basilicum
(sweet basil) |
Cotyledonary nodes and leaves (28 d) Cotyledons and hypocotyls (14 d) Direct organogenesis |
Cotyledonary nodes, cotyledons, and hypocotyls: MS+4 mg l–1 TDZ Leaves: MS+1 mg l–1 BAP |
– | 0.8% agar+ 10 mg l–1 citric acid+100 mg l–1 ascorbic acid (leaves and hypocotyls) |
Regeneration from all explants with highest frequency from cotyledonary nodes (2.6 shoots per explant) cf. other explants | Barberini et al. (2023) |
|
Prosopis cineraria
(spunge tree) |
Cotyledonary node (5 d) Direct organogenesis |
Shoot bud initiation: MS+2.22 μM BAP Shoot bud multiplication: MS+2.22 μM BAP+0.46 μM KIN |
0.59 μM AgNO3 | 3% sucrose +0.8% agar |
Max. multiple shoot bud regeneration (95.6%) observed in BAP+KIN+AgNO3 | Venkatachalam et al. (2017) |
|
Salvia plebeian
(common sage) |
Cotyledonary nodes and shoot tips (16 d) Direct organogenesis Hypocotyls (16 d) Indirect organogenesis |
Cotyledonary nodes: MS+1 mg l–1 TDZ+0.1 mg l–1 IAA Hypocotyls: MS+1 mg l–1 TDZ+0.1 mg l–1 IAA Shoot tips: MS+1 mg l–1 BAP+0.1 mg l–1 IAA |
– | 3% sucrose +0.6% agar |
Max. no. of shoots (37.5 ± 1.3) per explant observed in cotyledonary nodes in TDZ+IAA cf. BAP+IAA. Highest no. of globular bodies (17.4) per hypocotyl in TDZ+IAA cf. BAP+IAA |
Wu et al. (2022) |
|
Vigna mungo
(black gram) |
Cotyledonary nodes and shoot tips (3–5 d) Direct organogenesis |
MS+B5 vitamins+1 mg l–1 BAP+0.1 mg l–1 TDZ | 1 mg l–1 AgNO3 | 3% sucrose +0.8% agar+15 mg l–1 ADS |
Higher no. of shoots/explants in cotyledonary nodes obtained when BAP+TDZ used in combination with ADS+AgNO3 | Mookkan and Andy (2014) |
|
Withania somnifera
(Ashwagandha) |
Cotyledonary node (7 d) Direct organogenesis |
MS+2.5 µM BAP+0.5 µM NAA | – | 3% sucrose +0.8% agar |
90% regeneration frequency with highest number of shoots (29.3 ± 0.23) per explant and shoot length (5.62 ± 0.17 cm) in BAP+NAA cf. KIN+NAA and 2iP+NAA | Fatima and Anis (2021) |
|
Brassica rapa L. ssp. Pekinensis (Chinese cabbage) |
Cotyledon-petioles, hypocotyls, and roots Direct organogenesis |
MS+0.5 mg l–1 NAA+4 mg l–1 BAP | 4 mg l–1 AgNO3 | 3% sucrose +0.7% agar |
Higher differentiation rate (81.15%) in cotyledon-petioles cf. other explants, highest adventitious bud differentiation rate (79.2%) in BAP | Li et al. (2021) |
|
Cosmos bipinnatus
(garden cosmos) |
Cotyledons (7 d) Direct organogenesis |
MS+5 mg l–1 BAP | 5 mg l–1 AgNO3 | 3% sucrose +0.8% agar+40 mg l–1 ADS |
Highest shoot number (5.7) per explant induced on BAP+AgNO3+ADS | Jaberi et al. (2018) |
|
Eruca sativa
(rocket) |
Cotyledons, hypocotyls, and roots (5 d) Direct organogenesis |
MS+1 mg l–1 TDZ+0.1 mg l–1 NAA | 5 mg–1 AgNO3 | 2% sucrose+ 0.64% agar |
Enhanced shoot regeneration (25.38%) from hypocotyls in TDZ+NAA+AgNO3 cf. 2,4-D and BAP+NAA combinations | Banjac et al. (2023) |
|
Ficus religiosa
(sacred fig) |
Hypocotyls (8–10 d) Direct and indirect organogenesis |
Callusing: MS+0.5 mg l–1 2,4-D+0.05 mg l–1 BAP (dark) Direct and indirect regeneration: MS+1.5 mg l–1 BAP+0.15 mg l–1 IBA (light) |
– | 3% sucrose+ 0.6% agar |
Highest callus FW (2.43 g) observed in 2,4-D+BAP cf. IBA+BAP Indirect regeneration: highest regeneration frequency (86.66%) and max. shoot no. (4.13) in BAP+IBA cf. TDZ+IBA and KIN+IBA Direct regeneration: highest regeneration frequency (96.66%) and max. shoot number (6.26) in BAP+IBA cf. TDZ+IBA and KIN+IBA |
Hesami and Daneshvar (2018) |
|
Brassica napus
(rapeseed) |
Hypocotyls (5 d) Indirect organogenesis |
Pre-treatment: liquid B5 (for 1 h) Callusing: B5+1 mg l–1 2,4-D+0.1 mg l–1 IAA Regeneration: B5+2 mg l–1 BAP+1 mg l–1 Z |
Shooting medim: 5 mg l–1 AgNO3 | Pre-treatment: 2% sucrose+250 mg l–1 NH4NO3+750 mg l–1 CaCl2·2 H2O+250 mg l–1 xylose Callusing: pre-treatment media+0.6% agar Regeneration:pre-treatment media+0.6% agar |
Increased photosynthetic pigments in callus and improved regeneration efficiency from 0–0.8% to 8.3–10% in three of five varieties tested | Al Ramadan et al. (2021) |
|
Ficus carica
(common fig) |
Leaves from in vitro shoots Indirect organogenesis |
MS+2 mg l–1 IBA+0.5 or 1 mg l–1 TDZ+0.5 mM phloroglucinol (7 d dark followed by light) |
– | 3% sucrose+0.8% agar+3 mM MES, sealing with porous tape | Delayed tissue browning and enhanced shoot regeneration (8.1–10.8 multiple shoots per explant) in IBA+TDZ cf. IBA+BAP, 2,4-D+BAP, 2,4-D+TDZ, NAA+BAP and NAA+TDZ | Kim et al. (2007) |
|
Prunus persica
(peach) |
Leaves with petiole (21 d) Indirect organogenesis |
WPM+15.5 µM BAP | 10 µM STS | 3% sucrose+0.5% agar+210 µM cefotaxime+238 µM carbenicillin | Highest regeneration frequency (53%) and no. of regenerating shoots (0.77 ± 0.08) per leaf were observed in BAP+STS cf. KIN+NAA and TDZ+NAA | Ricci et al. (2020) |
|
Brassica napus
(rapeseed) |
Microspores Direct organogenesis |
Embryo formation: NN-13 (dark) Embryo maturation: B5+0.1 mg l–1 GA3 Regeneration: B5 |
– | Embryo formation: 13% sucrose ABA treatment: 0.5 mg l–1 for 12 h Embryo maturation: 2% sucrose+0.7% agar Regeneration: 1% sucrose+0.9% agar |
Enhanced embryogenesis (391.4 ± 18.1) in cultures exposed to ABA cf. jasmonic and salicylic acid, increased plant regeneration by 68% | Ahmadi et al. (2014) |
| Microspores Direct organogenesis |
Embryo formation: NN+0.1, 0.25, 0.5, and 1% PF-68 (dark) Embryo maturation: B5+1 mg l–1 Z Regeneration: B5+1 mg l–1 IBA |
– | Embryo formation: 8% sucrose+0.1% AC Cold treatment (4 °C) for 4 weeks Embryo maturation: 2% sucrose+0.8% agar Regeneration: 2% sucrose+0.8% agar |
Four of five recalcitrant populations showed increased shoot regeneration in presence of PF-68, dose–response effect did not show a consistent trend as optimum concentration was influenced by genotype | Barbulescu et al. (2011) | |
|
Brachypodium distachyon
(purple false brome) |
Seeds Indirect organogenesis |
Callusing: MS+2.5 mg l–1 2,4-D (dark) Regeneration: MS+75 µM FPX |
– | Callusing: 3% sucrose+0.3% phytagel+0.6 mg l–1 CuSO4 Regeneration: 3% sucrose+0.3% phytagel |
Highest regeneration rate (40.7%) in FPX cf. KIN and TDZ | Yu et al. (2019) |
|
Gloriosa superba
(flame lily) |
Leaf Indirect organogenesis |
Callusing: MS+3 mg l–1 2,4-D Regeneration: MS+2 mg l–1 BAP |
3% sucrose+ 0.8% agar |
Max. callus regeneration (84.5 ± 3.31%) in 2,4-D cf. BAP, picloram, BAP+NAA and max. no. of shoots per explants (5.25 ± 0.5) in BAP cf. BAP+NAA | Balamurugan et al. (2019) | |
|
Eremurus spectabilis
(foxtail lily) |
Roots Indirect organogenesis |
Callusing: MS+10 mg l–1 BAP+0.1 mg l–1 NAA Regeneration: MS+2 mg l–1 BAP+0.1 mg l–1 IBA |
– | Callusing: 3% sucrose+0.8% agar Regeneration: 3% sucrose+0.8% agar+200 mg l–1 AC |
Highest callus induction frequency (76.67%) and max. shoot proliferation (6.33) in MS medium cf. Schenk and Hildebrandt media. Max. shoot proliferation in intact callus cf. divided callus | Basiri et al. (2022) |
|
Veratrum dahuricum
(mountain corn) |
Immature embryos Indirect somatic embryogenesis |
Callusing 1: MS+8 mg l–1 picloram (dark) Callusing 2: AA+4 mg l–1 2,4-D Regeneration: R2M+3 mg l–1 BAP |
– | Callusing 1: 146 mg l–1 glutamine+ 200 mg l–1 casein hydrolysate+3% sucrose+3 g l–1 phytagel Callusing 2 and regeneration: 3% sucrose+3 g l–1 phytagel |
Highest frequency of embryogenic calli (56%) in picloram and green plant regeneration (95%) in BAP | Ma et al. (2020) |
Monocots are shaded in grey.
a MS, Murashige and Skoog; BAP, 6-benzylaminopurine; B5, Gamborg B5; NAA, 1-naphthaleneacetic acid; TDZ, thidiazuron; KIN, kinetin; IAA, indole-3-acetic acid; 2,4-D, 2,4-dichlorophenoxyacetic acid; IBA, indole-3-butyric acid; WPM, woody plant medium; NN-13, Nitsch and Nitsch-13; GA3, gibberellic acid; NN, Nitsch and Nitsch; PF-68, Pluronic F-68; Z, zeatin; FPX, fipexide; AA, amino acid medium; R2M, 190-2 medium.
b AgNO3, silver nitrate; STS, sodium thiosulfate; PVP, polyvinyl pyrrolidine; ADS, adenine sulfate; ABA, abscisic acid; NH4NO3, ammonium nitrate; CaCl2·2H2O, calcium chloride; AC, activated charcoal; CuSO4, copper sulfate.
The divergent TC responses between dicots and monocots has led to different transformation approaches being used (Kausch et al., 2019). Initially limited only to dicots, Agrobacterium-mediated gene transfer poses a challenge in monocot species due to their non-natural host status (Potrykus, 1990). Monocot transformation recalcitrance was overcome by the introduction of biolistic transformation and protoplast-based systems in which the plant cell wall is enzymically removed prior to DNA introduction (Kausch et al., 2019) (Fig. 2). Additionally, there are far fewer effective selectable markers available in monocots compared with dicots. While aminoglycoside resistance markers such as kanamycin, neomycin, and G418 (geneticin) have proven ineffective in most monocots, they have been used extensively in dicot transformation systems (Jones, 2009). Transformation selection of many monocots has been achieved using herbicide-resistant markers (e.g. phosphinothricin) and through the development of newer antibiotic selection marker systems (e.g. hygromycin).
While plants consist of various tissues and organs, not all are commonly used as explants due to difficulties in viable excision. Despite many tissues displaying totipotency or pluripotency, they are often inhibited from expressing this capability by neighbouring tissues. Isolation and in vitro culture of these tissues could free them from being recalcitrant (Bonga, 2017). For example, in Beta vulgaris, the guard cells exhibit high totipotency and have the remarkable ability to undergo SE when isolated from leaves (Hall et al., 1996). The choice of explants in medicinal plants becomes limited when the donor population is small, as in the case of endangered species, necessitating the use of mature tissues. Furthermore, factors such as a lack of dedifferentiation capacity (the process of specialized cells reverting to a more primitive state), limited cell division potential, or the presence of specialized metabolites can have an antagonistic effect on regeneration (Benson, 2000). In many cases, regeneration can be enabled through selection of organs that contain undifferentiated cells, such as young tissues of embryonic and meristematic origins.
Mature and immature zygotic embryo explants offer a higher proportion of undifferentiated cells and fewer specialized structures, and accumulate fewer inhibitory compounds. These traits are advantageous for initiating embryonic callus cultures or producing viable shoots through SE in many dicots and monocots (Benson, 2000). Zygotic embryos contain pre-embryogenic determined cells with embryogenic competence (Bhojwani and Dantu, 2013). In many monocots, immature embryos have proven to be efficiently transformable, with their size and growth conditions influencing transformation efficiency, but challenges persist in the consistent production of high-quality immature embryos year-round (Lee and Wang, 2023). Alternatively, mature seeds offer a cost-effective, easy to store, and reliable source of explants such as cotyledons, hypocotyls, epicotyls, and cotyledonary nodes, allowing for continuous supply under controlled conditions, and are used for callus induction and shoot proliferation. Cotyledonary node regions have axillary meristems at the junction between cotyledon and hypocotyl, which can proliferate and regenerate by the formation of multiple adventitious shoots on a culture medium containing cytokinin. A cotyledonary node as an explant offers several advantages such as simple accessibility, speedy response, and immense potential to favour shoot organogenesis and SE. Several examples showing high regeneration with the use of immature and seed-derived explants in various medicinal plants are listed in Table 1. Recently, half-seeds have become the trend for explants as they possess advantages of having a greater nutrition supply for shoot regeneration compared with a cotyledonary node alone. They can also be prepared within a shorter time frame, which reduces the total regeneration period and labour costs (Xu et al., 2022).
Another TC approach which takes advantage of the embryogenic process is co-culturing in which two different plant species are grown together in close proximity for promoting SE. In this system, one plant species which exhibits a higher frequency of SE releases specific molecules into the culture medium that stimulate and induce the formation of SEs in the co-cultivated species with a naturally lower rate of SE. Active components identified in embryogenic culture medium include arabinogalactan proteins (AGPs), endochitinases, and lipochitooligosaccharides (von Arnold et al., 2002). The beneficial effect of this strategy has been studied in the regeneration of wheat (Triticum aestivum) (Bakos et al., 2003) and to overcome recalcitrance in grapevine (Vitus sp.) (Ben Amar et al., 2007) and Cichorium species (Couillerot et al., 2012).
The shoot apical meristem (SAM), located at the cotyledon–embryo axis junction, possesses axillary meristems capable of developing into shoots without the need for dedifferentiation or redifferentiation (Sticklen and Oraby, 2005). It offers several advantages, including ease of in vitro culture, rapid regeneration, clonal multiplication, competence for genetic transformation, and the ability to be sustained in vitro for extended periods without cryopreservation. The strategy underlying the SAM-based transformation system involves multiplying transgenic SAM or germline cells in vitro and reprogramming them to differentiate (Baskaran and Dasgupta, 2012). The SAM-based biolistics or Agrobacterium-mediated transformation systems have achieved genotype-independent transformation in medicinal plants such as Catharanthus roseus (Madagascar periwinkle) (Bahari et al., 2019) and Tanacetum cinerariifolium (pyrethrum) (Li et al., 2022).
The use of male and female gametophytes has also been explored due to their ability to produce haploid and doubled haploid plants through gametic embryogenesis, allowing development of homozygous lines from heterozygous parents in a single step. However, not all species are amenable to this type of in vitro morphogenesis, and many medicinal species remain recalcitrant. Moreover, determining the optimum developmental stages of microspore explants is essential for maximum in vitro response (Benson, 2000). Also, the basis of microspore embryogenesis is the switching of the developmental process from normal gametophytic to sporophytic embryogenesis which requires pre-treatments such as cold or heat shock, carbohydrate, and nitrogen starvation, making the regeneration process more tedious (Sharma et al., 2018). Notwithstanding these challenges, isolated microspore cultures emerge as a promising technique to produce double haploids, surpassing anther and ovule cultures in terms of efficiency. Routinely used in vegetable crops, this method has recently been adapted to medicinal plants with encouraging outcomes, notably the recent successful induction of microspore-derived embryonic structures in Artemisia annua (Purnamaningsih et al., 2024).
Plant growth regulators and other chemical factors
Tissue culture medium provides the essential growth components to the explant, but it can also be considered as an interface for communication with the plant. The medium components can dictate certain growth behaviour, and the molecules intimately involved in reshaping plant development are PGRs. Phytohormones are ubiquitously used as PGRs in TC practices, cytokinins and auxins in particular having an impact on de novo shoot organogenesis (Raspor et al., 2021). Cytokinins, auxins, and other phytohormones have a diversity of molecular structures that either exist in nature or are of synthetic origin. For example, >20 different cytokinins and auxins are currently commercially available. Endogenous phytohormones control most aspects of plant growth and development, and modulate responses to abiotic/biotic stresses and other environmental cues. During the establishment and maintenance of meristematic cells of the embryo and SAM, phytohormones fall under the regulation of MG products, which sometime behave in intricate positive feedback loops, as exemplified by WUSCHEL (WUS) and cytokinin (Leibfried et al., 2005).
PGRs are important medium components that have a large impact on developmental and metabolic processes even at low concentrations. Optimizing PGR regimes, including application of novel, potent PGRs and removing the inhibitory interactive effects of endogenous and exogenous hormones, is often a first approach to overcome in vitro regeneration recalcitrance in many plant species. In this section, we will cover the roles of media components permitting regeneration capability to recalcitrant explants, but the reader needs to keep in mind that the enabling functions of cytokinin and other molecules are achieved through the involvement of a wide range of molecular players with morphogenic activities. Application of cytokinin is often viewed as the enabling factor in TC regeneration, but this must be viewed in a context of a cascade of events that occur in meristem cells or tissue of embryonic origins that requires the recruitment of morphogenic players that were silenced prior to the application of PGRs.
Exogenous phytohormones
Under in vitro cultivation, the fate of an explant hinges on the fundamental golden hormonal regeneration rule: a high ratio of cytokinin to auxin in the medium stimulates the formation of shoots, while a reversed ratio encourages the development of roots. Usually, under the influence of cytokinin, explants can produce elevated numbers of shooting units. Cytokinin can also trigger direct and indirect SE (Fig. 2) and enable regenerability of cells. Use of cytokinins for micropropagation and regeneration is so prevalent that it is often considered the first approach when studying micropropagation or regeneration in a new species (Šmeringai et al., 2023). Use of cytokinin in both micropropagation and calli regeneration protocols has provided excellent results in amenable species. With a wide range of natural and synthetic cytokinins, finding a desirable regeneration response in recalcitrant species is often a trial-and-error approach where a cytokinin’s molecular conformation, concentration, type of delivery to the explant, and interplay with other phytohormones form a complex matrix of conditions to test. In the last decade, thidiazuron (TDZ), a synthetic phytohormone (see below in the section on synthetic PGRs), has proven to be tremendously effective in a wide range of medicinal, woody, and other species. It is successfully used to promote de novo regeneration and SE initiation, and to stimulate shoot organogenesis and callus induction and proliferation. In the medicinal species Salvia bulleyana, direct organogenesis was observed from leaf explants while using TDZ (Grzegorczyk-Karolak et al., 2021) and in embryo explants of cannabis (Galán-Ávila et al., 2020; Zhang et al., 2021). Moreover, recent advancements in our understanding of auxin and cytokinin crosstalk have shed light on the complex world of regeneration phenomena, including SE, for the future of TC and transformation (Asghar et al., 2023). As for auxin, a wide variety of species require exogenous application of auxin in the medium as a pre-requisite to trigger a totipotency reversal in somatic cells. Calli produced under auxin acquire a competency for organogenesis that increases cell susceptibility to SE and shooting upon subsequent cytokinin exposure.
Endogenous phytohormones
Endogenous phytohormones are the native molecules already present in the explant when moved to in vitro growing conditions. Endogenous levels of phytohormones can be sufficient on their own to trigger a regeneration response from the right explant type, as seen in the previous section. In other instances, endogenous phytohormones are a hindrance to TC. As such, establishing cultures from vegetative explants such as leaves, petioles, and nodal segments acquired from mature medicinal trees can be difficult as they contain elevated levels of endogenous phytohormones, carbon sources, and other substances that can interfere with the effects of additives present in the growth medium, interfering with their regeneration potential and leading to potential developmental issues. Application of external phytohormones is often ineffective in mitigating the impact of endogenous levels of auxins. For instance, in plants with high endogenous auxin levels, including some medicinal species, the addition of auxin transport inhibitors, auxin antagonists, or auxin biosynthesis inhibitors positively affects shooting induction, as is the case in Carapichea ipecacuanha (Koike et al., 2020), and has been used to achieve successful regeneration in otherwise recalcitrant plants such as cannabis (Smýkalová et al., 2019). Applications of auxin transport inhibitors such as 1-naphthylphthalamic acid (NPA), 2,3,5-triiodobenzoic acid (TIBA), 2-(1-pyrenoyl) benzoic acid (PBA), and the flavonoid quercetin have seen increased regeneration rates in model organisms, fruit-bearing trees, and cereals (Yu et al., 2012; Hu et al., 2017; Ohbayashi et al., 2022). In medicinal species, the presence of TIBA in the medium has improved organogenesis from calli in mulberry (Bhau and Wakhlu, 2001), while NPA and TIBA have also shown a positive effect in cannabis (Dreger and Szalata, 2022), and quercetin too has shown increased regenerability in Oldenlandia umbellate (Saranya Krishnan and Siril, 2017). To flush out endogenous phytohormones or to load explants with PGRs, pre-treatment of explants in a liquid medium enriched with molecules such as cytokinins can stimulate or promote the regeneration process. For instance, shoot regeneration was successfully achieved in the woody medicinal plants G. biloba (Isah, 2020) and Pterocarpus marsupium (Ahmad et al., 2018) through pre-treatment with TDZ.
Similarly, endogenous levels of cytokinins play an important role in regeneration efficiency, and the technological approaches to measure endogenous concentration can subsequently be used to optimize the concentration of exogenous cytokinin to be applied to a culture, narrowing the window of the matrix of media conditions to be tested (Smýkalová et al., 2019). Novel rapid methods for quantifying endogenous phytohormones offer a tool for more effective TC protocols to be developed for cultivating recalcitrant species (Erland et al., 2017). A recent study in the woody medicinal plant Cyclocarya paliurus has highlighted the importance of seasonal variability of endogenous cytokinins when explants are isolated from perennial plant species (Cheng et al., 2023). The study demonstrated that similar adventitious shooting rates can be obtained across explants from different seasonal origins if the concentration of exogenously supplied 6-benzylademine, a cytokinin, is adjusted to match the endogenous level of phytohormone according to season (Cheng et al., 2023).
Novel synthetic plant growth regulators
Recalcitrance can also be overcome by substituting natural or commonly used PGRs with powerful synthetic counterparts that share similar physiological properties (Benson, 2000). Synthetic PGRs offer several advantages, including light insensitivity and resistance to degradation during autoclaving, exhibit potency levels 10–1000 times higher than natural hormones, and therefore are often required in lower concentrations for activity (Phillips and Garda, 2019). Some auxin-based herbicides such as dicamba, 2,4-dichlorophenoxyacetic acid, and picloram are used to induce SE in various species (Miroshnichenko et al., 2017). TDZ has found extensive application in TC as it demonstrates remarkable potency in propagating recalcitrant woody, legume, and medicinal species in vitro, including cannabis (Ali et al., 2022). TDZ’s efficacy is well established in TC, facilitating highly efficient regeneration across genotypes and explant types; hence it broadens the scope of transformation protocols to elite genotypes. However, it is worth noting that excessive TDZ concentration and prolonged exposure can lead to issues such as the formation of fasciated and compact shoots, hyperhydricity (shoot vitrification or glassiness), and downstream rooting challenges (Dewir et al., 2018).
Many plant species exhibit varied responses to the different cytokinins, and it becomes necessary to optimize TC protocols for individual species. Topolins in general, and meta-topolin in particular, were identified as a result of the continuous search for superior cytokinins. Meta-topolin and its derivatives are naturally occurring aromatic cytokinins that have shown promising effects in micropropagation of several medicinal plant species and promote induction of multiple shoots, improving physiological and biochemical traits and successful rooting (Ahmad and Anis, 2019). Additionally, several compounds such as brassinosteroids, jasmonates, salicylic acid, phloroglucinol, pluronic F-58, phytosulfokine-alpha, lignosulfonates, fipexide, abscisic acid, and trichostatin exhibit growth-modulating effects and have been used as PGRs in several species (Table 1), offering novel avenues for addressing recalcitrance issues in TC.
Ethylene inhibition and the role of silver compounds
Ethylene, a key regulator of physiological and developmental processes, exhibits contradictory impacts on regeneration, varying with species, genotypes, and explant type. While the concentration of auxins and cytokinins in culture media is precisely controlled, ethylene, being a gas, is typically released during in vitro culture, accumulating in closed vessels. Thus, understanding its role is critical for enhancing regeneration and addressing recalcitrance in certain species or tissues (Neves et al., 2021). Ethylene can adversely affect morphogenic responses, contributing to hyperhydricity. Strategies to regulate ethylene, using inhibitors such as salicylic acid, CoCl2, and AgNO3, show promise for improving TC protocols (Bashir et al., 2022). Interestingly, in some cases, ethylene has a positive influence, potentially reversing recalcitrance in genotypes with limited regeneration capacity (Neves et al., 2023).
Silver ions, especially in the form of AgNO3 and silver thiosulfate, are favoured due to their physical, chemical, and biological availability, water solubility, stability, non-toxicity, and specificity to inhibit ethylene action, disrupting its signalling pathway and impacting growth by enhancing polyamine biosynthesis (Pal Bais and Ravishankar, 2002; Kumar et al., 2009; Prem Kumar et al., 2016). Additionally, AgNO3 reduces aminocyclopropane-1-carboxylic acid, a precursor to ethylene, decreasing ethylene production and browning of explants (Gong et al., 2005). As a result of these properties, AgNO3, silver thiosulfate, and other Ag compounds are gaining prominence in refining TC protocols for addressing recalcitrance issues in various plant species, including medicinal plants (Table 1). In a later section, we describe the use of silver nanoparticles (AgNO3) to reduce the impact of ethylene in TC.
Other media components
Apart from phytohormones, other factors within the TC medium such as macro- and micronutrients, vitamins, carbon source, solidifying agents, and other additives all play a role in the in vitro growth of explants. Medium permutations affecting the type and concentration of these constituents have been shown to relieve TC recalcitrance (Fig. 1, Stage S1–S3) in multiple species (Long et al., 2022) (Table 1). For example, a doubling of the regeneration rate of indirect somatic embryogenesis was achieved in the recalcitrant rice elite cultivar IR64 with optimization of an established TC protocol by manipulating the type and concentration of carbon source and gelling agent, and by supplementation of the medium with additives such as free amino acids (Sundararajan et al., 2020). Similar increases in regeneration frequency have been observed in medicinal species such as the endangered TC-recalcitrant plant Oplopanax elatus, where regeneration frequencies could be increased by similarly modulating both carbon source and concentration and gelling agent in the cultivation medium (Moon et al., 2013; Sahoo et al., 2023). Use of maltose or a combination of maltose and sucrose has proven more effective in increasing regeneration in the medicinal plants Cymbopogon schoenanthus (Abdelsalam et al., 2018) and Kelussia odoratissima (Ebrahimi et al., 2018), respectively. Through the sugar sensing pathway, use of sucrose in the medium can have an antagonistic effect on cytokinin homeostasis (Ćosić et al., 2021) and other phytohormones (Raspor et al., 2021). Other media additives such as activated charcoal have provided some beneficial effects to ameliorate TC recalcitrance by sequestering and thereby rendering inert chemical inhibitors present in the media or secreted by the explant during their early stages of culture. However, the prolonged presence of activated charcoal in the medium can pose a challenge, as it has the potential to also absorb growth-promoting substances, ultimately diminishing the growth response or regeneration processes (Pinar et al., 2020).
Morphogenic genes
MGs are transcription factors that control cell fate and, consequently, govern plant development. Harnessing MGs can significantly improve and accelerate explant regeneration through their involvement in hormone biosynthesis, perception, and developmental signal transduction pathways, and hence transformation efficiency. Over the past two decades, MGs have been increasingly employed and have unlocked transformability in many recalcitrant crops, as outlined in two recent reviews (Maren et al., 2022; Lee and Wang, 2023).
Overexpression of MGs to stimulate an embryogenic or meristematic response to induce regeneration is classified into two categories: (i) genes that enhance a pre-existing embryogenic response under in vitro conditions; and (ii) genes involved in the direct formation of embryo or meristem-like structures without the need for induction conditions (Gordon-Kamm et al., 2019). An example of the first type of inducer is SOMATIC EMBRYOGENESIS RECEPTOR LIKE KINASE 1 (SERK1) which has been shown to be an enabler for cells to develop into somatic embryos, a change achieved through the modulation of auxin biosynthesis, transport, and perception (Yan et al., 2023). Previous studies have demonstrated its role in SE in both monocots and dicots (Sivanesan et al., 2022). Constitutive expression of SERK1 has enhanced SE initiation in Coffea canephora (Pérez-Pascual et al., 2018), Oryza sativa (Hu et al., 2005), and Arabidopsis thaliana (Hecht et al., 2001).
Major regulators of SAM formation and maintenance such as the homeobox genes WUSCHEL (WUS) and SHOOTMERISTEMLESS (STM) have also been used as MGs to improve embryonic responses (Lenhard et al., 2002). Expression of AtWUS in Medicago truncatula leaf explants induced callogenesis and the production of highly embryogenic calli, generating plantlets even in the absence of growth regulators in the medium (Kadri et al., 2021). Also, overexpression of WUS promoted SE and lateral branching in birch (Betula platyphylla) through an increased expression of SE-related genes such as BpSTM (Lou et al., 2022), and thus has proven to be a promising tool in developing plant growth regulator-free regeneration systems.
MGs in the second category have been extensively studied in various crops, as reviewed in detail elsewhere (Gordon-Kamm et al., 2019). One such gene is BABYBOOM (BBM) whose product belongs to the AP2/ERF superfamily of transcription factors (Boutilier et al., 2002). BBM plays a multifaceted role in processes such as cell proliferation, plant growth, and development, and notably it induces embryogenesis in differentiated cells. Its initial success in stimulating SE via ectopic expression without addition of external PGRs was observed in Brassica napus (Boutilier et al., 2002). Subsequently, BBM and BBM-like genes have been utilized in numerous plant species to improve transformation efficiency and regeneration (Jha and Kumar, 2018). Recently, there has been a shift in the use of BBM for enhancing transgenic plant regeneration beyond herbaceous plants and crops to include recalcitrant fruit trees. For example, the overexpression of MdBBM1 in apple has resulted in a remarkable enhancement of apple transformation efficiency (Chen et al., 2022; Xiao et al., 2023).
Beyond the promoter controlling gene expression, several factors influence the outcome of MG expression, including the target cell or tissue type(s), the source of the MG (i.e. whether it is derived from the native or another species), hormone dependency, and co-expression with other MGs. For instance, the gene LEAFY COTYLEDON1 (LEC1) plays a role in SE, and its overexpression can trigger embryo-like structures in vegetative tissues (Zhu et al., 2014). However, in conifers such as Picea abies (Norway spruce), overexpressing the LEC1-type gene PaHAP3A which is active during embryo development did not induce embryonic features in vegetative tissues. Instead, when activated during zygotic maturation, ectopic somatic embryos formed on the surface of zygotic embryos. This highlights that specific cells or tissue types are more receptive to MGs and that the spatiotemporal control of MG expression is an important consideration using this approach (Uddenberg et al., 2016). Additionally, the expression of endogenous genes may produce different developmental responses compared with homologues of other species. For example, ectopic AtBBM or BnBBM expression in Nicotiana tabacum produced developmental responses that differed from those observed using the endogenous tobacco BBM gene (Srinivasan et al., 2007).
Several MGs that play an important role in plant regeneration are hormone dependent and are also involved in phytohormone signal transduction. For example, CUP-SHAPED COTYLEDON genes (CUC1 and CUC2) contribute to SAM formation during embryogenesis and shoot regeneration (Aida et al., 1997). Overexpressing these genes in transgenic calli from A. thaliana hypocotyls promoted adventitious shoots (Daimon et al., 2003). However, when cultured on hormone-free medium, the same transgenic calli did not produce shoots, highlighting the need for an appropriate hormone context for CUC1 and CUC2 functionality. On the other hand, ENHANCER OF SHOOT REGENERATION genes (ERS1 and ERS2), involved in the cytokinin response pathway, and MONOPTEROS, an auxin-response gene, promoted a hormone-independent response in shoot meristem formation when overexpressed (Banno et al., 2001; Ikeda et al., 2006; Ckurshumova et al., 2014). Additionally, the levels of PGRs can influence the phenotypic response of genes involved in morphogenesis. For instance, transgenic A. thaliana explants overexpressing LEC2 produced somatic embryos and calli under low and high auxin concentrations, respectively (Wójcikowska et al., 2013). Similarly, when WUS was expressed in the root in the absence of phytohormones, shoots and leaves were observed; somatic embryos arose in the presence of auxin (Gallois et al., 2004). In the same study, floral structures were observed when WUS was induced along with LEAFY, a master regulator of floral development, providing evidence that unique phenotypes can be observed when MGs are co-expressed.
In many crops, achieving effective transformation often requires the use of selectable marker genes (Fig. 1) (Zuo et al., 2002). To address this issue, researchers have explored genes, including MGs, that enable the identification of transgenic events without the need for a selectable marker. The maize homeobox gene KNOTTED1 (KN1) is essential for meristem initiation and maintenance, and is normally expressed in shoot meristems. When KN1 was overexpressed in N. tabacum under non-selective conditions (without antibiotics) on a hormone-free medium, a 3-fold increase in transformation efficiency was observed relative to the kanamycin selection treatment, demonstrating its usefulness as a positive selection system for plant transformation (Luo et al., 2006). Similarly, co-expression of maize transcription factor genes BBM and WUS2 enabled regeneration of stable transgenics in the recalcitrant maize inbred line B73 and sorghum (Sorghum bicolor) P898012 (Mookkan et al., 2017). GROWTH-REGULATING FACTOR 4 (GRF4) and its cofactor GRF-INTERACTING FACTOR 1 (GIF1) form a transcription factor complex required for pluripotent cell formation in male and female reproductive structures (Lee et al., 2018). Expression of GRF4–GIF1 substantially increased the efficiency and speed of regeneration in wheat, triticale, and rice, and induced efficient wheat regeneration in the absence of exogenous cytokinins, facilitating selection of transgenic plants, thereby eliminating the need for antibiotic-based selectable markers (Debernardi et al., 2020).
While MGs have proven valuable in transforming and regenerating recalcitrant plant species, they come with a potential drawback—the risk of deleterious pleiotropic effects. When these MGs are expressed strongly and constitutively, they can lead to unwanted changes in plant morphology, reduced fitness, altered metabolism, and even infertility in regenerated plants (Gordon-Kamm et al., 2019). To maximize their benefits while minimizing these drawbacks, an additional step is needed to control MG expression after the transformation or regeneration process has occurred and their usefulness has expired. Several strategies to control the timing and level of expression of MGs have been developed, including their inducible expression, excision from the nuclear genome post-transformation, use of tissue-specific plant promoters, using GRF–GIF chimeras, innovative Agrobacterium-mediated delivery methods, and T-DNA border read-through. Some successful examples of the application of these approaches are listed in Table 2, with cannabis among the first medicinal plant species in which use of GRF–GIF chimeras was attempted (Zhang et al., 2021).
Table 2.
Different approaches to control MG expression levels to overcome negative pleiotropic effects
| Approach | Method | Plant species | MG used | Outcome | Reference |
|---|---|---|---|---|---|
| Inducible expression | Dexamethasone-inducible system (GR fusion) |
Capscium annum | BBM | Efficient regeneration of large numbers of fertile transgenic plants | Heidmann et al. (2011) |
| Arabidopis thaliana | BBM | Regeneration of fertile plants during extended cultivation in tissue culture | Lutz et al. (2015) | ||
| Theobroma cacao | LEC2 | Regeneration of secondary transgenics from the leaf-derived secondary embryos | Shires et al. (2017) | ||
| Estradiol-inducible system (OLexA promoter) |
Arabidopis thaliana | MYB115 | Vegetative to embryonic transition | Wang et al. (2009) | |
| Brassica rapa var. rapa | WUS | Fertile transgenic plants without developmental defects | Liu et al. (2022) | ||
| Excision-based | Desiccation-inducible CRE/LOXP | Zea mays | WUS2+BBM | Excision of the morphogenic genes to produce healthy, fertile transgenic plants | Lowe et al. (2016) |
| Heat shock-inducible FLP/FRT | Populus tomentosa | BBM | Transgenic plants with phenotypic alterations but fertile | Deng et al. (2009) | |
| Tissue-specific promoters | AXIG1 promoter PLTP promoter |
Zea mays | BBM+WUS2 | Robust and fertile transgenic plants even without excision of MG | Lowe et al. (2018) |
| GRF–GIF chimeras | – |
Triticum aestivum
Oryza sativa Citrus Cannabis sativa |
GRF4–GIF1
GRF3–GIF1 |
Fertile transgenic plants without developmental defects | Debernardi et al. (2020); Zhang et al. (2021) |
| Agrobacterium-mediated delivery methods | Two Agrobacterium strains with each harbouring a distinct T-DNA | Sorghum bicolor | WUS+CRC | Somatic embryo formation and regeneration of stable transgenic plants with only the selectable marker | Hoerster et al. (2020) |
| T-DNA border read-through | Positioning MG cassettes outside T-DNA left border | Zea mays |
WUS2
WUS2+BBM |
Non-excision method for creating a high-quality transgenic event | Gordon-Kamm et al. (2019) |
Over the past two decades, significant advancements in genetic transformation have been witnessed in major crop species such as rice, maize, wheat, sorghum, soybean, and cotton (Nalapalli et al., 2021). Comprehensive improvements in various aspects of the TC process have led to a high success rate in obtaining transgenic plants, with MGs playing a pivotal role. This transformation success has been particularly evident in monocot crops, where both Agrobacterium-mediated and particle bombardment gene delivery methods have been refined to achieve remarkable efficiency (Shrawat and Lörz, 2006). While recent advances in MG research, including genes such as BBM, WUS, GRF, and GRF–GIF chimeras, have effectively addressed transformation and regeneration challenges in many recalcitrant crop species, their application in the realm of medicinal plants has remained limited. This discrepancy presents a dual challenge and opportunity within the fields of plant biotechnology and medicinal plant research. The limited use of MGs in improving medicinal plants can be attributed to several factors such as complex biology and the diverse nature of medicinal plant species, lack of research funding and commercial investment, as well as regulatory and ethical considerations.
Novel techniques to improve transformation efficiency and overcome recalcitrance
Nanoparticles
In recent years, the field of TC has undergone a remarkable transformation with the use of nanoparticles (NPs). Nanoparticles are ultra-small structures measuring <100 nm in size that can be used to deliver molecular cargo through biolistic or transfection methods. They can also act as active agents themselves in TC medium. TC is often challenged by microbial contamination, and NPs provide a promising alternative to antibiotics for addressing this concern (Alfarraj et al., 2023). Endophytes found in many medicinal plants can also become problematic in in vitro cultures even though they may not be pathogenic as they can negatively impact plant health and vigour (Wu et al., 2021). While research on the use of NPs against endophytic bacteria is in its early stages, efforts are being made to tackle this issue (Rakhimol et al., 2023). NPs adhere to and penetrate the bacterial cell membrane, bind to the sulfhydryl group of enzymes involved in metabolic activities, and inactivate transport chain mechanisms, thus inhibiting their proliferation in the medium (Ahlawat et al., 2022) (Fig. 3). In addition, NPs generate reactive oxygen species which interact with the bases of microbial DNA and arrest their replication (Kim et al., 2011). These activities not only serve as a microbial deterrent but are also hypothesized to stimulate secondary metabolite production (Sena et al., 2023). NPs have been used as effective elicitors for the biosynthesis of medicinal compounds by causing changes in expression of key genes in diverse metabolite pathways (Ayoobi et al., 2024).
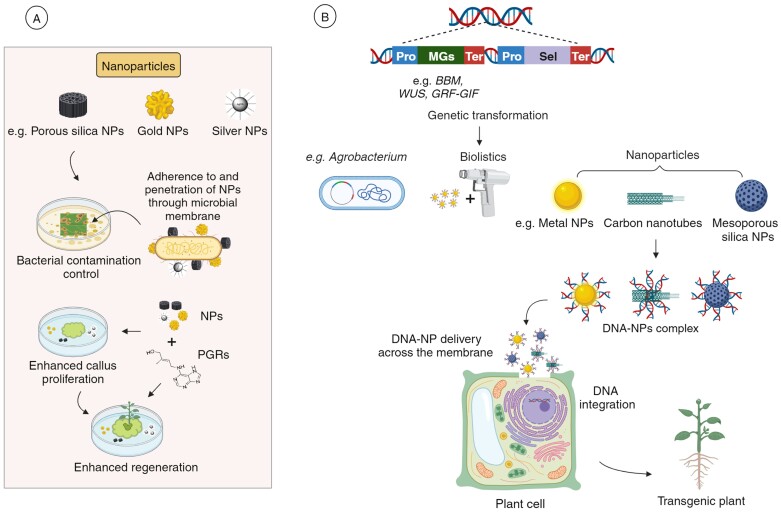
Fig. 3.
A schematic of different types of NPs and their application in plant tissue culture and transformation. (A) Role of NPs in eliminating microbial contamination and enhancing regeneration. (B) NPs as vehicles for delivering GOI(s), such as MG cargo, as an alternative to Agrobacterium-mediated transformation or biolistic transformation.
The favourable impact of NPs on overcoming barriers related to callus induction, SE, and organogenesis can be attributed to their ability to regulate key PGRs such as auxin, cytokinin, and gibberellins (Fig. 3). This regulation involves enhancing protein and enzyme activity, as well as improving photosynthesis by enhancing light absorption (Mandeh et al., 2012; Salih et al., 2021). Also, they reduce the rate of transpiration, maintaining cellular osmotic pressure, and facilitate water and nutrient uptake (Arruda et al., 2023). Recent studies have also underscored the effectiveness of NP combinations, demonstrating that blends of different NPs are more potent than single types in promoting callus biomass production and enhancing regeneration, especially when using mature embryo explants (Arruda et al., 2023). Silver NPs bind to ethylene receptors involved in signalling, thus hindering ethylene action, and reduce hyperhydricity. Likewise, the promotion by silver NPs of regeneration from callus cultures derived from diverse plants is linked to their capacity to increase antioxidant reserves (Phong et al., 2023). This dual action potentially mitigates oxidative stress and supports the regeneration process. Several successful although limited medicinal plant examples to date are shown in Table 3.
Table 3.
Use of NPs in medicinal plants for improved TC response and/or regeneration
| Plant species | Explants and TC/regeneration pathway | Nanoparticle typea | Size and concentration | Outcome | Reference |
|---|---|---|---|---|---|
|
-Bacopa monnieri
(water hyssop) |
Full leaf (FL), distal portion of half leaf (DPHL), and proximal portion of half leaf (PPHL) Indirect organogenesis |
TiO2 | 20–50 nm 2, 4, 6, 8, and 10 mg l–1 |
Max. callus FW (3.02 g) in PPHL at 8 mg l–1 and max. shoots/explant (30.43) in FL at 2 mg l–1 TiO2NP | Aasim et al. (2023) |
|
Gloriosa superba
(glory lily) |
Rhizome Indirect somatic embryogenesis |
ULAg | 5 nm and 50 nm 0.1, 0.2, 0.3, 0.4, and 0.5 mg l–1 |
Enhanced embryogenic callus (96%) in 0.1 mg l–1, highest somatic embryo production (95.7%) in 0.4 mg l–1, highest no. of mature embryos (24.3/culture) in 0.5 mg l–1, high-frequency embryo germination (98.1%) in 0.3 mg l–1 ULAgNP | Mahendran et al. (2018) |
|
Panax vietnamensis
(Vietnamese ginseng) |
Leaf-derived calli Indirect somatic embryogenesis |
Ag | <20 nm 0.4, 0.8, 1.2, 1.6, and 2.0 mg l–1 |
Max. no. of somatic embryos (140) and embryo-derived plantlets (14.66) in 1.6 mg l–1 | Manh Cuong et al. (2021) |
|
Prunella vulgaris
(common self-heal) |
Leaf Callogenesis |
Ag and Au | AgNP: 30 μg l–1 AgAu (1:3), AgAu (2:1), and AgAu (3:1) | 100% callus proliferation in 30 μg l–1 AgNP and AgAu (1:2) and AgAu (2:1) | Fazal et al. (2016) |
|
Satureja khuzestanica
(garden savoury) |
Leaf, stem, and root Callogenesis |
MWCNT | Outer diameter: 5–15 nm and length: 50 μm 0, 25, 50, 100, 250, and 500 mg l–1 |
Efficient callus induction in leaf explant cf. stem and root and highest callus proliferation rate (81%) in 100 µg ml– | Ghorbanpour and Hadian (2015) |
|
Scoparia dulcis
(goatweed) |
Young stem cuttings Indirect organogenesis |
Ag, Au, and CuO | AgNP: 13.5 nm, AuNP: 3.5 nm, and CuONP: 25 nm 2, 4, and 6 mg l–1 |
Optimum callus initiation in 4 mg l–1 AgNP, AuNP, and CuONP, and accelerated shoot regeneration in 4 mg l–1 CuONP cf. AgNP and AuNP | Rajan et al. (2021) |
|
Swertia chirata
(chirata) |
Shoot apices Direct organogenesis |
Biogenic Ag | 20 nm 1, 2, and 4 mg l–1 |
Max. no. of shoots per explant (10.24 ± 1.26), shoot length (2.4 ± 0.43 cm), and shoot regeneration percentage (98.26 ± 3.76) in 4 mg l–1 | Saha and Dutta Gupta (2018) |
|
Cannabis sativa
b
(cannabis) |
Leaf | Polyethylenimine cationic polymer-modified silicon dioxide-coated gold nanoparticles (PEI-Au@SiO2) | GmMYB29A2-pGWB6 and GmNAC42-1-pGWB6 | Infiltration of DNA-PEI-Au@SiO2 into leaf tissues resulted in transcription of both genes and localization of fluorescent-tagged transcription factor proteins in the nuclei of leaf cells including trichomes | Ahmed et al. (2021) |
a ULAg, Ulva lactuca silver; MWCNT, multi-walled carbon nanotubes; CuONP, copper oxide nanoparticles.
b NP used as a cargo delivery is shaded in grey.
Genetic engineering in plants is frequently limited by several factors such as the presence of a multilayered and rigid cell wall, cell damage, random DNA integration within the genome (excluding targeted gene edits), and negative effects of high antibiotic concentrations when traditional gene delivery methods are used, reducing transformation efficiency, regeneration, and compromising the genetic stability of resulting plants (Sarmast and Salehi, 2016; Dong and Ronald, 2021). To address these issues, researchers have turned to NP-mediated gene delivery methods (Fig. 3). These methods, free from the external forces utilized in biolistics or electroporation, deliver biomolecules to intact plant cells and offer advantages such as the ability to traverse biological membranes and target specific tissues or cells, protect cargoes (DNA, RNA, proteins, and ribonucleoproteins) from degradation and release them in controlled quantities and intervals (Cunningham et al., 2018; Squire et al., 2023). Delivery of cargo inside liposome NPs, for example, is an efficient method in species with protoplast-amenable regeneration protocols. In cannabis, passive diffusion of silicon polymer-coated gold NPs to which two Agrobacterium vectors were fused was successfully used to transiently transform intact leaves with two transcription factors (Ahmed et al., 2021). NPs have therefore emerged as a promising and biocompatible tool for manipulating a plant’s genome or for the transient expression of genes of interest.
NP–MG combinations have the potential to make significant advances in the field of plant genetic engineering (Squire et al., 2023). Addressing the pleiotropic effects of MGs, DNA-free direct delivery of transcription factors has emerged as a promising solution. For instance, AtWUS was successfully delivered into tobacco using cell-penetrating peptides through a method known as delivered complementation in planta (Wang et al., 2023). These short peptides, forming cell-penetrating peptide–cargo complexes, enable cytosolic delivery of cargo molecules through the plasma membrane by covalent conjugation, overcoming the need for introducing foreign DNA (Guo et al., 2019). Additionally, nanomaterial-based small-molecule approaches are being explored to mimic endogenous transcription factor proteins, replicating their multidomain structure and gene-regulating functions (Patel et al., 2014). Furthermore, NPs can deliver CRISPR-associated protein 9 (Cas9)/gRNA ribonucleoproteins into regenerative tissues with the aim of generating targeted DNA modifications in transgene-free plants (Demirer et al., 2021). These approaches hold enormous promise for application in plants. Such innovative techniques provide greater precision and control over gene expression, ultimately advancing our ability to manipulate medicinal plants for various purposes.
Overexpression of histone genes
Many economically important crops remain highly recalcitrant to Agrobacterium infection. The success of plant transformation depends on complex interactions between the plant and Agrobacterium, involving numerous genes from both organisms (Rahman et al., 2023). Several strategies have been attempted to enhance transformation efficiency, such as using highly virulent Agrobacterium strains or super binary vectors with extra Vir genes, and optimizing plant culture conditions (De Saeger et al., 2021). Despite these efforts, there are limits to improving transformation in recalcitrant crops using these methods. An alternative approach to boosting plant transformation involves modifying the plant itself. This can be achieved by identifying plant genes that play roles in the transformation process. Some candidate plant genes have been identified through genetic screening (Mysore et al., 2000).
One of the identified genes, the A. thaliana histone H2A gene HTA1 (RAT5), is involved in the integration of T-DNA into the plant genome. Overexpression of AtHTA1 has been shown to increase Agrobacterium transformation efficiency of A. thaliana plants (Mysore et al., 2000). Similarly, expression of other histone genes such as HTR and HFO, whether in their native host or in alternative plant species, has also led to increased transformation susceptibility, suggesting that exploring the manipulation of plant genes involved in the process offers a promising avenue for expanding the range of recalcitrant crops that can be effectively transformed using Agrobacterium (Tenea et al., 2009).
Other tissue culture-independent transformation methods
The reproducibility of transformation protocols involving TC is a complex puzzle, particularly in recalcitrant plant species (Gharghi et al., 2023). In planta transformation offers a simpler, faster, and TC-independent alternative which involves direct uptake of foreign DNA into plant tissues through techniques such as microinjection, electroporation, or by protoplasts without the use of any vector (Su et al., 2023). Various improvements in Agrobacterium-mediated transformation efficiency have been achieved by modifying factors such as pre-culture conditions, chemoattractant concentration (acetosyringone and chloroxynil), and Agrobacterium strains (Karthik et al., 2018). Apart from biolistics, other common in planta methods include injecting Agrobacterium into the SAM, floral dip or spray, pollen uptake, and embryo/seed imbibition (Kaur and Devi, 2019). Another promising solution comes in the form of a rapid, reliable imbibed seed-piercing method, which has the potential to be applied to fibre-producing crops (Majumder et al., 2020). Pollen magnetofection is being explored to overcome the plant cell wall barrier in some crops which makes them resistant to DNA delivery and recalcitrant to transformation. It involves coupling DNA with magnetic NPs in the presence of a magnetic field (Dobson, 2006). This method takes advantage of the unique characteristics of pollen, which has surface apertures (5–10 µm diameter) with either reduced wall thickness or devoid of walls, facilitating DNA uptake (Ressayre et al., 1998). This technique has been successfully demonstrated to produce transgenic seeds in cotton and other crops such as pepper and pumpkin (Zhao et al., 2017), recalcitrant maize inbred lines (Wang et al., 2022), and okra (Farooq et al., 2022). Despite its advantages, pollen magnetofection has some limitations, not being suitable for certain plant species with incompatible pollen apertures, and it is not effective for introducing genetic material into maternally inherited organelles such as chloroplasts and mitochondria (Lv et al., 2020). Another recent TC-free transformation method has shown great potential for transforming herbaceous, tuberous, and woody species by taking advantage of the shooting regenerability of their roots, tubers, or stem sections, respectively (Cao et al., 2023). In the cut–dip–budding gene delivery system, the method utilizes a scion donor (cut) that is challenged with Agrobacterium rhizogenes (dip) to enable the generation of transgenic shoots (budding). It has been successfully applied to various medicinal plants with root-suckering capabilities, in species such as Clerodendrum spp (Lu et al., 2024), Taraxacum mongolicum (Pugongying), and Rehmannia glutinosa (Dihuang) (Cao et al., 2024).
Non-transformation methods
Due to ethical, regulatory, and other concerns regarding the production of transgenic plants, significant effort has been invested in developing methods that do not rely on DNA integration to overcome transformation or regeneration recalcitrance. For example, new Agrobacterium strains are being developed that can transiently express but do not integrate T-DNA into the host genome. Additionally, advancements in CRISPR/Cas technology have improved the robustness of this process by allowing genetic changes to be accomplished without any integration of foreign DNA through transient expression of a site-specific nuclease using viral vectors in the form of either mRNA, which is unstable and quickly degrades, or protein, which is not transmitted from parent to offspring (Sedeek et al., 2019). Gene edits can also be implemented through the transfection of gRNA-loaded Cas9 ribonucleoproteins by polyethylene glycol (PEG) in species where regeneration from protoplasts is possible, or by particle bombardment in regenerative explants. Multiple examples in non-medicinal plant species are covered in a recent review (Gu et al., 2021). MGs can essentially be co-delivered in the same way to produce gene edits in regeneration-recalcitrant medicinal plants.
Future advances using artificial intelligence
The numerous environmental and genetic factors on which a successful TC process depends are complex, non-linear, and non-deterministic due to the highly interactive nature of these variables. Their unravelling can be a time-consuming and costly endeavour. To assist with this challenge, artificial intelligence models and optimization algorithms are now being applied to enhance different stages of TC (Hesami and Jones, 2020). For instance, a combination of a generalized regression neural network (GRNN) and a genetic algorithm (GA) was used to model and predict in vitro shoot regeneration outcomes of wheat. Metadata collected from previous in vitro shoot regeneration studies on the basis of 10 factors, including genotypes, explants, PGR type, and concentration, were considered to develop and optimize genotype-independent regeneration protocols (Hesami et al., 2020). Similarly, other input variables such as digitized images have been used to capture visual data, for example to classify non-embryonic callus and somatic embryos during SE and to recognize different phases of embryo development (Hesami and Jones, 2020). These advances in data-driven modelling demonstrate the potential of artificial intelligence for overcoming genotype-related challenges in medicinal plants and promoting more efficient and widespread crop trait improvement through genetic engineering and TC techniques.
Conclusion
Although MGs have an undisputed impact on explant regeneration, in some instances they require a specific cellular context to enable their morphogenic functions. We have highlighted studies where the right balance of exogenous phytohormones (Daimon et al., 2003) or explant type (Uddenberg et al., 2016) was needed to trigger a regeneration response. Overcoming regeneration in recalcitrant species foremostly requires an understanding of how explant, phytohormones, and MGs, both endogenous and exogenously supplied, interact and enable each other. The use of MGs and NPs to enhance transformation and regeneration in medicinal plants represents a promising field of research, with the potential to radically transform cultivation practices and up-scale the production of valuable therapeutic compounds. While various MGs associated with embryogenesis and meristem development have been identified, their individual and combined effects on medicinal plant transformation need thorough evaluation (Duan et al., 2022). Given the diverse nature of medicinal plants, a universal solution is unlikely, necessitating the exploration of new MG combinations for different species and even within the same species.
Over the past two decades, extensive basic research has elucidated many MGs, with ongoing discoveries providing continued insights for testing and refining their use. These advancements are complemented by the emergence of faster, more affordable, and efficient genome sequencing tools, paving the way for a deeper genetic understanding of medicinal plants. Furthermore, innovative strategies for controlling or limiting MG expression hold promise for enhancing transformation efficiency, making it more routine and accessible for diverse medicinal plant species. Additionally, the integration of artificial intelligence stands to further revolutionize this field by streamlining the research process, offering predictive insights into gene functions and interactions. This, in turn, facilitates CRISPR/Cas-mediated genome modifications in many important species and accelerates cultivar development. Ultimately, progress in in vitro cultivation, genetic transformation, and regeneration techniques is essential for ensuring the conservation and sustainable use of medicinal plants for present and future generations. The convergence of cutting-edge biotechnology and computational tools points towards a future where medicinal plant production is more predictable, efficient, and sustainable.
Glossary
Abbreviations:
- BBM
BABY BOOM
- Cas9
CRISPR-associated protein 9
- CRISPR
clustered regularly interspaced short palindromic repeats
- GIF
GRF-INTERACTING FACTOR 1
- GRF
GROWTH-REGULATING FACTOR
- gRNA
guide RNA
- MG
morphogenic gene
- NP
nanoparticle
- PGR
plant growth regulator
- SAM
shoot apical meristem
- SE
somatic embryogenesis
- TC
tissue culture
- TDZ
thidiazuron
- WUS
WUSCHEL
Contributor Information
Praveen Lakshman Bennur, Australian Research Council (ARC) Industrial Transformation Research Hub for Medicinal Agriculture, La Trobe Institute for Sustainable Agriculture and Food (LISAF), Department of Animal, Plant and Soil Sciences, La Trobe University, Victoria 3086, Australia.
Martin O’Brien, Australian Research Council (ARC) Industrial Transformation Research Hub for Medicinal Agriculture, La Trobe Institute for Sustainable Agriculture and Food (LISAF), Department of Animal, Plant and Soil Sciences, La Trobe University, Victoria 3086, Australia.
Shyama C Fernando, Australian Research Council (ARC) Industrial Transformation Research Hub for Medicinal Agriculture, La Trobe Institute for Sustainable Agriculture and Food (LISAF), Department of Animal, Plant and Soil Sciences, La Trobe University, Victoria 3086, Australia.
Monika S Doblin, Australian Research Council (ARC) Industrial Transformation Research Hub for Medicinal Agriculture, La Trobe Institute for Sustainable Agriculture and Food (LISAF), Department of Animal, Plant and Soil Sciences, La Trobe University, Victoria 3086, Australia.
Rainer Melzer, University College Dublin, Ireland.
Author contributions
PLB, MOB, and MSD: conceptualization; PLB: reviewing the referenced articles, and writing the original draft, and figure preparation; PLB and SCF: table preparation; MOB and MSD: supervision; PLB, MOB, and MSD: review and editing. All authors approved the final version of the text.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Australian Research Council to the Industrial Transformation Research Hub for Medicinal Agriculture (ARC MedAg Hub; IH180100006).
References
- Aasim M, Korkmaz E, Culu A, Kahveci B, Sonmezoglu OA.. 2023. TiO2 nanoparticle synthesis, characterization and application to shoot regeneration of water hyssop (Bacopa monnieri L. Pennel) in vitro. Biotechnic & Histochemistry 98, 29–37. [DOI] [PubMed] [Google Scholar]
- Abdelsalam A, Chowdhury K, El Bakry A.. 2018. Efficient adventitious morphogenesis from in vitro cultures of the medicinal plant Cymbopogon schoenanthus. Plant Tissue Culture and Biotechnology 28, 147–160. [Google Scholar]
- Ahlawat J, Sehrawat AR, Choudhary R, Yadav SK.. 2022. Biologically synthesized silver nanoparticles eclipse fungal and bacterial contamination in micropropagation of Capparis decidua (FORSK.) Edgew: a substitute to toxic substances. Indian Journal of Experimental Biology 58, 336–343. [Google Scholar]
- Ahmad A, Ahmad N, Anis M.. 2018. Preconditioning of nodal explants in thidiazuron-supplemented liquid media improves shoot multiplication in Pterocarpus marsupium (Roxb.). In: Ahmad N, Faisal M, eds. Thidiazuron: from urea derivative to plant growth regulator. Singapore: Springer, 175–187. [Google Scholar]
- Ahmad A, Anis M.. 2019. Meta-topolin improves in vitro morphogenesis, rhizogenesis and biochemical analysis in Pterocarpus marsupium Roxb.: a potential drug-yielding tree. Journal of Plant Growth Regulation 38, 1007–1016. [Google Scholar]
- Ahmadi B, Shariatpanahi ME, Teixeira da Silva JA.. 2014. Efficient induction of microspore embryogenesis using abscisic acid, jasmonic acid and salicylic acid in Brassica napus L. Plant Cell, Tissue and Organ Culture 116, 343–351. [Google Scholar]
- Ahmed S, Gao X, Jahan MA, Adams M, Wu N, Kovinich N.. 2021. Nanoparticle-based genetic transformation of Cannabis sativa. Journal of Biotechnology 326, 48–51. [DOI] [PubMed] [Google Scholar]
- Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M.. 1997. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. The Plant Cell 9, 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam N, Ahmad A, Ahmad N, Anis M.. 2023. Polyamines mediated in vitro morphogenesis in cotyledonary node explants of Mucuna pruriens (L.) DC.: a natural source of l-Dopa. Journal of Plant Growth Regulation 42, 5203–5215. [Google Scholar]
- Alfarraj NS, Tarroum M, Al-Qurainy F, Nadeem M, Khan S, Salih AM, Shaikhaldein HO, Al-Hashimi A, Alansi S, Perveen K.. 2023. Biosynthesis of silver nanoparticles and exploring their potential of reducing the contamination of the in vitro culture media and inducing the callus growth of Rumex nervosus explants. Molecules 28, 3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali HM, Khan T, Khan MA, Ullah N.. 2022. The multipotent thidiazuron: a mechanistic overview of its roles in callogenesis and other plant cultures in vitro. Biotechnology and Applied Biochemistry 69, 2624–2640. [DOI] [PubMed] [Google Scholar]
- Al Ramadan R, Karas M, Ranušová P, Moravčíková J.. 2021. Effect of silver nitrate on in vitro regeneration and antioxidant responses of oilseed rape cultivars (Brassica napus L.). Journal of Microbiology, Biotechnology and Food Sciences 10, e4494–e4494. [Google Scholar]
- Alttaher AGA, Balia Yusof ZN, Mahmood M, Shaharuddin N.. 2020. High-frequency induction of multiple shoots and plant regeneration from cotyledonary node explants of Tonghat Ali (Eurycoma longifolia Jack). Applied Ecology and Environmental Research 18, 6321–6333. [Google Scholar]
- Arruda MAZ, da Silva ABS, Kato LS.. 2023. There is plenty of room in plant science: nanobiotechnology as an emerging area applied to somatic embryogenesis. Journal of Agricultural and Food Chemistry 71, 3651–3657. [DOI] [PubMed] [Google Scholar]
- Asghar S, Ghori N, Hyat F, Li Y, Chen C.. 2023. Use of auxin and cytokinin for somatic embryogenesis in plant: a story from competence towards completion. Plant Growth Regulation 99, 413–428. [Google Scholar]
- Ayoobi A, Saboora A, Asgarani E, Efferth T.. 2024. Iron oxide nanoparticles (Fe3O4-NPs) elicited Artemisia annua L. in vitro, toward enhancing artemisinin production through overexpression of key genes in the terpenoids biosynthetic pathway and induction of oxidative stress. Plant Cell, Tissue and Organ Culture 156, 85. [Google Scholar]
- Bahari Z, Sazegari S, Niazi A, Afsharifar A.. 2019. The application of an Agrobacterium-mediated in planta transformation system in a Catharanthus roseus medicinal plant. Czech Journal of Genetics and Plant Breeding 56, 34–41. [Google Scholar]
- Bakos F, Darkó E, Pónya Z, Barnabás B.. 2003. Regeneration of fertile wheat (Triticum aestivum) plants from isolated zygotes using wheat microspore culture as nurse cells. Plant Cell, Tissue and Organ Culture 74, 243–247. [Google Scholar]
- Balamurugan V, Amal TC, Karthika P, Selvakumar S, Vasanth K.. 2019. Somatic embryogenesis and plant regeneration in Gloriosa superba L.: an endangered medicinal plant. In: Kumar M, Muthusamy A, Kumar V, Bhalla-Sarin N, eds. In vitro plant breeding towards novel agronomic traits: biotic and abiotic stress tolerance. Singapore: Springer, 27–42. [Google Scholar]
- Banjac N, Krstić-Milošević D, Mijalković T, Petrović M, Ćosić T, Stanišić M, Vinterhalter B.. 2023. In vitro shoot multiplication and regeneration of the recalcitrant rocket (Eruca sativa Mill.) variety Domaća Rukola. Horticulturae 9, 533. [Google Scholar]
- Banno H, Ikeda Y, Niu QW, Chua NH.. 2001. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. The Plant Cell 13, 2609–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbulescu DM, Burton WA, Salisbury PA.. 2011. Pluronic F-68: an answer for shoot regeneration recalcitrance in microspore-derived Brassica napus embryos. In Vitro Cellular & Developmental Biology - Plant 47, 282–288. [Google Scholar]
- Barberini S, Forti C, Laura M, Ciorba R, Mascarello C, Giovannini A, Ruffoni B, Savona M.. 2023. An optimized protocol for in vitro regeneration of Ocimum basilicum cv. FT Italiko. Horticulturae 9, 407. [Google Scholar]
- Bashir MA, Silvestri C, Salimonti A, Rugini E, Cristofori V, Zelasco S.. 2022. Can ethylene inhibitors enhance the success of olive somatic embryogenesis? Plants 11, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basiri Y, Etemadi N, Alizadeh M, Alizargar J.. 2022. In vitro culture of Eremurus spectabilis (Liliaceae), a rare ornamental and medicinal plant, through root explants. Horticulturae 8, 202. [Google Scholar]
- Baskaran P, Dasgupta I.. 2012. Gene delivery using microinjection of Agrobacterium to embryonic shoot apical meristem of elite indica rice cultivars. Journal of Plant Biochemistry and Biotechnology 21, 268–274. [Google Scholar]
- Ben Amar A, Cobanov P, Boonrod K, Krczal G, Bouzid S, Ghorbel A, Reustle GM.. 2007. Efficient procedure for grapevine embryogenic suspension establishment and plant regeneration: role of conditioned medium for cell proliferation. Plant Cell Reports 26, 1439–1447. [DOI] [PubMed] [Google Scholar]
- Benson EE. 2000. In vitro plant recalcitrance: an introduction. In Vitro Cellular & Developmental Biology - Plant 36, 141–148. [Google Scholar]
- Bhau BS, Wakhlu AK.. 2001. Effect of genotype, explant type and growth regulators on organogenesis in Morus alba. Plant Cell, Tissue and Organ Culture 66, 25–29. [Google Scholar]
- Bhojwani SS, Dantu PK.. 2013. Somatic embryogenesis. In: Bhojwani SS, Dantu PK, eds. Plant tissue culture: an introductory text. India: Springer, 75–92. [Google Scholar]
- Bonga JM. 2017. Can explant choice help resolve recalcitrance problems in in vitro propagation, a problem still acute especially for adult conifers? Trees 31, 781–789. [Google Scholar]
- Booker A, Johnston D, Heinrich M.. 2012. Value chains of herbal medicines—research needs and key challenges in the context of ethnopharmacology. Journal of Ethnopharmacology 140, 624–633. [DOI] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, et al. 2002. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. The Plant Cell 14, 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto JB. 2019. The role of natural products in modern drug discovery. Annals of the Brazilian Academy of Sciences 91, e20190105. [DOI] [PubMed] [Google Scholar]
- Canter PH, Thomas H, Ernst E.. 2005. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends in Biotechnology 23, 180–185. [DOI] [PubMed] [Google Scholar]
- Cao X, Xie H, Song M, et al. 2023. Cut–dip–budding delivery system enables genetic modifications in plants without tissue culture. Innovation 4, 100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Xie H, Song M, Zhao L, Liu H, Li G, Zhu JK.. 2024. Simple method for transformation and gene editing in medicinal plants. Journal of Integrative Plant Biology 66, 17–19. [DOI] [PubMed] [Google Scholar]
- Chaturvedi HC, Jain M, Kidwai NR.. 2007. Cloning of medicinal plants through tissue culture—a review. Indian Journal of Experimental Biology 45, 937–948. [PubMed] [Google Scholar]
- Chen J, Tomes S, Gleave AP, Hall W, Luo Z, Xu J, Yao JL.. 2022. Significant improvement of apple (Malus domestica Borkh.) transgenic plant production by pre-transformation with a Baby boom transcription factor. Horticulture Research 9, uhab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Cui Y, Shang X, Fu X.. 2023. Fitting levels of 6-benzylademine matching seasonal explants effectively stimulate adventitious shoot induction in Cyclocarya paliurus (Batal.) Iljinskaja. Plant Cell, Tissue and Organ Culture 156, 38. [Google Scholar]
- Ckurshumova W, Smirnova T, Marcos D, Zayed Y, Berleth T.. 2014. Irrepressible MONOPTEROS/ARF5 promotes de novo shoot formation. New Phytologist 204, 556–566. [DOI] [PubMed] [Google Scholar]
- Condic ML. 2014. Totipotency: what it is and what it is not. Stem Cells and Development 23, 796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćosić T, Motyka V, Savić J, Raspor M, Marković M, Dobrev PI, Ninković S.. 2021. Sucrose interferes with endogenous cytokinin homeostasis and expression of organogenesis-related genes during de novo shoot organogenesis in kohlrabi. Scientific Reports 11, 6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillerot JP, Windels D, Vazquez F, Michalski JC, Hilbert JL, Blervacq AS.. 2012. Pretreatments, conditioned medium and co-culture increase the incidence of somatic embryogenesis of different Cichorium species. Plant Signaling & Behavior 7, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham FJ, Goh NS, Demirer GS, Matos JL, Landry MP.. 2018. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends in Biotechnology 36, 882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimon Y, Takabe K, Tasaka M.. 2003. The CUP-SHAPED COTYLEDON genes promote adventitious shoot formation on calli. Plant and Cell Physiology 44, 113–121. [DOI] [PubMed] [Google Scholar]
- Debernardi JM, Tricoli DM, Ercoli MF, Hayta S, Ronald P, Palatnik JF, Dubcovsky J.. 2020. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nature Biotechnology 38, 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirer GS, Silva TN, Jackson CT, Thomas JB, Ehrhardt DW, Rhee SY, Mortimer JC, Landry MP.. 2021. Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nature Nanotechnology 16, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Luo K, Li Z, Yang Y.. 2009. A novel method for induction of plant regeneration via somatic embryogenesis. Plant Science 177, 43–48. [Google Scholar]
- De Saeger J, Park J, Chung HS, Hernalsteens J-P, Van Lijsebettens M, Inzé D, Van Montagu M, Depuydt S.. 2021. Agrobacterium strains and strain improvement: present and outlook. Biotechnology Advances 53, 107677. [DOI] [PubMed] [Google Scholar]
- Dewir YH, Nurmansyah, Naidoo Y, Teixeira da Silva JA.. 2018. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Reports 37, 1451–1470. [DOI] [PubMed] [Google Scholar]
- Dobson J. 2006. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Therapy 13, 283–287. [DOI] [PubMed] [Google Scholar]
- Dong OX, Ronald PC.. 2021. Targeted DNA insertion in plants. Proceedings of the National Academy of Sciences, USA 118, e2004834117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger M, Szalata M.. 2022. The effect of TIBA and NPA on shoot regeneration of Cannabis sativa L. epicotyl explants. Agronomy 12, 104. [Google Scholar]
- Duan H, Maren NA, Ranney TG, Liu W.. 2022. New opportunities for using WUS/BBM and GRF-GIF genes to enhance genetic transformation of ornamental plants. Ornamental Plant Research 2, 1–7. [Google Scholar]
- Ebrahimi M, Mokhtari A, Amirian R.. 2018. A highly efficient method for somatic embryogenesis of Kelussia odorotissima Mozaff., an endangered medicinal plant. Plant Cell, Tissue and Organ Culture 132, 99–110. [Google Scholar]
- Ebrahimzadegan R, Maroufi A.. 2022. In vitro regeneration and Agrobacterium-mediated genetic transformation of Dragon’s Head plant (Lallemantia iberica). Scientific Reports 12, 1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erland LAE, Shukla MR, Glover WB, Saxena PK.. 2017. A simple and efficient method for analysis of plant growth regulators: a new tool in the chest to combat recalcitrance in plant tissue culture. Plant Cell, Tissue and Organ Culture 131, 459–470. [Google Scholar]
- Farooq N, Ather L, Shafiq M, et al. 2022. Magnetofection approach for the transformation of okra using green iron nanoparticles. Scientific Reports 12, 16568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima N, Anis M.. 2021. Regulation of in vitro morphogenesis by modulation of culture conditions in Withania somnifera L. using cotyledonary node explants. In: Siddique I, ed. Propagation and genetic manipulation of plants. Singapore: Springer, 121–137. [Google Scholar]
- Fazal H, Abbasi BH, Ahmad N, Ali M.. 2016. Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Applied Biochemistry and Biotechnology 180, 1076–1092. [DOI] [PubMed] [Google Scholar]
- Fehér A. 2019. Callus, dedifferentiation, totipotency, somatic embryogenesis: what these terms mean in the era of molecular plant biology? Frontiers in Plant Science 10, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán-Ávila A, García-Fortea E, Prohens J, Herraiz FJ.. 2020. Development of a direct in vitro plant regeneration protocol from Cannabis sativa L. seedling explants: developmental morphology of shoot regeneration and ploidy level of regenerated plants. Frontiers in Plant Science 11, 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois JL, Nora FR, Mizukami Y, Sablowski R.. 2004. WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes and Development 18, 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharghi A, Nazeri A, Niazi A.. 2023. In planta Agrobacterium-mediated transformation of l-asparaginase gene into potato (Solanum tuberosum L.) cv. Agria: an efficient and novel method. Plant Biotechnology Reports 17, 149–158. [Google Scholar]
- Ghorbanpour M, Hadian J.. 2015. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 94, 749–759. [Google Scholar]
- Gómez-Galera S, Pelacho AM, Gené A, Capell T, Christou P.. 2007. The genetic manipulation of medicinal and aromatic plants. Plant Cell Reports 26, 1689–1715. [DOI] [PubMed] [Google Scholar]
- Gong Y, Gao F, Tang K, Debergh P.. 2005. In vitro high frequency direct root and shoot regeneration in sweet potato using the ethylene inhibitor silver nitrate. South African Journal of Botany 71, 110–113. [Google Scholar]
- Gordon-Kamm B, Sardesai N, Arling M, Lowe K, Hoerster G, Betts S, Jones AT.. 2019. Using morphogenic genes to improve recovery and regeneration of transgenic plants. Plants 8, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegorczyk-Karolak I, Hnatuszko-Konka K, Krzemińska M, Olszewska MA, Owczarek A.. 2021. Cytokinin-based tissue cultures for stable medicinal plant production: regeneration and phytochemical profiling of Salvia bulleyana shoots. Biomolecules 11, 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Liu L, Zhang H.. 2021. Transgene-free genome editing in plants. Frontiers in Genome Editing 3, 805317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Itami J, Oikawa K, Motoda Y, Kigawa T, Numata K.. 2019. Native protein delivery into rice callus using ionic complexes of protein and cell-penetrating peptides. PLoS One 14, e0214033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RD, Riksen-Bruinsma T, Weyens G, Lefebvre M, Dunwell JM, Krens FA.. 1996. Stomatal guard cells are totipotent. Plant Physiology 112, 889–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U, de Vries SC.. 2001. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiology 127, 803–816. [PMC free article] [PubMed] [Google Scholar]
- Heidmann I, De Lange B, Lambalk J, Angenent GC, Boutilier K.. 2011. Efficient sweet pepper transformation mediated by the BABY BOOM transcription factor. Plant Cell Reports 30, 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesami M, Condori-Apfata JA, Valderrama Valencia M, Mohammadi M.. 2020. Application of artificial neural network for modeling and studying in vitro genotype-independent shoot regeneration in wheat. Applied Sciences 10, 5370. [Google Scholar]
- Hesami M, Daneshvar MH.. 2018. In vitro adventitious shoot regeneration through direct and indirect organogenesis from seedling-derived hypocotyl segments of Ficus religiosa L.: an important medicinal plant. HortScience 53, 55–61. [Google Scholar]
- Hesami M, Jones AMP.. 2020. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Applied Microbiology and Biotechnology 104, 9449–9485. [DOI] [PubMed] [Google Scholar]
- Hoerster G, Wang N, Ryan L, Wu E, Anand A, McBride K, Lowe K, Jones T, Gordon-Kamm B.. 2020. Use of non-integrating Zm-Wus2 vectors to enhance maize transformation. In Vitro Cellular & Developmental Biology - Plant 56, 265–279. [Google Scholar]
- Hu H, Xiong L, Yang Y.. 2005. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 222, 107–117. [DOI] [PubMed] [Google Scholar]
- Hu W, Fagundez S, Katin-Grazzini L, et al. 2017. Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Horticulture Research 4, 17071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Banno H, Niu QW, Howell SH, Chua NH.. 2006. The ENHANCER OF SHOOT REGENERATION 2 gene in Arabidopsis regulates CUP-SHAPED COTYLEDON 1 at the transcriptional level and controls cotyledon development. Plant and Cell Physiology 47, 1443–1456. [DOI] [PubMed] [Google Scholar]
- Isah T. 2020. Nodal segment explant type and preconditioning influence in vitro shoot morphogenesis in Ginkgo biloba L. Plant Physiology Reports 25, 74–86. [Google Scholar]
- Jaberi M, Azadi P, Gharehyazi B, Khosrowchahli M, Sharafi A, Aboofazeli N, Bagheri H.. 2018. Silver nitrate and adenine sulphate induced high regeneration frequency in the recalcitrant plant Cosmos bipinnatus using cotyledon explants. The Journal of Horticultural Science and Biotechnology 93, 204–208. [Google Scholar]
- Jha P, Kumar V.. 2018. BABY BOOM (BBM): a candidate transcription factor gene in plant biotechnology. Biotechnology Letters 40, 1467–1475. [DOI] [PubMed] [Google Scholar]
- Jones TJ. 2009. Maize tissue culture and transformation: the first 20 years. In: Kriz AL, Larkins BA, eds. Molecular genetic approaches to maize improvement. Berlin, Heidelberg: Springer, 7–27. [Google Scholar]
- Juturu VN, Mekala GK, Kirti PB.. 2015. Current status of tissue culture and genetic transformation research in cotton (Gossypium spp.). Plant Cell, Tissue and Organ Culture 120, 813–839. [Google Scholar]
- Kadri A, Grenier De March G, Guerineau F, Cosson V, Ratet P.. 2021. WUSCHEL overexpression promotes callogenesis and somatic embryogenesis in Medicago truncatula Gaertn. Plants 10, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthik S, Pavan G, Sathish S, Siva R, Kumar PS, Manickavasagam M.. 2018. Genotype-independent and enhanced in planta Agrobacterium tumefaciens-mediated genetic transformation of peanut [Arachis hypogaea (L.)]. Biotech 8, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur RP, Devi S.. 2019. In planta transformation in plants: a review. Agricultural Reviews 40, 159–174. [Google Scholar]
- Kausch AP, Nelson-Vasilchik K, Hague J, Mookkan M, Quemada H, Dellaporta S, Fragoso C, Zhang ZJ.. 2019. Edit at will: genotype independent plant transformation in the era of advanced genomics and genome editing. Plant Science 281, 186–205. [DOI] [PubMed] [Google Scholar]
- Kenta T, Edwards JE, Butlin RK, Burke T, Quick WP, Urwin P, Davey MP.. 2016. Tissue culture as a source of replicates in nonmodel plants: variation in cold response in Arabidopsis lyrata ssp. petraea. G3 6, 3817–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-M, Kim MY, Yun PY, Chandrasekhar T, Lee H-Y, Song P-S.. 2007. Production of multiple shoots and plant regeneration from leaf segments of fig tree (Ficus carica L.). Journal of Plant Biology 50, 440–446. [Google Scholar]
- Kim S-H, Lee H-S, Ryu D-S, Choi S-J, Lee D-S.. 2011. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean Journal of Microbiology and Biotechnology 39, 77–85. [Google Scholar]
- Koike I, Watanabe S, Okazaki K, Hayashi K-i, Kasahara H, Shimomura K, Umehara M.. 2020. Endogenous auxin determines the pattern of adventitious shoot formation on internodal segments of ipecac. Planta 251, 73. [DOI] [PubMed] [Google Scholar]
- Krishna H, Alizadeh M, Singh D, Singh U, Chauhan N, Eftekhari M, Sadh RK.. 2016. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 6, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Parvatam G, Ravishankar GA.. 2009. AgNO3: a potential regulator of ethylene activity and plant growth modulator. Electronic Journal of Biotechnology 12, 1–15. [Google Scholar]
- Lee K, Wang K.. 2023. Strategies for genotype-flexible plant transformation. Current Opinion in Biotechnology 79, 102848. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee BH, Jung JH, Park SK, Song JT, Kim JH.. 2018. GROWTH-REGULATING FACTOR and GRF-INTERACTING FACTOR specify meristematic cells of gynoecia and anthers. Plant Physiology 176, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, To JPC, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU.. 2005. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438, 1172–1175. [DOI] [PubMed] [Google Scholar]
- Lenhard M, Jürgens G, Laux T.. 2002. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 129, 3195–3206. [DOI] [PubMed] [Google Scholar]
- Li J, Xu Z, Zeng T, Zhou L, Li J, Hu H, Luo J, Wang C.. 2022. Overexpression of TcCHS increases pyrethrin content when using a genotype-independent transformation system in Pyrethrum (Tanacetum cinerariifolium). Plants 11, 1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li H, Zhao Y, Zong P, Zhan Z, Piao Z.. 2021. Establishment of a simple and efficient Agrobacterium-mediated genetic transformation system to Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Horticultural Plant Journal 7, 117–128. [Google Scholar]
- Liu Y, Zhang L, Li C, Yang Y, Duan Y, Yang Y, Sun X.. 2022. Establishment of Agrobacterium-mediated genetic transformation and application of CRISPR/Cas9 genome-editing system to Brassica rapa var. rapa. Plant Methods 18, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Yang Y, Pan G, Shen Y.. 2022. New insights into tissue culture plant-regeneration mechanisms. Frontiers in Plant Science 13, 926752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Huang Y, Wang W, Cai Z, Cai H, Liu Z, Sun L, Xu Q.. 2022. Overexpression of the AtWUSCHEL gene promotes somatic embryogenesis and lateral branch formation in birch (Betula platyphylla Suk.). Plant Cell, Tissue and Organ Culture 150, 371–383. [Google Scholar]
- Lowe K, La Rota M, Hoerster G, Hastings C, Wang N, Chamberlin M, Wu E, Jones T, Gordon-Kamm W.. 2018. Rapid genotype ‘independent’ Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cellular & Development Biology - Plant 54, 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe K, Wu E, Wang N, et al. 2016. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. The Plant Cell 28, 1998–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Lu S, Su C, Deng S, Wang M, Tang H, Wang Z, Li G, Lang Z, Zhu J-K.. 2024. Tissue culture-free transformation of traditional Chinese medicinal plants with root suckering capability. Horticulture Research 11, uhad290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Zheng X, Chen Y, Xiao Y, Zhao D, McAvoy R, Pei Y, Li Y.. 2006. The maize Knotted1 gene is an effective positive selectable marker gene for Agrobacterium-mediated tobacco transformation. Plant Cell Reports 25, 403–409. [DOI] [PubMed] [Google Scholar]
- Lutz KA, Martin C, Khairzada S, Maliga P.. 2015. Steroid-inducible BABY BOOM system for development of fertile Arabidopsis thaliana plants after prolonged tissue culture. Plant Cell Reports 34, 1849–1856. [DOI] [PubMed] [Google Scholar]
- Lv Z, Jiang R, Chen J, Chen W.. 2020. Nanoparticle-mediated gene transformation strategies for plant genetic engineering. The Plant Journal 104, 880–891. [DOI] [PubMed] [Google Scholar]
- Ma R, Yu Z, Cai Q, Li H, Dong Y, Oksman-Caldentey KM, Rischer H.. 2020. Agrobacterium-mediated genetic transformation of the medicinal plant Veratrum dahuricum. Plants 9, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran D, Kavi Kishor P, Geetha N, Venkatachalam P.. 2018. Phycomolecule-coated silver nanoparticles and seaweed extracts induced high-frequency somatic embryogenesis and plant regeneration from Gloriosa superba L. Journal of Applied Phycology 30, 1425–1436. [Google Scholar]
- Majumder S, Sarkar C, Datta K, Datta SK.. 2020. Establishment of the ‘imbibed seed piercing’ method for Agrobacterium-mediated transformation of jute and flax bast fibre crops via phloem-specific expression of the β-glucuronidase gene. Industrial Crops and Products 154, 112620. [Google Scholar]
- Mandeh M, Omidi M, Rahaie M.. 2012. In vitro influences of TiO2 nanoparticles on barley (Hordeum vulgare L.) tissue culture. Biological Trace Element Research 150, 376–380. [DOI] [PubMed] [Google Scholar]
- Manh Cuong D, Cong Du P, Tung HT, Ngan HTM, Luan VQ, Phong TH, Khai HD, Phuong TTB, Nhut DT.. 2021. Silver nanoparticles as an effective stimulant in micropropagation of Panax vietnamensis—a valuable medicinal plant. Plant Cell, Tissue and Organ Culture 146, 577–588. [Google Scholar]
- Maren NA, Duan H, Da K, Yencho GC, Ranney TG, Liu W.. 2022. Genotype-independent plant transformation. Horticulture Research 9, uhac047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnichenko D, Chaban I, Chernobrovkina M, Dolgov S.. 2017. Protocol for efficient regulation of in vitro morphogenesis in einkorn (Triticum monococcum L.), a recalcitrant diploid wheat species. PLoS One 12, e0173533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookkan M, Andy G.. 2014. AgNO3 boosted high-frequency shoot regeneration in Vigna mungo (L.) Hepper. Plant Signaling & Behavior 9, e972284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookkan M, Nelson-Vasilchik K, Hague J, Zhang ZJ, Kausch AP.. 2017. Selectable marker independent transformation of recalcitrant maize inbred B73 and sorghum P898012 mediated by morphogenic regulators BABY BOOM and WUSCHEL2. Plant Cell Reports 36, 1477–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HK, Kim YW, Hong YP, Park SY.. 2013. Improvement of somatic embryogenesis and plantlet conversion in Oplopanax elatus, an endangered medicinal woody plant. Springerplus 2, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore KS, Nam J, Gelvin SB.. 2000. An Arabidopsis histone H2A mutant is deficient in Agrobacterium T-DNA integration. Proceedings of the National Academy of Sciences, USA 97, 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapalli S, Tunc-Ozdemir M, Sun Y, Elumalai S, Que Q.. 2021. Morphogenic regulators and their application in improving plant transformation. Methods in Molecular Biology 2238, 37–61. [DOI] [PubMed] [Google Scholar]
- Neves M, Correia S, Canhoto J.. 2023. Ethylene inhibition reduces de novo shoot organogenesis and subsequent plant development from leaf explants of Solanum betaceum Cav. Plants 12, 1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves M, Correia S, Cavaleiro C, Canhoto J.. 2021. Modulation of organogenesis and somatic embryogenesis by ethylene: an overview. Plants 10, 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi I, Sakamoto Y, Kuwae H, Kasahara H, Sugiyama M.. 2022. Enhancement of shoot regeneration by treatment with inhibitors of auxin biosynthesis and transport during callus induction in tissue culture of Arabidopsis thaliana. Plant Biotechnology 39, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal Bais H, Ravishankar GA.. 2002. Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell, Tissue and Organ Culture 69, 1–34. [Google Scholar]
- Pandey V, Misra P, Chaturvedi P, Mishra MK, Trivedi PK, Tuli R.. 2010. Agrobacterium tumefaciens-mediated transformation of Withania somnifera (L.) Dunal: an important medicinal plant. Plant Cell Reports 29, 133–141. [DOI] [PubMed] [Google Scholar]
- Patel S, Jung D, Yin PT, Carlton P, Yamamoto M, Bando T, Sugiyama H, Lee KB.. 2014. NanoScript: a nanoparticle-based artificial transcription factor for effective gene regulation. ACS Nano 8, 8959–8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C, Montalbán IA, Pedrosa A, Tavares J, Pestryakov A, Bogdanchikova N, Canhoto J, Moncaleán P.. 2021. Regeneration of Pinus halepensis (Mill.) through organogenesis from apical shoot buds. Forests 12, 363. [Google Scholar]
- Pérez-Pascual D, Jiménez-Guillen D, Villanueva-Alonzo H, Souza-Perera R, Godoy-Hernández G, Zúñiga-Aguilar JJ.. 2018. Ectopic expression of the Coffea canephora SERK1 homolog-induced differential transcription of genes involved in auxin metabolism and in the developmental control of embryogenesis. Physiologia Plantarum 163, 530–551. [DOI] [PubMed] [Google Scholar]
- Phillips GC, Garda M.. 2019. Plant tissue culture media and practices: an overview. In Vitro Cellular & Developmental Biology - Plant 55, 242–257. [Google Scholar]
- Phong TH, Hieu T, Tung HT, Mai NTN, Khai HD, Cuong DM, Luan VQ, Nam NB, Nhut DT.. 2023. Silver nanoparticles enhance the in vitro plant regeneration via thin cell layer culture system in purple passion fruit. Plant Cell, Tissue and Organ Culture 155, 403–415. [Google Scholar]
- Pinar H, Mutlu N, Yildiz S, Simsek D, Shams M.. 2020. Transferring the cultured anther to a medium without activated charcoal overcomes the recalcitrance in pepper genotypes. Canadian Journal of Plant Science 101, 151–156. [Google Scholar]
- Potrykus I. 1990. Gene transfer to cereals: an assessment. Bio/Technology 8, 535–542. [Google Scholar]
- Prem Kumar G, Sivakumar S, Siva G, Vigneswaran M, Senthil Kumar T, Jayabalan N.. 2016. Silver nitrate promotes high-frequency multiple shoot regeneration in cotton (Gossypium hirsutum L.) by inhibiting ethylene production and phenolic secretion. In Vitro Cellular & Developmental Biology - Plant 52, 408–418. [Google Scholar]
- Purnamaningsih R, Dewi IS, Sukmadjaya D, Apriana A, Purwoko BS.. 2024. Isolated microspore culture for embryoid production in Artemisia annua (L). Plant Cell, Tissue and Organ Culture 157, 5. [Google Scholar]
- Rahman SU, Khan MO, Ullah R, Ahmad F, Raza G.. 2023. Agrobacterium-mediated transformation for the development of transgenic crops; present and future prospects. Molecular Biotechnology doi: https://doi.org/ 10.1007/s12033-023-00826-8. [DOI] [PubMed] [Google Scholar]
- Rajan RK, Sabu T, Nandakumar K, Kochupurakkal J.. 2021. Casein stabilized metal and metal oxide nanoparticles for the efficient in vitro culturing of Scoparia dulcis L. Journal of the Siberian Federal University. Biology 14, 498–509. [Google Scholar]
- Rakhimol KR, Ashitha A, Thomas S, Kalarikkal N, Jayachandran K.. 2023. Casein stabilized metal and metal oxide nanoparticle to eradicate the endophytic bacterial contamination from in vitro culturing of Scoparia dulcis L. Plant Cell, Tissue and Organ Culture 155, 521–529. [Google Scholar]
- Raspor M, Motyka V, Kaleri AR, Ninković S, Tubić L, Cingel A, Ćosić T.. 2021. Integrating the roles for cytokinin and auxin in de novo shoot organogenesis: from hormone uptake to signaling outputs. International Journal of Molecular Sciences 22, 8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressayre A, Godelle B, Mignot A, Gouyon PH.. 1998. A morphogenetic model accounting for pollen aperture pattern in flowering plants. Journal of Theoretical Biology 193, 321–334. [DOI] [PubMed] [Google Scholar]
- Ricci A, Capriotti L, Mezzetti B, Navacchi O, Sabbadini S.. 2020. Adventitious shoot regeneration from in vitro leaf explants of the peach rootstock hansen 536. Plants 9, 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha N, Dutta Gupta S.. 2018. Promotion of shoot regeneration of Swertia chirata by biosynthesized silver nanoparticles and their involvement in ethylene interceptions and activation of antioxidant activity. Plant Cell, Tissue and Organ Culture 134, 289–300. [Google Scholar]
- Sahoo S, Lenka J, Kar B, Nayak S.. 2023. Clonal fidelity and phytochemical analysis of in vitro propagated Kaempferia rotunda Linn.—an endangered medicinal plant. In Vitro Cellular & Developmental Biology - Plant 59, 329–339. [Google Scholar]
- Salih AM, Al-Qurainy F, Khan S, Tarroum M, Nadeem M, Shaikhaldein HO, Gaafar A-RZ, Alfarraj NS.. 2021. Biosynthesis of zinc oxide nanoparticles using Phoenix dactylifera and their effect on biomass and phytochemical compounds in Juniperus procera. Scientific Reports 11, 19136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranya Krishnan SR, Siril EA.. 2017. Enhanced in vitro shoot regeneration in Oldenlandia umbellata L. by using quercetin: a naturally occurring auxin-transport inhibitor. Proceedings of the National Academy of Sciences, India Section B 87, 899–904. [Google Scholar]
- Sarmast MK, Salehi H.. 2016. Silver nanoparticles: an influential element in plant nanobiotechnology. Molecular Biotechnology 58, 441–449. [DOI] [PubMed] [Google Scholar]
- Sedeek KEM, Mahas A, Mahfouz M.. 2019. Plant genome engineering for targeted improvement of crop traits. Frontiers in Plant Science 10, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena S, Ochatt SJ, Kumar V.. 2023. Application of green synthesized nanoparticles in medicinal plant research: revisiting an emerging eco-friendly approach. Plant Cell, Tissue and Organ Culture 155, 345–384. [Google Scholar]
- Sharma S, Satardekar KV, Barve SS.. 2018. Genetic improvement of medicinal and aromatic plants through haploid and double haploid development. In: Kumar N, ed. Biotechnological approaches for medicinal and aromatic plants: conservation, genetic improvement and utilization. Singapore: Springer, 523–556. [Google Scholar]
- Shires ME, Florez SL, Lai TS, Curtis WR.. 2017. Inducible somatic embryogenesis in Theobroma cacao achieved using the DEX-activatable transcription factor–glucocorticoid receptor fusion. Biotechnology Letters 39, 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrawat AK, Lörz H.. 2006. Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnology Journal 4, 575–603. [DOI] [PubMed] [Google Scholar]
- Sivanesan I, Nayeem S, Venkidasamy B, Kuppuraj SP, Rn C, Samynathan R.. 2022. Genetic and epigenetic modes of the regulation of somatic embryogenesis: a review. Biologia Futura 73, 259–277. [DOI] [PubMed] [Google Scholar]
- Šmeringai J, Schrumpfová PP, Pernisová M.. 2023. Cytokinins—regulators of de novo shoot organogenesis. Frontiers in Plant Science 14, 1239133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smýkalová I, Vrbová M, Cvečková M, Plačková L, Žukauskaitė A, Zatloukal M, Hrdlička J, Plíhalová L, Doležal K, Griga M.. 2019. The effects of novel synthetic cytokinin derivatives and endogenous cytokinins on the in vitro growth responses of hemp (Cannabis sativa L.) explants. Plant Cell, Tissue and Organ Culture 139, 381–394. [Google Scholar]
- Squire HJ, Tomatz S, Voke E, González-Grandío E, Landry M.. 2023. The emerging role of nanotechnology in plant genetic engineering. Nature Reviews. Bioengineering 1, 314–328. [Google Scholar]
- Srinivasan C, Liu Z, Heidmann I, et al. 2007. Heterologous expression of the BABY BOOM AP2/ERF transcription factor enhances the regeneration capacity of tobacco (Nicotiana tabacum L.). Planta 225, 341–351. [DOI] [PubMed] [Google Scholar]
- Sticklen MB, Oraby HF.. 2005. Shoot apical meristem: a sustainable explant for genetic transformation of cereal crops. In Vitro Cellular & Developmental Biology - Plant 41, 187–200. [Google Scholar]
- Su W, Xu M, Radani Y, Yang L.. 2023. Technological development and application of plant genetic transformation. International Journal of Molecular Sciences 24, 10646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan S, Rajendran V, Nayeem S, Ramalingam S.. 2020. Physicochemical factors modulate regeneration and Agrobacterium-mediated genetic transformation of recalcitrant indica rice cultivars—ASD16 and IR64. Biocatalysis and Agricultural Biotechnology 24, 101519. [Google Scholar]
- Tenea GN, Spantzel J, Lee LY, Zhu Y, Lin K, Johnson SJ, Gelvin SB.. 2009. Overexpression of several Arabidopsis histone genes increases Agrobacterium-mediated transformation and transgene expression in plants. The Plant Cell 21, 3350–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddenberg D, Abrahamsson M, von Arnold S.. 2016. Overexpression of PaHAP3A stimulates differentiation of ectopic embryos from maturing somatic embryos of Norway spruce. Tree Genetics and Genomes 12, 18. [Google Scholar]
- Venkatachalam P, Jinu U, Gomathi M, Mahendran D, Ahmad N, Geetha N, Sahi SV.. 2017. Role of silver nitrate in plant regeneration from cotyledonary nodal segment explants of Prosopis cineraria (L.) Druce.: a recalcitrant medicinal leguminous tree. Biocatalysis and Agricultural Biotechnology 12, 286–291. [Google Scholar]
- von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L.. 2002. Developmental pathways of somatic embryogenesis. Plant Cell, Tissue and Organ Culture 69, 233–249. [Google Scholar]
- Wang JW, Squire HJ, Goh NS, Ni HM, Lien E, Wong C, González-Grandío E, Landry MP.. 2023. Delivered complementation in planta (DCIP) enables measurement of peptide-mediated protein delivery efficiency in plants. Communications Biology 6, 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Niu Q-W, Teng C, Li C, Mu J, Chua N-H, Zuo J.. 2009. Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Research 19, 224–235. [DOI] [PubMed] [Google Scholar]
- Wang ZP, Zhang ZB, Zheng DY, Zhang TT, Li XL, Zhang C, Yu R, Wei JH, Wu ZY.. 2022. Efficient and genotype independent maize transformation using pollen transfected by DNA-coated magnetic nanoparticles. Journal of Integrative Plant Biology 64, 1145–1156. [DOI] [PubMed] [Google Scholar]
- Wójcikowska B, Jaskóła K, Gąsiorek P, Meus M, Nowak K, Gaj MD.. 2013. LEAFY COTYLEDON2 (LEC2) promotes embryogenic induction in somatic tissues of Arabidopsis, via YUCCA-mediated auxin biosynthesis. Planta 238, 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhang C, Yang H, Hu J, Zou L.. 2022. In vitro propagation via organogenesis and formation of globular bodies of Salvia plebeia: a valuable medicinal plant. In Vitro Cellular & Developmental Biology - Plant 58, 51–60. [Google Scholar]
- Wu W, Chen W, Liu S, Wu J, Zhu Y, Qin L, Zhu B.. 2021. Beneficial relationships between endophytic bacteria and medicinal plants. Frontiers in Plant Science 12, 646146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Zhang C, Liu Y, Wang X, You C.. 2023. Functional identification of apple Baby Boom in genetic transformation and somatic embryogenesis. In Vitro Cellular & Developmental Biology - Plant 59, 1–13. [Google Scholar]
- Xu H, Guo Y, Qiu L, Ran Y.. 2022. Progress in soybean genetic transformation over the last decade. Frontiers in Plant Science 13, 900318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Hou Q, Wei X, Qi Y, Pu A, Wu S, An X, Wan X.. 2023. Promoting genotype-independent plant transformation by manipulating developmental regulatory genes and/or using nanoparticles. Plant Cell Reports 42, 1395–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Saand MA, Huang L, Abdelaal WB, Sirohi MH.. 2021. Applications of multi-omics technologies for crop improvement. Frontiers in Plant Science 12, 563953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz M. 2012. The prerequisite of the success in plant tissue culture: high frequency shoot regeneration. In: Leva A, Rinaldi LMR, eds. Recent advances in plant in vitro culture. Rijeka: Intech, 63–90. [Google Scholar]
- Yu G, Wang J, Miao L, Xi M, Wang Q, Wang K.. 2019. Optimization of mature embryo-based tissue culture and Agrobacterium-mediated transformation in model grass Brachypodium distachyon. International Journal of Molecular Sciences 20, 5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang W, Wang Y, Hou B.. 2012. High frequency wheat regeneration from leaf tissue explants of regenerated plantlets. Advances in Bioscience and Biotechnology 03, 46–50. [Google Scholar]
- Zhang X, Xu G, Cheng C, et al. 2021. Establishment of an Agrobacterium-mediated genetic transformation and CRISPR/Cas9-mediated targeted mutagenesis in Hemp (Cannabis sativa L.). Plant Biotechnology Journal 19, 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Meng Z, Wang Y, et al. 2017. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nature Plants 3, 956–964. [DOI] [PubMed] [Google Scholar]
- Zhu S-P, Wang J, J-l Y, Zhu A-D, Guo W-W, Deng X-X.. 2014. Isolation and characterization of LEAFY COTYLEDON 1-LIKE gene related to embryogenic competence in Citrus sinensis. Plant Cell, Tissue and Organ Culture 119, 1–13. [Google Scholar]
- Zuo J, Niu QW, Ikeda Y, Chua NH.. 2002. Marker-free transformation: increasing transformation frequency by the use of regeneration-promoting genes. Current Opinion in Biotechnology 13, 173–180. [DOI] [PubMed] [Google Scholar]