Abstract
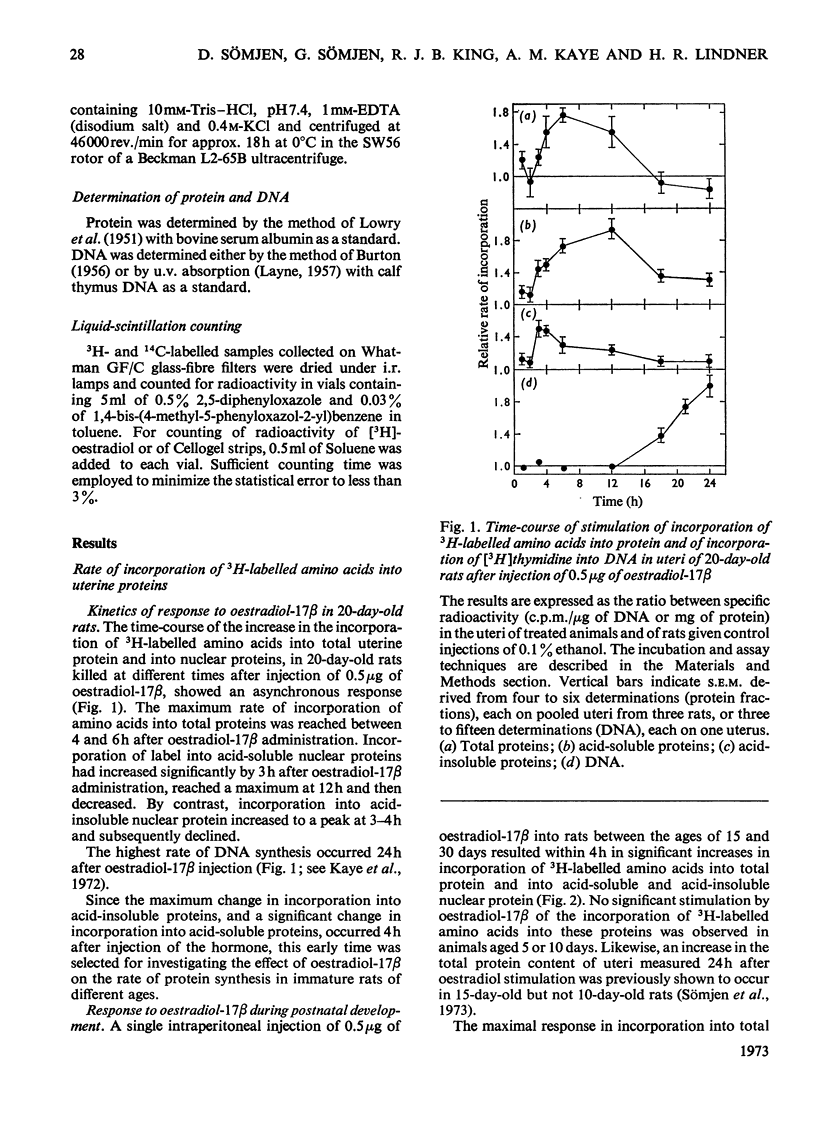
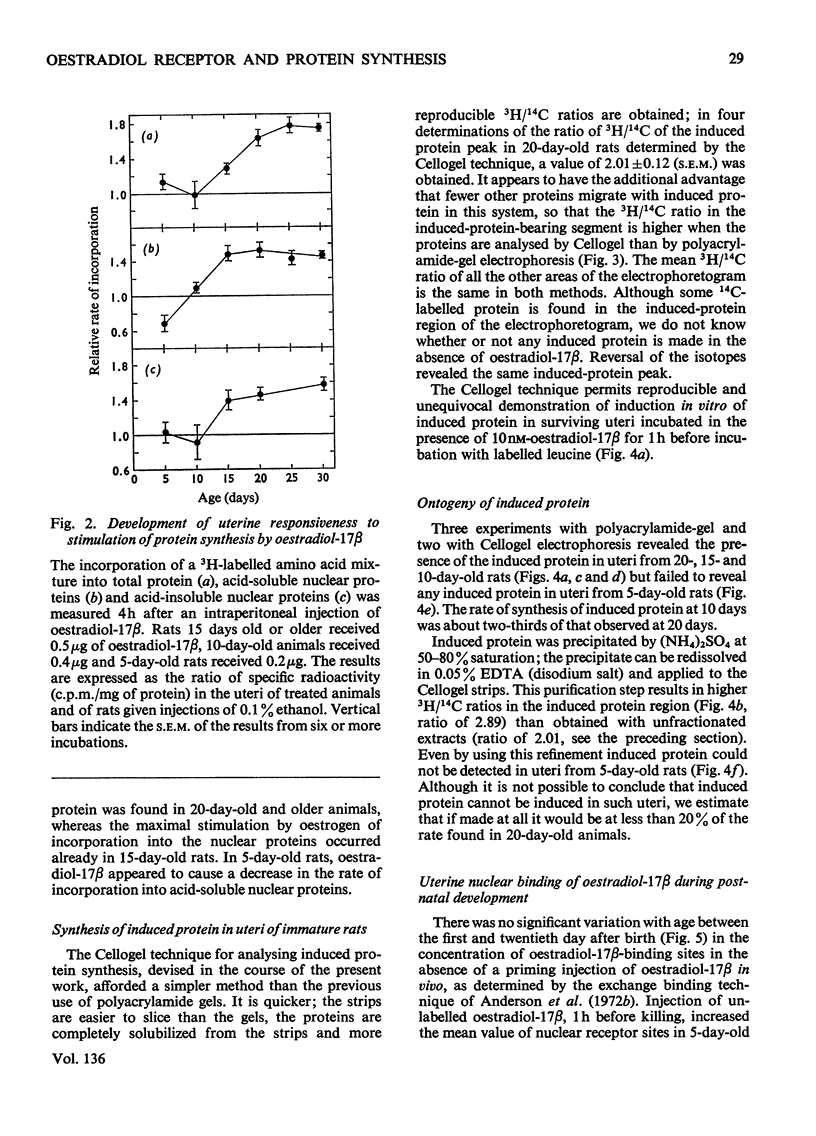
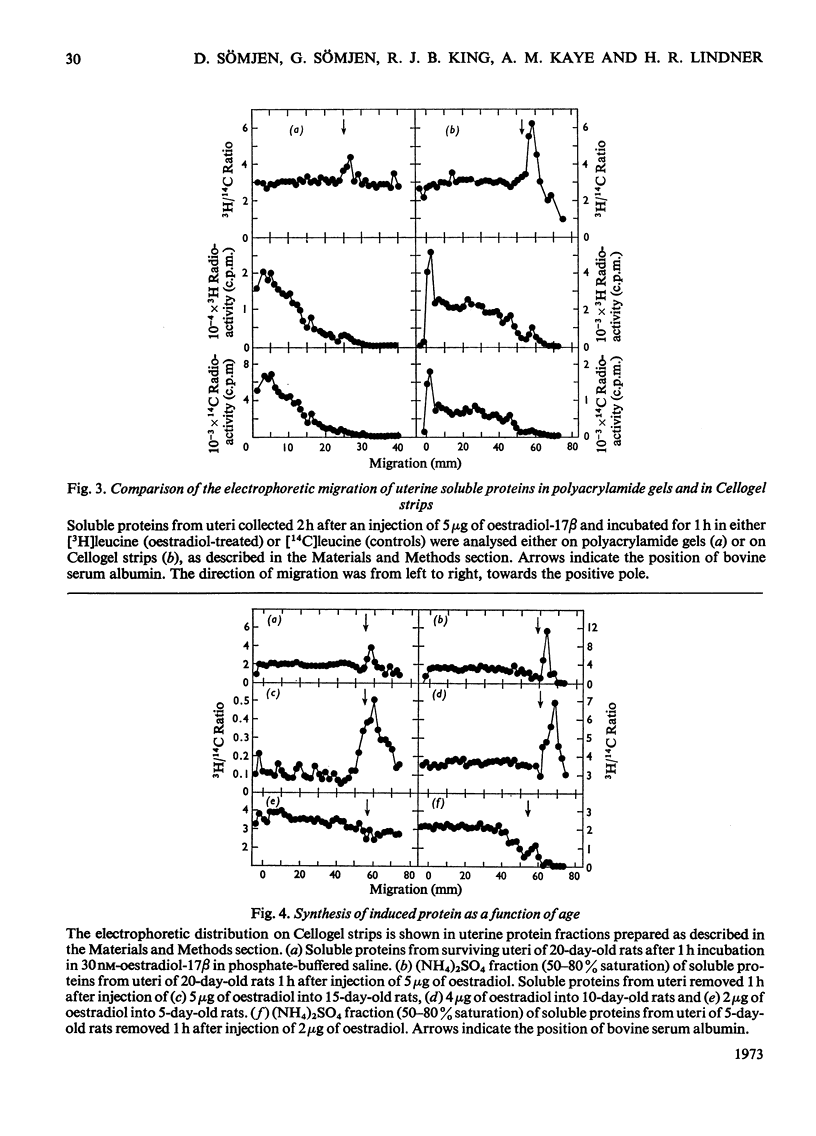
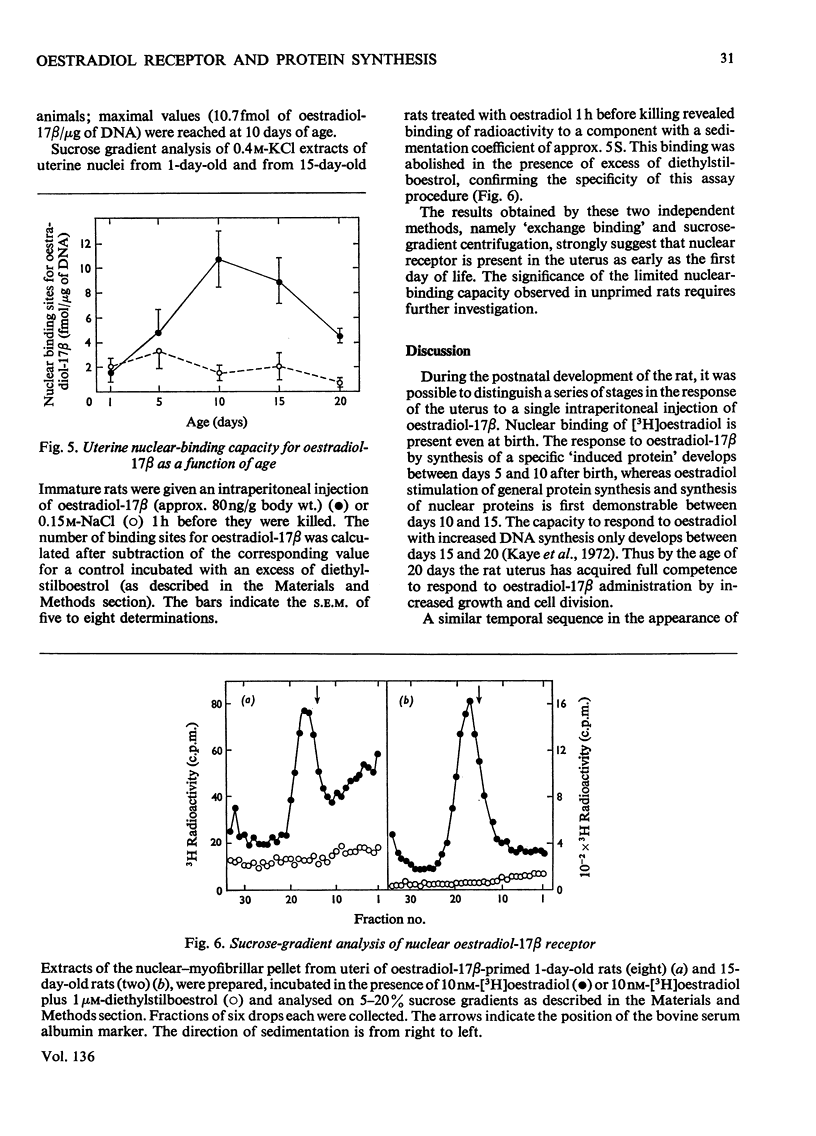
1. The uterine response to a single injection of oestradiol-17β during postnatal development of the rat was studied with respect to (i) nuclear binding of oestradiol-17β; (ii) induction of the synthesis of a specific cytoplasmic protein (`induced protein' of Gorski); (iii) rate of incorporation of 3H-labelled amino acids into total protein and into nuclear acid-soluble and acid-insoluble protein; and (iv) rate of [3H]thymidine incorporation into DNA. 2. Specific nuclear binding of oestradiol-17β could be demonstrated even at birth. Administration of oestradiol-17β in vivo caused a significant increase in the number of nuclear binding sites in rats aged 10 days or older. 3. A rapid method is described for the detection of the `induced protein', based on cellulose acetate electrophoresis. Induction of this protein could be demonstrated at the age of 10, 15 and 20 days, but not in 5-day-old rats. 4. In 20-day-old rats the rate of 3H-labelled amino acid incorporation into protein increased by 3h after oestradiol administration. Incorporation into the different protein fractions reached peak values asynchronously: at 3–4h for acid-insoluble nuclear protein, at 6h for total protein and at about 12h for acid-soluble protein. 5. Treatment with oestradiol failed to stimulate amino acid incorporation into protein in 5- or 10-day-old rats; at the age of 15 to 30 days the hormone caused a significant increase in incorporation into total protein and into both types of nuclear protein. 6. Since the capacity for nuclear binding of oestradiol and for synthesis of the induced protein is demonstrable in the rat uterus before it acquires the ability to respond to the hormone with enhanced general protein synthesis and DNA synthesis, it appears that nuclear binding and the synthesis of the induced protein may be necessary but not sufficient conditions for the trophic action of oestradiol.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. N., Clark J. H., Peck E. J., Jr The relationship between nuclear receptor-estrogen binding and uterotrophic responses. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1460–1468. doi: 10.1016/0006-291x(72)90878-9. [DOI] [PubMed] [Google Scholar]
- Anderson J., Clark J. H., Peck E. J., Jr Oestrogen and nuclear binding sites. Determination of specific sites by ( 3 H)oestradiol exchange. Biochem J. 1972 Feb;126(3):561–567. doi: 10.1042/bj1260561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSH I. E. Methods of paper chromatography of steroids applicable to the study of steroids in mammalian blood and tissues. Biochem J. 1952 Jan;50(3):370–378. doi: 10.1042/bj0500370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A., Gorski J. Estrogen-induced protein. Time course of synthesis. Biochemistry. 1970 Apr 28;9(9):1899–1904. doi: 10.1021/bi00811a006. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Campbell P. S., Peck E. J., Jr Receptor-estrogen complex in the nuclear fraction of the pituitary and hypothalamus of male and female immature rats. Neuroendocrinology. 1972;10(4):218–228. doi: 10.1159/000122091. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Gorski J. Ontogeny of the estrogen receptor during early uterine development. Science. 1970 Jul 3;169(3940):76–78. doi: 10.1126/science.169.3940.76. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelo A. B., Gorski J. Role of RNA synthesis in the estrogen induction of a specific uterine protein. Proc Natl Acad Sci U S A. 1970 Jul;66(3):693–700. doi: 10.1073/pnas.66.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Hamilton T. H. Control by estrogen of genetic transcription and translation. Binding to chromatin and stimulation of nucleolar RNA synthesis are primary events in the early estrogen action. Science. 1968 Aug 16;161(3842):649–661. doi: 10.1126/science.161.3842.649. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Gorski J. Estrogen action in vitro. Induction of the synthesis of a specific uterine protein. J Biol Chem. 1972 Feb 25;247(4):1299–1305. [PubMed] [Google Scholar]
- Kaye A. M., Icekson I., Lindner H. R. Stimulation by estrogens of ornithine and S-adenosylmethionine decarboxylases in the immature rat uterus. Biochim Biophys Acta. 1971 Oct;252(1):150–159. doi: 10.1016/0304-4165(71)90103-6. [DOI] [PubMed] [Google Scholar]
- Kaye A. M., Sheratzky D., Lindner H. R. Kinetics of DNA synthesis in immature rat uterus: age dependence and estradiol stimulation. Biochim Biophys Acta. 1971 Feb 28;261(2):475–486. doi: 10.1016/0304-4165(72)90072-4. [DOI] [PubMed] [Google Scholar]
- King R. J., Gordon J. Involvement of DNA in the acceptor mechanism for uterine oestradiol receptor. Nat New Biol. 1972 Dec 6;240(101):185–187. doi: 10.1038/newbio240185a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mueller G. C., Vonderhaar B., Kim U. H., Le Mahieu M. Estrogen action: an inroad to cell biology. Recent Prog Horm Res. 1972;28:1–49. doi: 10.1007/BF02558535. [DOI] [PubMed] [Google Scholar]
- Notides A., Gorski J. Estrogen-induced synthesis of a specific uterine protein. Proc Natl Acad Sci U S A. 1966 Jul;56(1):230–235. doi: 10.1073/pnas.56.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Spelsberg T. C., Scharder W. T., Chytil F., Steggles A. W. Mechanisms of interaction of a hormone--receptor complex with the genome of a eukaryotic target cell. Nature. 1972 Jan 21;235(5334):141–144. doi: 10.1038/235141a0. [DOI] [PubMed] [Google Scholar]
- Shyamala G. Estradiol receptors in mouse mammary tumors: absence of the transfer of bound estradiol from the cytoplasm to the nucleus. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1623–1630. doi: 10.1016/0006-291x(72)90795-4. [DOI] [PubMed] [Google Scholar]
- Shyamala G., Gorski J. Estrogen receptors in the rat uterus. Studies on the interaction of cytosol and nuclear binding sites. J Biol Chem. 1969 Mar 10;244(5):1097–1103. [PubMed] [Google Scholar]
- Smith J. A., Martin L., King R. J., Vértes M. Effects of oestradiol-17-beta and progesterone on total and nuclear-protein synthesis in epithelial and stromal tissues of the mouse uterus, and of progesterone on the ability of these tissues to bind oestradiol-17-beta. Biochem J. 1970 Oct;119(4):773–784. doi: 10.1042/bj1190773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen R. H., Cole R. D. Chromosomal proteins. Annu Rev Biochem. 1969;38:951–990. doi: 10.1146/annurev.bi.38.070169.004511. [DOI] [PubMed] [Google Scholar]
- Sömjen D., Kaye A. M., Lindner H. R. Postnatal development of uterine response to estradiol-17beta in the rat. Dev Biol. 1973 Apr;31(2):409–412. doi: 10.1016/0012-1606(73)90275-3. [DOI] [PubMed] [Google Scholar]
- Williams D., Gorski J. Kinetic and equilibrium analysis of estradiol in uterus: a model of binding-site distribution in uterine cells. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3464–3468. doi: 10.1073/pnas.69.11.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]


