Abstract
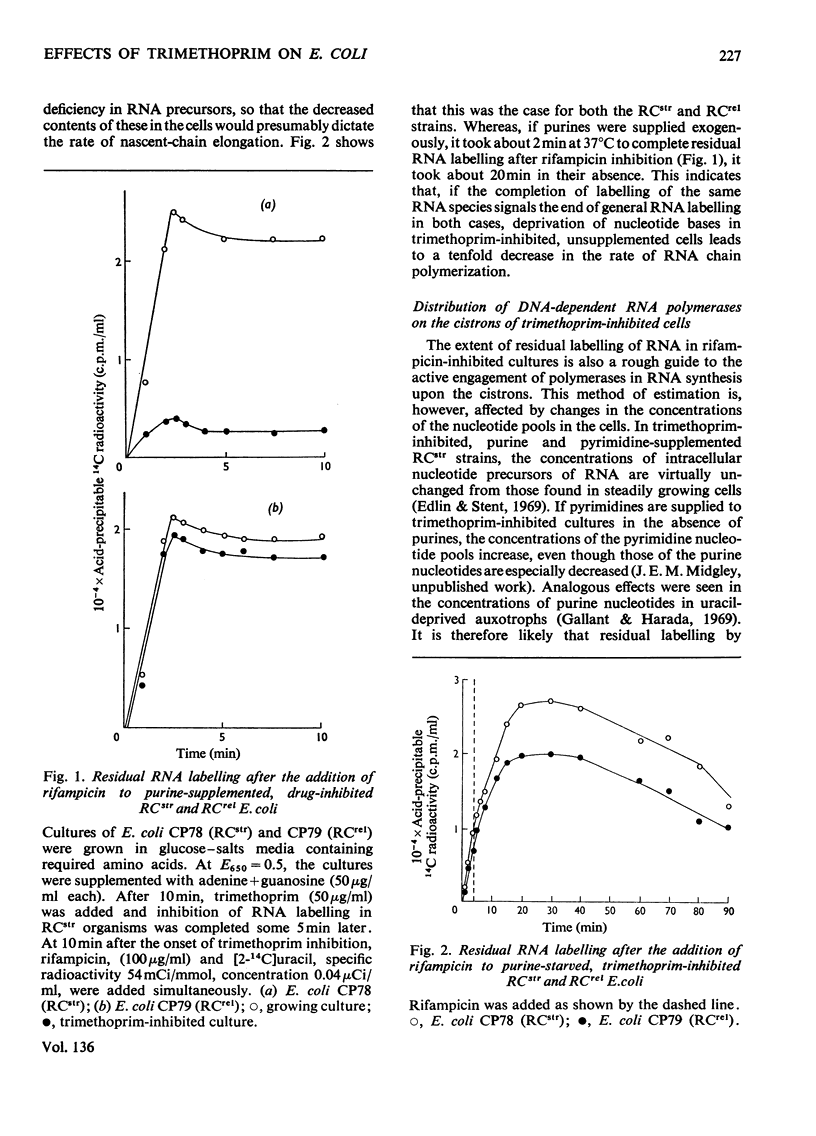
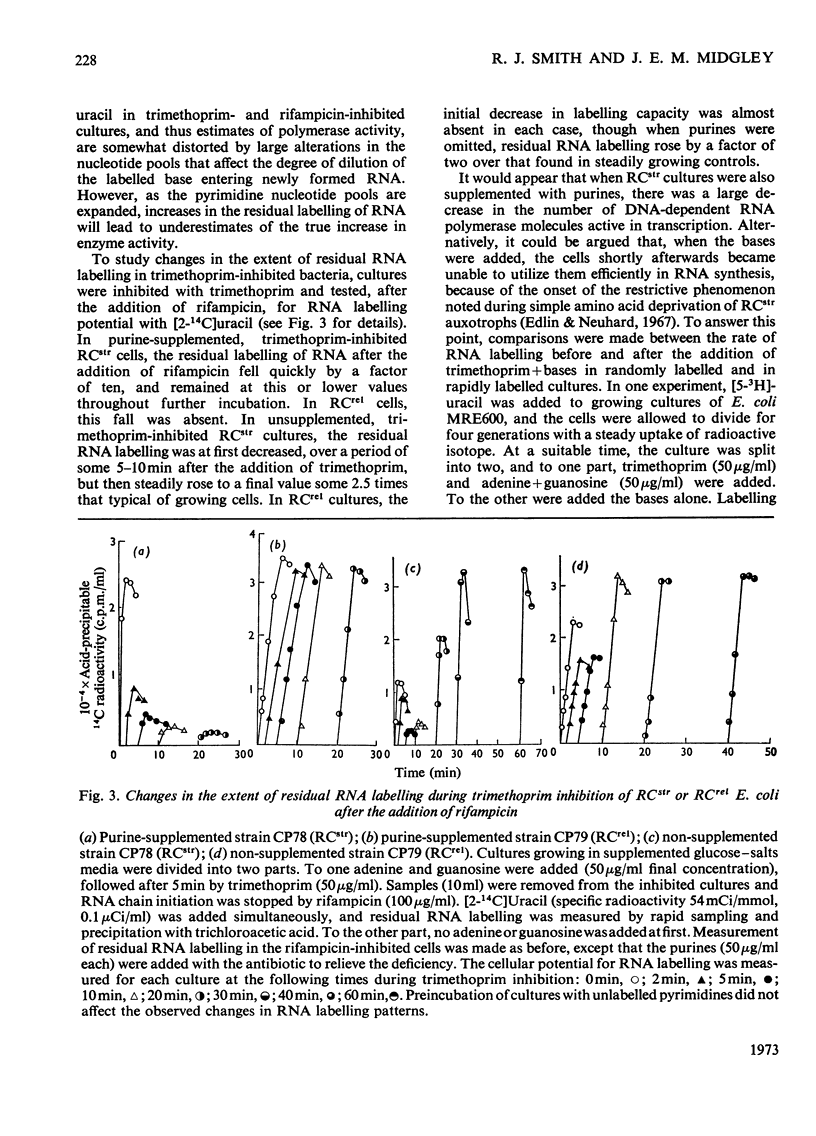
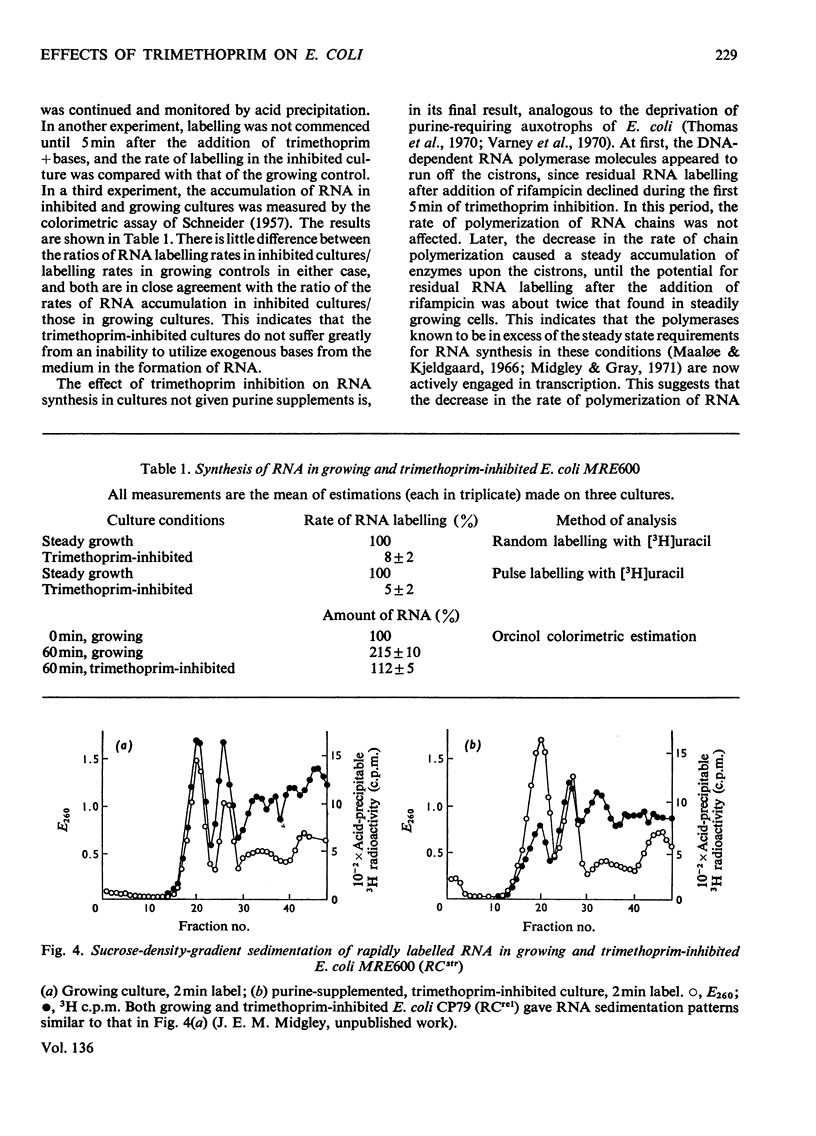
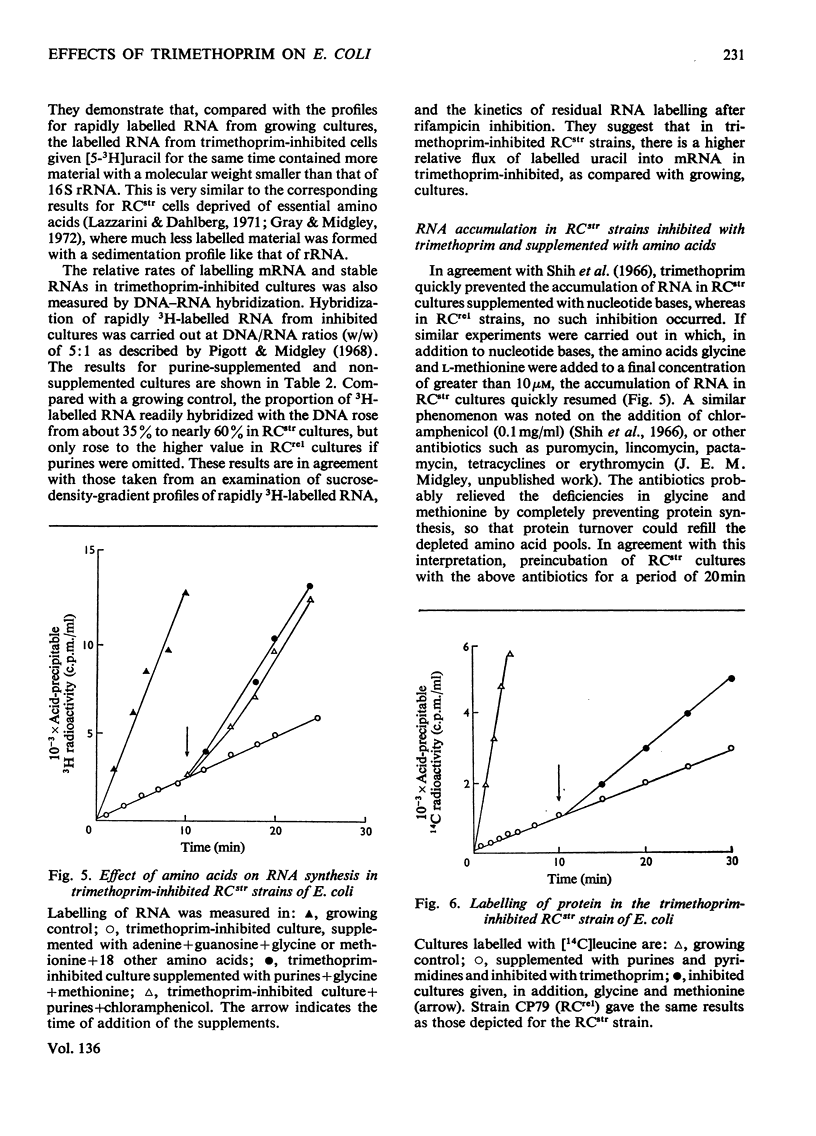
In trimethoprim-inhibited RCstr strains of Escherichia coli, the expression of the RC control of stable RNA synthesis arose primarily from a decrease in the intracellular concentrations of glycine and methionine, and not from inhibition of the initiation of new protein chains. In non-supplemented cultures, experiments with rifampicin showed that the immediate response to the addition of trimethoprim was a rapid decrease in the rate of initiation of RNA chains. This was followed after a few minutes by a sufficiently large fall in the rate of endogenous synthesis of nucleotide bases to cause a decrease in the rate of RNA chain polymerization. Inhibition of RNA chain initiation was thus overridden by an accumulation of DNA-dependent RNA polymerases upon the cistrons. RCrel strains also accumulated polymerases upon the DNA in similar circumstances, but did not suffer the initial effects on chain initiation. If purines were supplied before adding trimethoprim, RCstr strains polymerized RNA chains at normal rates, but initiation rates were permanently decreased. In either situation, an increased% of the RNA formed was mRNA. However, in RCrel strains supplemented with bases, trimethoprim did not affect either the rate of initiation of new chains or their rates of polymerization or the relative rates of synthesis of stable RNA and mRNA. Protein synthesis was also severely inhibited by trimethoprim. Though the addition of glycine and methionine to base-supplemented, trimethoprim-inhibited RCstr strains did not apparently affect the decreased rate of protein synthesis, RNA accumulation resumed at its normal rate. Thus, the inhibition of protein chain initiation had no effect on the rate of RNA accumulation in either RCstr or RCrel bacteria. The RC control does not express itself through inhibitions of protein synthesis at this level.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery R. J., Midgley J. E. A new approach to the analysis of hybridization of bacterial nucleic acids. Analysis of the ribosomal ribonucleic acids of Bacillus subtilis. Biochem J. 1969 Nov;115(3):383–394. doi: 10.1042/bj1150383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Berry L. Co-transcription of 16S and 23S ribosomal RNA in Escherichia coli. Nat New Biol. 1971 Nov 17;234(46):81–83. doi: 10.1038/newbio234081a0. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Dale B. A., Greenberg G. R. Effect of the folic acid analogue, trimethoprim, on growth, macromolecular synthesis, and incorporation of exogenous thymine in Escherichia coli. J Bacteriol. 1972 Jun;110(3):905–916. doi: 10.1128/jb.110.3.905-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Pace N. R. Transcriptional organization of the ribosomal RNA cistrons in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1786–1790. doi: 10.1073/pnas.68.8.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Neuhard J. Regulation of nucleoside triphosphate pools in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):225–230. doi: 10.1016/0022-2836(67)90328-2. [DOI] [PubMed] [Google Scholar]
- Edlin G., Stent G. S. Nucleoside triphosphate pools and the regulation of RNA synthesis in E. coli. Proc Natl Acad Sci U S A. 1969 Feb;62(2):475–482. doi: 10.1073/pnas.62.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt J., Lengyel P. Formylmethionyl-tRNA dependence of amino acid incorporation in extracts of trimethoprim-treated Escherichia coli. Science. 1966 Oct 28;154(3748):524–527. [PubMed] [Google Scholar]
- GUEST J. R., WOODS D. D. Cobalamin and the enzymic formation of a factor concerned in the synthesis of methionine by Escherichia coli. Biochem J. 1960 Dec;77:422–430. doi: 10.1042/bj0770422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969 Jun 25;244(12):3125–3132. [PubMed] [Google Scholar]
- Gray W. J., Midgley J. E. The control of ribonucleic acid synthesis in bacteria. Steady-state content of messenger ribonucleic acid in Escherichia coli M.R.E. 600. Biochem J. 1970 Nov;120(2):279–288. doi: 10.1042/bj1200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. J., Midgley J. E. The control of ribonucleic acid synthesis in bacteria. The synthesis and stability of ribonucleic acids in relaxed and stringent amino acid auxotrophs of Escherichia coli. Biochem J. 1972 Aug;128(5):1007–1020. doi: 10.1042/bj1281007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. J., Midgley J. E. The control of ribonucleic acid synthesis in bacteria. The synthesis and stbility of ribonucleic acid in rifampicin-inhibited cultures of Escherichia coli. Biochem J. 1971 Apr;122(2):161–169. doi: 10.1042/bj1220161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. J., Vickers T. G., Midgley J. E. The control of ribonucleic acid synthesis in bacteria. Polymerization rates for ribonucleic acids in amino acid-starved relaxed and stringent auxotrophs of Escherichia coli. Biochem J. 1972 Aug;128(5):1021–1031. doi: 10.1042/bj1281021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUENNEKENS F. M. The role of dihydrofolic reductase in the metabolism of one-carbon units. Biochemistry. 1963 Jan-Feb;2:151–159. doi: 10.1021/bi00901a027. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Gallant J. A. Effect of the RC gene product on constitutive enzyme synthesis. J Mol Biol. 1971 Oct 14;61(1):271–273. doi: 10.1016/0022-2836(71)90225-7. [DOI] [PubMed] [Google Scholar]
- Hitchings G. H., Burchall J. J. Inhibition of folate biosynthesis and function as a basis for chemotherapy. Adv Enzymol Relat Areas Mol Biol. 1965;27:417–468. doi: 10.1002/9780470122723.ch9. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- Midgley J. E., Gray W. J. The control of ribonucleic acid synthesis in bacteria. The synthesis and stability of ribonucleic acid in chloramphenicol-inhibited cultures of Escherichia coli. Biochem J. 1971 Apr;122(2):149–159. doi: 10.1042/bj1220149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley J. E., Smith R. J. The effect of trimethoprim on macromolecular synthesis in Escherichia coli. Ribosome maturation in RCstr and RCrel strains. Biochem J. 1973 Oct;136(2):235–247. doi: 10.1042/bj1360235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miovic M., Pizer L. I. Effect of trimethoprim on macromolecular synthesis in Escherichia coli. J Bacteriol. 1971 Jun;106(3):856–862. doi: 10.1128/jb.106.3.856-862.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott G. H., Midgley J. E. Characterization of rapidly labelled ribonucleic acid in Escherichia coli by deoxyribonucleic acid-ribonucleic acid hybridization. Biochem J. 1968 Nov;110(2):251–263. doi: 10.1042/bj1100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdom I., Bishop J. O., Birnstiel M. L. Scattered arrangement of the bacterial ribosomal cistrons. Nature. 1970 Jul 18;227(5255):239–242. doi: 10.1038/227239a0. [DOI] [PubMed] [Google Scholar]
- Shih A. Y., Eisenstadt J., Lengyel P. On the relation between ribonucleic acid synthesis and peptide chain initiation in E. coli. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1599–1605. doi: 10.1073/pnas.56.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Midgley J. E. The effect of trimethoprim on macromolecular synthesis in Escherichia coli. Regulation of ribonucleic acid synthesis by 'Magic Spot' nucleotides. Biochem J. 1973 Oct;136(2):249–257. doi: 10.1042/bj1360249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNOCK G., WILD D. G. THE SYNTHESIS OF RIBOSOMES BY A MUTANT OF ESCHERICHIA COLI. Biochem J. 1965 Jun;95:597–607. doi: 10.1042/bj0950597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then R., Angehrn P. Effects of trimethoprim and its antagonists on RNA synthesis in Escherichia coli. Biochim Biophys Acta. 1972 Nov 16;287(1):98–105. doi: 10.1016/0005-2787(72)90333-4. [DOI] [PubMed] [Google Scholar]
- Thomas G. A., Varney N. F., Burton K. Nucleic acid synthesis and nucleotide pools in purine-deficient Escherichia coli. Biochem J. 1970 Nov;120(1):117–124. doi: 10.1042/bj1200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney N. F., Thomas G. A., Burton K. Synthesis of ribonucleic acid in purine-deficient Escherichia coli and a comparison with the effects of amino acid starvation. Biochem J. 1970 Nov;120(1):125–132. doi: 10.1042/bj1200125. [DOI] [PMC free article] [PubMed] [Google Scholar]


