Abstract
Understanding the molecular mechanisms which drive and modulate host-pathogen interactions are essential when designing effective therapeutic and diagnostic approaches aimed at controlling infectious diseases. Certain large and giant viruses have recently been discovered as components of the human virome, yet little is known about their interactions with the host immune system. We have dissected the role of viral N-linked glycans during the interaction between the glycoproteins from six chloroviruses (belonging to three chlorovirus classes: NC64A, SAG, and Osy viruses) and the representative carbohydrate-binding receptors of the innate immune system. Using solid-phase assays we have identified the binding of viral glycoproteins to different C-type lectins in a carbohydrate-dependent manner. These experiments verified the importance of D-rhamnose in modulating their binding to C-type lectins DC-SIGN and Langerin. In vitro assays further determined the ability of the chlorovirus glycoproteins to trigger secretion of cytokines Interleukins 6 and 10 (IL-6 and IL-10) in human monocyte-derived dendritic cells and mouse macrophages. Additionally, IgG from healthy human controls recognized certain chlorovirus glycoproteins, indicating the significance of human environmental viral exposures. Collectively, these results demonstrate the ability of the innate and adaptive immune systems to recognize chlorovirus glycoproteins, a process dependent on their specific N-glycan structures.
Subject terms: Glycoconjugates, Glycoconjugates
This study explores how viral N-linked glycans play a role in the interaction between chloroviruses glycoproteins and key receptors of the innate immune system.
Introduction
Studies have identified certain large and giant viruses as being a component of the human virome. Yolken et al. in 2014 detected chlorovirus Acanthocystis turfacea chlorella virus 1, (ATCV-1, family Phycodnaviridae) genome sequences in throat swabs of 40 out of 92 individuals who participated in a study measuring cognitive function. The presence of ATCV-1 DNA was not associated with demographic variables but was associated with a statistically significant decrease in performance on cognitive assessments of visual processing and visual motor speed. Subsequent experiments focused on mouse macrophage responses, wherein strong production of the inflammatory cytokine IL-6 together with the expression of both IFN-β and IRF7 were reported in response to ATCV1. These responses are typical for viral infection during the interaction with macrophage cells2. Interestingly, viral titre increased slightly during the macrophage encounter with ATCV-1, while two other giant chloroviruses, CVM-1 and PBCV-1, failed to induce a significant response and showed a rapid decline in viral titre during their encounter with mouse macrophages1.
Another study identified prototype chlorovirus Paramecium bursaria chlorella virus 1 (PBCV-1) genome sequences in the human intestine following a faecal microbiota transplantation3,4. Phycodnavirus genome sequences were also reported in an infant gastrointestinal virome that is formed during breastfeeding5.
Chloroviruses are routinely found within inland aqueous environments, often reaching thousands of plaque-forming units per mL of indigenous water6. Consequently, one would expect humans to encounter chloroviruses multiple times during their lifetime.
These observations led us to postulate the probable interactions which chloroviruses and humans have, at both the molecular and immunological levels. Accordingly, the present study examined the interaction of glycan-containing chlorovirus surface molecules with human monocyte-derived dendritic cells and mouse macrophages, presumably through glycan-containing pathogen-associated molecular patterns (PAMP) of microbes that bind to Pattern Recognition Receptors (PRRs) of the innate immune system7. The focus on viral glycan-containing molecules arose from the discovery that chloroviruses differ from other viruses that infect eukaryotic organisms in that they encode most, if not all, of the enzymes necessary to synthesize their own glycans. Furthermore, the glycan synthesis occurs in the cytoplasm of the cell8.
Protein N-glycosylation is a key process in all domains of life9 with N-glycans occurring in a conserved sequon of the protein, NXS/T (with X being any amino acid except proline). Viruses are no exception, and many viruses have N-glycosylated capsids10. Most viruses use host-encoded glycosyltransferases and glycosidases located in the endoplasmic reticulum and Golgi apparatus to add and remove sugar residues from viral N-linked glycoproteins. As a result, viral glycoproteins resemble those of the host10–12.
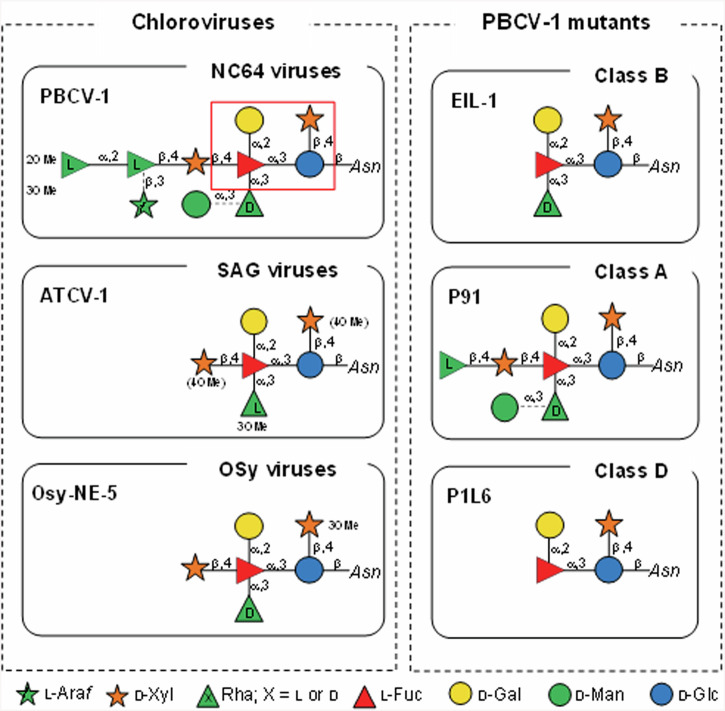
Giant viruses, as reported for chloroviruses and a few members of the Mimiviridae family, have changed this perception8. Focusing on chloroviruses, the reference virus PBCV-1 has a 330 kb genome and encodes many proteins that are not present in smaller viruses including enzymes involved in protein glycosylation synthesis. The PBCV-1 major capsid protein (MCP), named Vp54, is decorated with a N-glycan that is unusual in terms of structure (Fig. 1) and is linked to asparagine in non-canonical protein sequences, NIPG, NTAT, NTET and NTGT13 (Fig. 1). To date, several spontaneous mutants (or antigenic variants) of PBCV-1 have been isolated that feature truncated forms of the N-glycan. The study of their glycans has been pivotal for the structure-to-function assignment of PBCV-1 mutated genes14,15.
Fig. 1. The N-glycan structures attached to the chlorovirus glycoproteins used in this study.
The left panel reports the three different viruses along with the class they belong to (upper part of each individual panel). On the right, the N-glycan structures of PBCV-1 antigenic variants are reported along with their antigenic classification (upper part of each individual panel). Note that PBCV-1 glycoprotein has 4 N-linked sites, none in a typical consensus sequon13,16,18,20. Dotted linkages in PBCV-1 and P91 N-glycans indicate that certain monosaccharides are non-stoichiometric substituents. The red box in the PBCV-1 N-glycan encloses the four monosaccharide residues conserved in all the chloroviruses studied to date. In ATCV-1, the methyl groups are enclosed in brackets because they are nonstoichiometric substituents.
Chloroviruses are divided into four classes depending on their algal host; the size of the N-glycans attached to their MCPs varies from 4 to 10 residues. Notably, all these glycans share a conserved core region composed of 4 residues16,17 (Fig. 1), with the smallest native structure reported for virus P1L6, a spontaneous mutant of PBCV-118.
To link the viral-encoded glycan structures to the host immune system, we selected representatives from three chlorovirus classes; PBCV-1 that infects Chlorella variabilis NC64A (NC64A viruses); ATCV-1 that infects Chlorella heliozoae SAG 3.83 (SAG viruses); and Osy-NE-5 that infects Chlorella variabilis Syngen cells (Osy viruses). The first two viruses were chosen because they were associated with the human virome or because they were suspected to be pathogens3,4,19, while Osy-NE-5 was included due to the similarity between its N-glycan and that of ATCV-1 except for the absolute configuration of the rhamnose (Rha) unit (Fig. 1). These three viruses have a particular N-glycan attached to their MCP (Fig. 1) and the working hypothesis was that it could be recognized by the human innate system glycan recognizing PRRs. To determine which structural features of the glycans were important, this study also included three antigenic variants of PBCV-1 (P91, PIL6 and EIL-1) wherein their glycans are truncated versions of the wild-type virus, while still attached to the same capsid protein (Fig. 1). Thus, these variants are the perfect tools to understand the impact that viral glycans have on recognition by the innate immune system.
The analyses adopted a stepwise approach. First, we focused on the interactions between the viral MCPs and a set of human/plant carbohydrate-binding proteins (lectins) due to the role that these proteins play in the host immune system. Second, the effects of the MCPs on the induction of cytokines IL-6 and IL-10 were monitored in two vertebrate systems: human, by using monocytes-derived dendritic cells (moDC), and mice, by using the macrophage cell line RAW264.7. Finally, human sera from healthy donors were screened for the presence of IgG capable of recognizing the different glycosylated MCPs, which would indicate the MCPs stimulated the human adaptive immune system. Collectively, these experiments established that glycosylated molecular patterns on chloroviruses are recognized by the human innate and adaptive immune systems.
Results
Binding viral glycoproteins to human and plant lectins
Lectins are ubiquitous in nature and recognize glycosylation patterns either from the same organism (self) or other entities (non-self). Certain lectins are evolutionarily conserved receptors of the innate immune system that recognize glycosylated patterns on pathogens and induce innate immune responses.
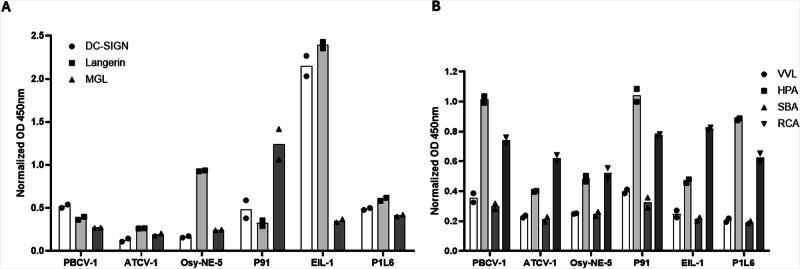
The interactions between glycoproteins isolated from chloroviruses and the human C-type lectins (DC-SIGN, Langerin and MGL) are reported in Fig. 2A. The three chloroviruses (PBCV-1, ATCV-1 and OSy-NE-5) bound to C-type lectins to varying degrees, and among the non-antigenic variants, PBCV-1 showed the stronger binding to DC-SIGN and Osy-NE-5 showed stronger binding to Langerin. In contrast, the PBCV-1 antigenic variant, EIL-1, had more robust binding to DC-SIGN and Langerin, while the P91 variant had a robust binding to MGL. The PBCV-1 antigenic variant PIL6, which had the shortest N-glycan, interacted poorly with the tested C-type lectins. However, the interaction of DC-SIGN and Langerin with variant EIL-1, which differs from PIL6 in having an additional D-Rha unit, increased significantly. The interaction decreased with the other MCPs where the D-Rha was further substituted and/or hidden by sugar residues attached to other regions of the surface N-glycan, as occurs for the variant P91 and PBCV-1. Interestingly, both P91 and PBCV-1 have an additional Rha unit (L-Rha) at the end of the surface N-glycan, nevertheless the interaction was weaker. Hence, in this assay, both lectins appear to bind terminal D-Rha rather than L-Rha, i.e., they discriminate between the D- and L- enantiomers. This conclusion was confirmed by the fact that Langerin interacts with OSy-NE-5, that has an external D-Rha and not with ATCV-1, which has an exposed L-Rha. Collectively, these results are consistent with the specificities reported for these two C-type lectins in the literature21–23. Indeed, it is known that both Langerin and DC-SIGN interact with mannose (Man) or fucose (Fuc)24,25, and D-Rha (not the L isomer) is structurally similar to Man with the only difference being the lack of a hydroxyl function on carbon-6.
Fig. 2. Binding of viral glycoproteins to human and plant lectins.
A Human C-type lectins ELISA results: wells were coated with viral glycoproteins and the binding to DC-SIGN, Langerin and MGL was evaluated in a calcium-containing buffer. B Plant lectins ELISA results: wells were coated with viral glycoproteins and the binding to VVL, HPA, SBA and RCA was evaluated. The experiments were performed in duplicate and data were normalized over signal from BSA-coated wells. Error bars indicate standard deviations. OD Optical density, VVL Vicia villosa lectin, HPA Helix pomatia agglutinin, SBA Soybean agglutinin, RCA Ricinus communis agglutinin.
Solid-phase competition experiments pre-incubating DC-SIGN and Langerin with their bona fide multivalent ligands (Polyacrylamide polymers, PAA, coated with LeX or LeY) were also performed. The pre-incubation of DC-SIGN with PAA-LeX or PAA-LeY clearly showed a decrease in binding confirming the involvement of the viral glycans in this interaction and the carbohydrate-recognition domain of DC-SIGN. The pre-incubation of Langerin with PAA-LeY showed also a clear binding reduction in these experimental conditions (see Fig. S1 in the Supplementary Information).
Of note, both P91 and PBCV-1 MCPs were poorly recognized by DC-SIGN and Langerin even though they also have an exposed D-Man unit. In both cases, the reduced recognition is likely due to the steric hindrance exerted by the other sugar residues that elongate the oligosaccharide from the non-reducing end (Fig. 1).
To validate this experimental approach, namely, to check if the viral proteins were coating the ELISA wells, the binding of 4 viral MCPs to plant lectins (VVL, HPA, SBA, RCA) was also examined. The selected plant lectins are able to recognize Gal and/or GalNAc-containing glyco-conjugates. In Fig. 2B the accessibility of Gal for the plant lectins has confirmed not only the effectiveness of the ELISA-coating strategy but also the glycans-accessibility to lectins in these experimental conditions. This analysis revealed that all the MCPs to varying degrees were recognized by all four lectins (Fig. 2B), thus supporting the validity of the experimental set-up with the human lectins. Among these plant lectins, the RCA lectin showed the strongest binding with the 4 viral MCPs while HPA had a marked preference for PBCV-1 and PIL6 MCPs. These results structurally fit with RCA having a reported preference to bind terminal Gal and GalNAc as well as the lectin HPA26,27.
Chlorovirus MCPs and induction of cytokines IL-6 and IL-10 from antigen-presenting cells
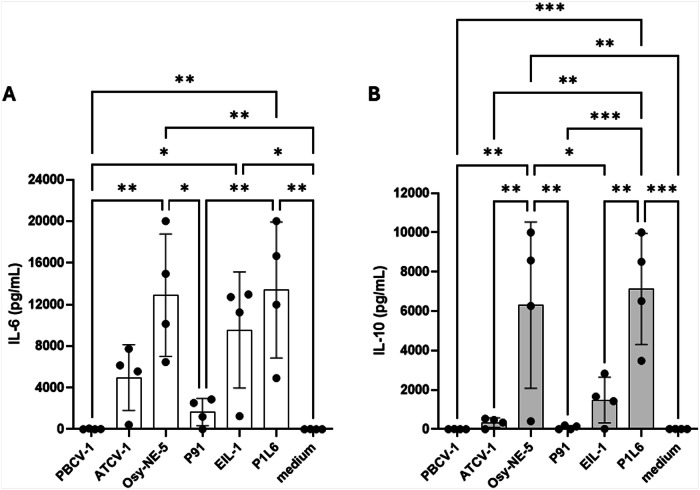
Antigen-presenting cells, such as macrophages and DCs, produce IL-6, an inflammatory cytokine, and IL-10, an anti-inflammatory cytokine, after exposure to viruses28 and other PAMPs. Therefore, interleukin secretion from human moDCs stimulated with viral MCPs from the six chloroviruses was examined. Stimulation of moDC with PBCV-1 MCP did not trigger the production of either interleukin while P91 MCP showed only a very weak induction (Fig. 3). In contrast, ATCV-1 MCP induced production of IL-6 but not IL-10, while Osy-NE-5 MCP strongly upregulated the secretion of both IL-6 and IL-10. Interestingly, EIL-1 and PIL6 variant MCPs of PBCV-1 induced the production of IL-6 (Fig. 3), while PIL6 MCP also upregulated IL-10. A dose-effect trend was also observed by titrating different concentrations of the viral glycoproteins (Fig. S2 in the Supplementary Information).
Fig. 3. IL-6 and IL-10 ELISA detection from moDC.
IL-6 (A) and IL-10 (B) secretion from human monocytes-derived dendritic cells (n = 4) stimulated with the viral glycoproteins at 20 μg/mL, detected via ELISA in the supernatants after 20 h stimulation. Mean values from four different experiments (four different human donors) are reported. Error bars indicate standard deviations. Experimental data were analysed by ANOVA multiple comparison (Tukey’s multiple comparison test), with a statistical significance level of alpha=0.05. Significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
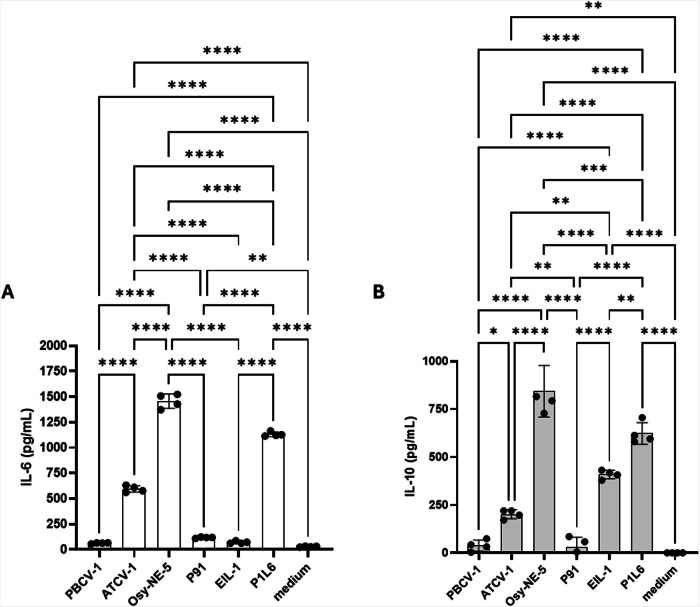
To confirm that the viral glycoproteins stimulated IL-6 and IL-10 production from macrophages we used the RAW264.7 Lucia mouse macrophage cell line. As reported with human dendritic cells, OSy-NE-5 MCP strongly stimulated expression of both IL-6 and IL-10, whereas ATCV-1 MCP stimulated less IL-6 and IL-10. In contrast, PBCV-1 MCP failed to stimulate either cytokine. In contrast PBCV-1 MCP variant, PIL6, induced high levels of IL-6 and IL-10, while EIL-1 MCP variant stimulated IL-10 but not IL-6 (Fig. 4). Glycoproteins from variant P91 did not trigger production of either IL-6 or IL-10 (Fig. 4).
Fig. 4. IL-6 and IL-10 ELISA detection from RAW264.7 cells.
Concentration of IL-6 (A) and IL-10 (B) secreted from RAW264.7 Lucia macrophage cell line challenged with 20 µg/mL of the MCP glycans of PBCV-1, ATCV-1, OSy-NE5, P91, EIL-1, and PIL6. Accumulated IL-6 and IL-10 concentrations in 20 h supernatants were measured with ELISA. Data are means ± standard error (n = 4). Experimental data were analysed by ANOVA multiple comparison (Tukey’s multiple comparison test), with a statistical significance level of alpha=0.05. Significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
Serological IgG binding to viral glycoproteins
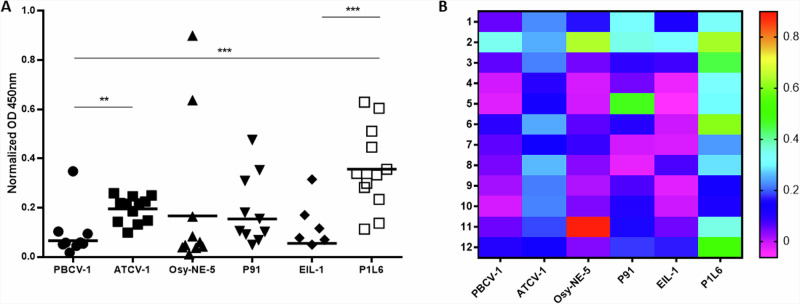
Detection of IgG antibodies can establish the immune status of an individual following pathogens infection, including viruses. Previously, we reported that a significant percentage of humans that were screened expressed IgG1 antibodies to ATCV-129. Therefore, individual plasma from 12 healthy donors was tested, with the aim of verifying if they had antibodies to different chlorovirus glycoproteins (Fig. 5). Consistent with our previous report, most of the donor sera contained IgG antibodies to ATCV-1 MCP with a statistical difference compared to PBCV-1. In contrast, nearly all sera contained low or undetectable levels of IgG antibodies to PBCV-1. Unexpectedly, all the donor sera contained high levels of IgG antibodies to PBCV-1 variant P1L6 with a statistically significant difference compared to the variant EIL-1 having only the extra D-Rha. In addition, the IgG response to ATCV-1 was more homogeneous among the different donors compared to the more disperse/heterogenous P1L6 IgG titre. These results confirm that humans have antibodies to certain chloroviruses MCPs and their variants, suggesting significant environmental exposures and/or suggesting the presence of conserved glycan patterns in the environment.
Fig. 5. Healthy donors (n = 12) IgG binding to the viral glycoproteins.
A IgG ELISA results: wells were coated with viral glycoproteins and human IgG was specifically detected. Single OD normalized values are reported from 12 different donors, as well as the mean value from each group. Data were normalized over signal from BSA-coated wells. B Heat map from the IgG ELISA results. OD: Optical density. Experimental data were analysed by ANOVA multiple comparison (Tukey’s multiple comparison test), with a statistical significance level of alpha=0.05. Significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
Determining the responses of the human immune system to viral molecules is essential for the appropriate development of new therapeutic agents, diagnostics, and molecular adjuvants. We have demonstrated via solid-phase assays the carbohydrate-dependent binding of six chlorovirus-encoded glycoproteins to different human C-type lectins. These lectins represent some of the PRRs responsible for the induction of innate immune responses, and also help to bridge the adaptive and innate systems. The PBCV-1 variant EIL-1 MCP bound strongly to the C-type lectins DC-SIGN and Langerin while the PBCV-1 variants P91 and P1L6 showed much weaker binding. These C-type lectins are known to recognize Fuc and/or Man residues of glycans, thereby triggering cytokine responses in a carbohydrate-dependent manner and modulating the entry of many pathogens into different antigen-presenting cells30,31. We have shown that EIL-1 also stimulated cytokines IL-6 and IL-10 from macrophage lineage cells. Furthermore, we discovered that human antigen-presenting cells, moDC, respond to different chlorovirus glycoproteins in a carbohydrate-dependent manner. For example, moDCs were sensitive to D vs L Rha on the viral glycoproteins (Osy-NE-5 vs ATCV-1) in triggering both IL-6 and IL-10, and in the presence vs absence of D-Rha on the viral glycoproteins (EIL-1 vs P1L6) in triggering only IL-10. These results suggest that in addition to Fuc and Man, D-Rha is recognized by DC-SIGN and Langerin. Moreover, because IL-6 is a proinflammatory cytokine while IL-10 is an anti-inflammatory cytokine the data suggest that only certain chlorovirus glycoproteins stimulate inflammatory responses. These two cytokines have been selected as representative to study these type of glycan-based host–pathogens interactions as previously reported and our results are in line with other studies that demonstrated a balance between different PRRs during host–pathogens interactions31. In contrast, PBCV-1 variant, P1L6, did not bind very well to DC-SIGN or Langerin but was able to upregulate production of IL-6 and IL-10, while the variant P91 was unable to induce these cytokines. These data suggest that other C-type lectin receptors, or other lectins, for ATCV-1 and P1L6 MCP glycans need to be determined in future studies.
When analysing data from the human moDCs and the mouse macrophage cell line, we speculate that the N-glycan “core” is responsible for the innate cytokine responses. The inability of PBCV-1 and P91 to stimulate these innate cytokine responses could be explained by the more complex N-glycan structures that “hide” the reactive core of the viral N-glycan. The data suggest that for these types of responses the glycosylated molecular pattern recognized by the innate system is the Fuc-D-Rha and/or the Xyl-Fuc epitopes. In both cases, the inner core Fuc appears to be playing a crucial role in triggering these responses.
It is interesting to note that EIL-1, P1L6 and P91 are spontaneous mutants of PBCV-1 isolated in laboratory conditions, even though their glycans are truncated forms of those of the wild-type virus (Fig. 1) they infect the algal host with the same efficiency as the parent strain. These pieces of evidence prompt the question about what advantage the virus receives by maintaining such complex and energy-demanding glycosylation machinery. The finding that cytokine induction occurs only for the heavily truncated glycans of P1L6 and EIL-1, suggests that a selection mechanism is in place and that it favours the maintenance of the full-length glycan of PBCV-1. Since PBCV-1 inhabits inland waters, it likely enters in contact with many forms of life, including humans, along with the potential risk of being neutralized by their immune system. Thus, it is tempting to speculate that the conservation of the glycosylation machinery is driven by the need of PBCV-1 to be stealth to the immune system given the fact that only the full-length N-glycan does not trigger any signalling.
One of the unresolved issues in the research described herein is identification of the lectin and its signalling pathway that recognizes and responds to P1L6, since it stimulated high levels of both IL-6 and IL-10. Future investigations may focus on these lectin pathways for recognition of P1L6.
In this context, an improved understanding of the viral molecular components which trigger cell signalling pathways in antigen-presenting cells will be essential for understanding the interplay between chloroviruses and the environment, independently from their ability to act as potential pathogens. Such information may also lead to the development of new therapeutic agents to control and/or to monitor viral pathogenesis32 in case it is confirmed that any other chlorovirus, such as ATCV-1, becomes a threat to human health.
Linked to these evolutionary-conserved interactions with innate immune system molecules (i.e., the lectins-glycoproteins interactions and signalling), we also investigated the presence of IgG antibodies in human plasma to glycosylated viral proteins. Anti-carbohydrate antibodies are extremely important from a diagnostic perspective, representing one of the first responses triggered during an infection. Additionally, these anti-carbohydrate antibodies against the chlorovirus glycoproteins could also be similar to the antibodies against ancient glycosylated molecular patterns (like anti-Rha IgG) conserved in the human adaptive immune system that have been linked to human disease33,34. For instance, we demonstrated a robust IgG response among all the human donors against P1L6, demonstrating that the antibody responses to the chloroviral glycoproteins could be used for diagnostic purposes in the event that any of these viruses are linked to human diseases, such as Amyotrophic Lateral Sclerosis29.
The combined results from these experiments have revealed that the host innate and adaptive systems are able to recognize the viral surface glycoproteins from giant viruses, with convincing evidence linking these responses to the unique viral N-glycans structures.
Methods
Production of the viruses
Virus PBCV-1 and its antigenic mutants were grown by infection of Chlorella variabilis NC64A cells, virus ATCV-1 was grown by infection of Chlorella heliozoae SAG 3.83, and virus Osy-NE-5 was grown by infection of Chlorella variabilis Syngen 2-3, as described previously6. The virus purification procedure is described in detail elsewhere35. Briefly, 3-day-old chlorella cell lysates were treated with 1% Triton X-100 at room temperature with constant mixing for 1 h. Then cell lysates were centrifuged at 4000 × g for 5 min at 4 °C. The supernatant was then centrifuged at 4 °C for 50 min at 53,000 × g to pellet the virus. The virus pellet was resuspended in a small volume of virus suspension buffer (VSB, 50 mM Tris-HCl, 10 mM MgCl2 pH 7.8) and placed on 10 to 40% linear sucrose density gradients equilibrated with VSB in a swinging bucket rotor and centrifuged at 4 °C for 20 min at 72,000 × g. The virus band was removed from the gradient with a sterile needle, diluted with VSB, and centrifuged for 50 min at 53,000 × g to pellet the virus. The virus in the pellet was re-suspended and treated with proteinase K (0.02 mg/mL) for 1 h at 45 °C. After proteinase K treatment the virus was subjected to a second sucrose density gradient centrifugation and resuspension.
Isolation of the virus MCPs
The MCPs were purified to near homogeneity as reported16. Briefly, viruses were suspended in 1 mL of 100 mM Tris, 50 mM NaCl, and 10 mM MgCl2 at pH 7.5 and heated at 70 °C for 20 min. A first supernatant was obtained by centrifugation (4 °C, 15 min. 10,000 g) and the pellet was again extracted with the same buffer. The supernatants from the first and the second extractions were treated separately with 5 volumes of cold acetone and left at −20 °C overnight. MCPs were recovered as precipitates by centrifugation (4 °C, 15 min. 10,000 g) and subjected to SDS-PAGE, which revealed a homogeneous band at ~54 kD. MCPs were contained in both extracts which were pooled, yielding 0.5–2 mg of MCP for each virus. Staining of SDS-PAGE was performed both by Coomassie brilliant blue or PAS (Periodic Acid-Schiff) staining, this last stain is selective for glycoproteins.
Lectin assays
A solution of 50 µL of the different glycosylated MCPs (5 µg/mL), in PBS (10 mM, pH 7.4), was used to coat a Nunc MaxiSorp plate for 2 h at room temperature. After discarding and washing (2 ×;150 µL) with calcium and magnesium-containing buffer TSM (20 mM tris(hydroxymethyl)aminomethane (Tris)-HCl, pH 8.0; 150 mM NaCl; 1 mM CaCl2; 2 mM MgCl2), the wells were blocked with 100 µL of 1% BSA (Sigma-Aldrich, lyophilized powder, ≥96%, agarose gel electrophoresis) in TSM at 37 °C for 30 min. The blocking solution was discarded and 50 µL of different C-type-lectins including human-Fc (DC-SIGN, Langerin and MGL) or biotinylated plant lectins (VVL, HPA, SBA and RCA) at 1 µg/mL in assay buffer (TSM, 0.5% BSA) were added to the wells. After 1 h at room temperature, the wells were washed with TSM (2 × 150 µL) and then 100 µL of anti-human horseradish peroxidase (0.3 µg/mL, Goat anti-human IgG-HRP from JacksonImmuno) was added. For the plant lectins, Streptavidin-HRP conjugate (Merck) was directly added. After 30 min at room temperature, the wells were washed with TSM (2 × 150 µL). Finally, 100 µL of substrate solution (3,3ʹ,5,5ʹ- tetramethylbenzidine, TMB, in citric/acetate buffer, pH 4, and H2O2) were added and after 15 min incubation at room temperature the reaction was stopped with 50 µL of H2SO4 (0.8 M) and the optical density (OD) was measured at 450 nm in an ELISA reader. The experiment was performed three times (each in duplicate) with similar results, and data were normalized over the signal at 450 nm from the BSA-containing wells.
Human moDC isolation, stimulation cytokine quantification by ELISA
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy volunteers (Sanquin, Amsterdam, The Netherlands) by centrifugation on a Ficoll gradient as previously described36. Briefly, blood was mixed with PBS, 1% citrate and layered on the Ficoll. After 30 min centrifugation, the interphase containing monocytes and lymphocytes was collected, washed with PBS/citrate and the pellet was resuspended in complete RPMI medium (Lonza, Basel, Switzerland). PBMCs were then loaded on a Percoll layer (GE 5 Healthcare, Chicago, U.S.) and after centrifugation, the interphase was collected, washed, and resuspended in complete RPMI medium. moDCs were generated by culturing monocytes for 5–7 days at a concentration of 1.25 × 106/mL in RPMI medium containing 500 U/mL IL-4 (ImmunoTools, Friesoythe, Germany) and 800 U/mL Granulocyte Macrophage Colony-stimulating Factor (GM-CSF) (ImmunoTools). moDCs (0.5–1 × 105) at day 4 after the monocytes stimulation were challenged with 20 μg/mL of different glycosylated MCPs in RPMI-1640, supplemented with 10% foetal calf serum, L-glutamine (2 mM), and penicillin/streptomycin (100 U/mL) at 37 °C, 5% CO2 for 16 h.
Human IL-10 and IL-6 ELISA Kit Duo Set R&D systems were used following the manufacturer’s instructions. Briefly, anti-human IL-10 or IL-6 was coated overnight in 50 mM Na2CO3, pH 9.7, in Nunc Maxisorp plates. After washing with PBS, 0.05% Tween, and blocked for 30 min with 1% BSA in PBS, moDC supernatants were added together with the corresponding detection antibody for 2 h at room temperature. Cytokines were detected with Streptavidin-PO (Biosource Finnigan, Waltham, U.S.) adding substrate buffer (110 mM citric acid (Merck), 110 mM sodium acetate (Fisher Scientific, Waltham, U.S.), pH 4 and 100 μg/mL TMB solution (Sigma) with a catalytic amount of H2O2) and stopping the enzymatic reaction with 0.8 M H2SO4. UV absorbance was measured at 450 nm on an ELISA reader (BioRad Benchmark, Hercules, U.S.).
IgG serology
Human plasma was taken during the isolation of human PBMCs from buffy coats of healthy donors upon their consent in accordance with the Declaration of Helsinki (Sanquin Bloodbank, Amsterdam, Netherlands). The tested glycosylated MCPs were diluted to 5 µg/mL in PBS (10 mM, pH 7.4) and 50 μL were used to coat the Nunc MaxiSorp plate for 2 h at room temperature. After washing with PBS (2 × 200 μL), 1% BSA (protease-free, Roche) in PBS was used for blocking at room temperature for 30 min. Wells were discarded and subsequently 100 µL human plasma diluted 1:100 in PBS (0.5% BSA) was added to the wells and incubated for 1 h at room temperature. After further washing with PBS (2 × 200 μL), 100 µL of 0.8 µg/mL of goat anti-human IgG-horse radish peroxidase (HRP, Invitrogen) was added and incubated for 30 min at room temperature. After washing with PBS (2 × 200 μL), 100 μL of TMB solution (Sigma-Aldrich) was added. The reaction was stopped after approximately 10 min with 50 μL of 0.8 M H2SO4, and the IgG binding was measured at an OD of 450 nm. Experimental data were analysed by ANOVA multiple comparison (Tukey’s multiple comparison test), with GraphPad 8.0, with a statistical significance level of alpha=0.05. Significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
Disclaimer for the use of human moDCs
All ethical regulations relevant to human research participants were followed. Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats obtained from Sanquin Blood bank, Amsterdam, Netherlands, from healthy adult volunteers (blood donors) following written informed consent in accordance with the Declaration of Helsinki. For this study no ethical approval was required, in accordance with the Sanquin Blood Bank adult volunteers accord number NVT0203.01.
Cell lines
Mouse macrophage cell line, RAW264.7-Lucia-ISG (InvivoGen, San Diego, CA), was grown in complete cell culture medium composed of Dulbecco’s modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% foetal bovine serum (FBS; Invitrogen) and 50 μg /mL gentamicin (Invitrogen).
Macrophage challenge with Chloroviruses MCPs
2 × 105 cells of RAW264.7-Lucia-ISG were incubated overnight in 24-well plates at 37 °C in DMEM, after which they were challenged with 20 µg/mL of viral MCPs. The plates were re-incubated at 37 °C and the supernatants were collected at 20 h post-stimulation.
Mouse cytokine ELISA
To measure IL-6 and IL-10 cytokines in supernatants of RAW cells treated with 20 µg/mL of viral glycoproteins, antibody pairs were obtained from BioLegend and Invitrogen-ThermoFisher, respectively. Briefly, 96-well ELISA plates were coated with anti-mouse IL-6 or anti-mouse IL-10 capture antibodies in coating buffer, sealed, and incubated overnight at 4 °C. The captured antibody was then decanted, and the wells were washed once with PBS/0.05% Tween 20 and blocked for 1 h at room temperature with a blocking buffer (PBS-10% FBS). Wells were washed with PBS/0.05% Tween 20 and supernatants or serial dilutions of recombinant IL-6 or IL-10 standards were added to the corresponding wells. After 2 h incubation, wells were washed and 1 μg/mL of biotinylated antibody to mouse IL-6 or mouse IL-10 was added to each well. After 1 h at room temperature, wells were washed and avidin conjugated to horseradish peroxidase was added for 30 min at room temperature. After washing, tetramethylbenzidine substrate followed by the addition of stopping reagent was added to each well. ODs at 450 nm with a reference wavelength of 570 nm were measured with an ELISA plate reader. IL-6 and IL-10 concentrations were measured per mL in the supernatant using the standard curve readings.
Antibodies used
Coating and biotinaylated IL-6 antibody from the PeliKine human IL-6 ELISA kit REF. M1906, Coating and biotinaylated IL-10 antibody from the PeliKine human IL-10 ELISA kit REF. These sandwich ELISA antibodies were used following the Kit instructions. M1910, Jacksonimmuno Goat Anti-Human IgG AB_2337539, Goat anti-Human IgG (H + L) Secondary Antibody HRP A18805 were used at 0.3 or 0.8 μg/mL.
Statistics and reproducibility
For experiment with n > 3, error bars indicate standard deviations among the different donors or animals. Experimental data were analysed by ANOVA multiple comparison (Tukey’s multiple comparison test), with a statistical significance level of alpha=0.05. Significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001. For ELISA experiment with n < 3, experiments were performed in duplicate.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This study was funded in part by the University of Nebraska Foundation Algal Virus Research Funds (JVE), the Stuart Nichols Research Foundation (JVE), FC thanks the Amsterdam Infection and Immunity Institute (AII postdoc grant) for funding. This study was support by the projects PRIN2022 2022SHW3KY CLariFY and PRIN2022PNRR P202293ZMC GIVING to FC. CDC gratefully acknowledges STARPLUS 2020 (project no. 21‐UNINA‐EPIG‐042) from the University of Napoli for financial support.
Author contributions
JVE, CDC, FC conceived the study, wrote the manuscript with input from all authors and have analysed the experimental data and discussed them in the manuscript. IS, AN performed the isolation, purification and characterization of the studied glyco-proteins. SB, FC, FB and ER performed the lectins studies, in vitro experiments and serological work. AE, TMP, IVA, GLP performed the in vitro mouse macrophage experiments and the related data analysis and data discussion. YvK, AM provided critical feedback and helped shape the research. All authors contributed to the editing of the manuscript. All authors discussed the results and contributed to the final manuscript.
Peer review
Peer review information
Communications Biology thanks Hirokazu Yagi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Johannes Stortz. A peer review file is available.
Data availability
All the data (numerical source) from the results represented in all the Figures are accessible in the Supplementary data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cristina De Castro, Email: decastro@unina.it.
Fabrizio Chiodo, Email: f.chiodo@icb.cnr.it.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-07244-9.
References
- 1.Petro, T. M. et al. Response of mammalian macrophages to challenge with the chlorovirus Acanthocystis turfacea chlorella virus 1. J. Virol.89, 12096–12107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore, T. C., Bush, K. L., Cody, L., Brown, D. M. & Petro, T. M. Control of early Theiler’s murine encephalomyelitis virus replication in macrophages by interleukin-6 occurs in conjunction with STAT1 activation and nitric oxide production. J. Virol.86, 10841–10851 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broecker, F. et al. Long-term changes of bacterial and viral compositions in the intestine of a recovered Clostridium difficile patient after fecal microbiota transplantation. Cold Spring Harb. Mol. Case Stud.2, a000448 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, W. et al. Metagenomic analysis of microbiome in colon tissue from subjects with inflammatory bowel diseases reveals interplay of viruses and bacteria. Inflamm. Bowel Dis.21, 1419–1427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pannaraj, P. S. et al. Shared and distinct features of human milk and infant stool viromes. Front. Microbiol.9, 1162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Etten, J. L., Agarkova, I. V. & Dunigan, D. D. Chloroviruses. Viruses12, E20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, G. D., Willment, J. A. & Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol.18, 374–389 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Speciale, I. et al. N-Glycans from Paramecium bursaria chlorella virus MA-1D: Re-evaluation of the oligosaccharide common core structure. Glycobiology32, 260–273 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Schwarz, F. & Aebi, M. Mechanisms and principles of N-linked protein glycosylation. Cur. Opin. Struct. Biol.21, 576–582 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Bagdonaite, I. & Wandall, H. H. Global aspects of viral glycosylation. Glycobiology28, 443–467 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vigerust, D. J. & Shepherd, V. L. Virus Glycosylation: Role in virulence and immune interactions. Trends Microbiol.15, 211–218 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe, Y., Bowden, T. A., Wilson, I. A. & Crispin, M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta Gen. Subj.1863, 1480–1497 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Castro, C. et al. Structure of the N-linked oligosaccharides attached to virus PBCV-1 major capsid protein: an unusual class of complex N-glycans. Proc. Natl Acad. Sci. USA110, 13956–13960 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speciale, I. et al. Chlorovirus PBCV-1 protein A064R has three of the transferase activities necessary to synthesize its capsid protein N-linked glycans. Proc. Natl Acad. Sci.117, 28735–28742 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel, E. et al. Chlorovirus PBCV-1 multidomain protein A111/114R has three glycosyltransferase functions involved in the synthesis of atypical N-glycans. Viruses13, 87 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Castro, C. et al. N-linked glycans of chloroviruses sharing a core architecture without precedent. Angew. Chem. Int. Ed.55, 654–658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speciale, I. et al. The astounding world of glycans from giant viruses. ACS Chem. Rev.122, 15717–15766 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speciale, I. et al. Glycan structures of chlorovirus PBCV-1 major capsid protein antigenic variants help identify virus-encoded glycosyltransferases. J. Biol. Chem.294, 5688–5699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yolken, R. H. et al. Chlorovirus ATCV-1 is part of the human oropharyngeal virome and is associated with changes in cognitive functions in humans and mice. Proc. Natl Acad. Sci.111, 16106–16111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quispe, C. F. et al. Characterization of a new chlorovirus type with permissive and non-permissive features on phylogenetically related strains. Virology500, 103–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tateno, H. et al. Dual specificity of Langerin to sulfated and mannosylated glycans via a single C-type carbohydrate recognition domain. J. Biol. Chem.285, 6390–6400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Liempt et al. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett.580, 6123–6131 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Geijtenbeek, T. B. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell100, 575–585 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Feinberg, H., Powlesland, A. S., Taylor, M. E. & Weis, W. I. Trimeric structure of Langerin. J. Biol. Chem.285, 13285–13293 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holla, A. & Skerra, A. Comparative analysis reveals selective recognition of glycans by the dendritic cell receptors DC-SIGN and Langerin. Protein Eng., Des. Selection24, 659–669 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Wu, A. M. Recognition factors of Ricinus communis agglutinin 1 (RCA(1). Mol. Immunol43, 1700–1715 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Sanchez, J. F. et al. Biochemical and structural analysis of Helix pomatia agglutinin: a hexameric lectin with a novel fold. J. Biol. Chem.281, 20171–20180 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Esmael, A. & Petro, T. M. IL-33 promotes increased replication of Theiler’s Murine Encephalomyelitis Virus in RAW264.7 macrophage cells with an IRF3-dependent response. Virus Res.323, 199007 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petro, T. M. et al. Chlorovirus ATCV-1 accelerates motor deterioration in SOD1-G93A transgenic mice and Its SOD1 augments Induction of inflammatory factors from murine macrophages. Front. Neurol.13, 821166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Kooyk, Y. & Geijtenbeek, T. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol.3, 697–709 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Gringhuis, S. I., den Dunnen, J., Litjens, M., van der Vlist, M. & Geijtenbeek, T. B. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol.10, 1081–1088 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Kremsreiter, S. M., Kroell, A. H., Weinberger, K. & Boehm, H. Glycan-lectin interactions in cancer and viral infections and how to disrupt them. Int J. Mol. Sci.22, 10577 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temme, J. S., Butler, D. L. & Gildersleeve, J. C. Anti-glycan antibodies: roles in human disease. Biochem. J.478, 1485–1509 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Kappler, K. & Hennet, T. Emergence and significance of carbohydrate-specific antibodies. Genes Immun.21, 224–239 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarkova, I. V., Dunigan, D. D. & Van Etten, J. L. Virion-associated restriction endonucleases of chloroviruses. J. Virol.80, 8114–8123 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolk, D. A. et al. Lipo-based vaccines as an approach to target dendritic cells for induction of T-and iNKT cell responses. Front. Immunol.11, 990 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All the data (numerical source) from the results represented in all the Figures are accessible in the Supplementary data.