Abstract
This study provides a comprehensive proteomic and metabolomic analysis of novel anthocyanin‐ and carotenoid‐rich wheat varieties to assess their immunogenicity in the context of Celiac Disease. Using (semi)‐quantitative mass spectrometry, the research found that gliadin expression and peptide release, particularly those containing immunostimulatory γ‐gliadin epitopes, vary significantly across different wheat varieties. While non‐targeted mass spectrometry provided valuable insights, the study acknowledged potential methodological biases, such limitations of ion current intensity as a measure of peptide abundance. Despite promising results, further research is required to determine the safety and efficacy of coloured wheat varieties for Celiac Disease patients, considering the complex interplay of gluten proteins, food processing, digestion and matrix effects. The ongoing studies hold potential for developing nutritionally beneficial wheat alternatives for Celiac Disease management.
Keywords: Celiac Disease, gut homeostasis, pigmented wheat grains, wheat Phenolics
SUMMARY
Over the past few decades, Celiac Disease has become one of the most common chronic gastrointestinal disorders. Currently, the only available treatment is a lifelong gluten‐free diet, which is challenging to maintain and compliance is quite low, especially in young patients. Many patients do not achieve full normalisation of duodenal lesions, even with strict adherence to the diet, symptom relief and negative Celiac Disease serology. Persistent villous atrophy in long‐term treated patients, even without symptoms, increases the risk of lymphoproliferative malignancies and mortality compared to those with mucosal healing.
While a gluten‐free diet is beneficial for the great majority of patients, its nutritional adequacy has come under scrutiny. Gluten‐free products are typically made with starches and refined flours, leading to nutritional imbalances such as inadequate micronutrient intake, higher consumption of unhealthy fats and increased glycaemic load. Therefore, new interventions for product development and reformulation are needed to meet nutritional recommendations while aligning with consumer preferences. Based on recent findings – and industrial trends for the production of minimally processed foods – one strategy to ameliorate immune reactivity to gluten proteins could rely, for instance, on the use of polyphenol‐rich whole wheat grains to preventively reduce gluten immunotoxicity and downstream intestinal damage.
Aligned with the United Nations' ‘Decade of Action’ plan and the FOOD 2030 Policy Framework, this programme integrated chemical, biochemical and nutritional assessments of purple, blue and dark‐coloured wheat flours. The goal was to inform food safety and public health strategies for Celiac Disease management, including updates to dietary advice, food processing technologies and wheat breeding programmes that enhance the health benefits of polyphenols. This fellowship was conducted at the Institute of Biochemistry and Cell Biology of the National Council of Research of Italy (IBBC‐CNR, Naples), in collaboration with the European Laboratory for the Investigation of Food‐Induced Diseases, University of Naples Federico II (ELFID‐UNINA, Naples) and LAQV‐REQUIMTE (Porto, Portugal). The fellow spent 4 months at the hosting site under the supervision of Dra. Carmen Gianfrani, a leading expert in Celiac Disease immunogenesis.
1. INTRODUCTION
Wheat, the most widely cultivated cereal globally, has been a crucial food source since its domestication 10,000 years ago (Erenstein et al., 2022). It provides not only carbohydrates for energy but also essential macro and micronutrients such as protein, fibre, vitamins, minerals, lipids and phytochemicals, all of which contribute to a healthy diet (Shewry & Hey, 2015). Wheat consumption, especially in its whole grain form, is linked to health benefits like reduced risks of type 2 diabetes, cardiovascular diseases and cancer mortality (Reynolds et al., 2020; Zhang et al., 2018). However, most wheat undergoes milling, where the germ and bran are removed to achieve desired flour properties, significantly reducing its nutritional value by stripping away fibre, vitamins, minerals and phenolic compounds (Awika, 2011; Cappelli & Cini, 2021). The challenge lies in developing wheat‐based products that satisfy consumer preferences for texture and flavour while retaining the health benefits of whole grain.
Consumers today are increasingly health‐conscious and seek natural foods with functional benefits. Naturally coloured foods, valued for their nutritional and antioxidant properties, are gaining popularity (Saini et al., 2021) and coloured wheat varieties are no exception. Research into these wheat varieties is growing, highlighting their potential advantages over traditional red and white wheat flours (Garg et al., 2022).
While common flour is derived from red or white wheat grains (Triticum aestivum L.), which have an amber colour, coloured wheat varieties are richer in phytochemicals like anthocyanins, flavonoids, carotenoids and phenolic compounds. These compounds not only give coloured wheat its distinctive hues but also enhance its antioxidant properties and health benefits. For example, yellow wheat contains higher carotenoid levels in the endosperm, while purple wheat has more anthocyanins in the pericarp, blue wheat in the aleurone layer and black wheat in both layers (Garg et al., 2022; Paznocht et al., 2021). These coloured wheat species result from hybridisation rather than natural occurrence.
The yellow colour in Triticum durum and other wheat varieties is due to carotenoids like lutein and zeaxanthin in the endosperm, controlled by two loci: Psy1 on chromosome 7 and Psy2 on chromosome 5 (Lachman et al., 2017; Paznocht et al., 2021). Purple wheat pigmentation, on the other hand, arises from purple pericarp genes transferred from Ethiopian tetraploid wheat Triticum turgidum L. subsp. abyssinicum Vavilov to common wheat. The genetic control of this purple pericarp involves two genes: Pp1, with alleles Pp‐B1 on chromosome 7B in T. durum and Pp‐D1 on chromosome 7D in T. aestivum, and Pp3 on chromosome 2A. The purple colour develops only when complementary alleles, either Pp‐B1 + Pp3 or Pp‐D1 + Pp3, are present (Garg et al., 2022; Lachman et al., 2017). Blue wheat's origin is more complex, involving the introgression of genes from wild relatives like Thinopyrum ponticum and Agropyron species into T. aestivum. The blue aleurone colour is influenced by various genes, such as Ba1 on chromosome 4B of Th. ponticum or 4AgL of Ag. elongatum and Ba2 on chromosome 4A of T. boeoticum. However, the genetic mechanisms behind this trait are not fully understood (Garg et al., 2022; Lachman et al., 2017). Black wheat results from hybridising purple pericarp and blue aleurone wheats, combining the genetic profiles of both to produce a dark purple colour due to anthocyanins in both layers (Efremova et al., 2023).
Yellow endosperm wheat is mainly rich in carotenoids, with lesser amounts of other pigments (Lachman et al., 2017). Purple, blue and black varieties, on the opposite, contain a broader range of phenolic compounds, including anthocyanins and flavonoids. The dominant anthocyanin in purple wheat is cyanidin‐3‐glucoside, while blue wheat is rich in delphinidin derivatives and black wheat has variable concentrations of cyanidin and delphinidin derivatives (Abdel‐Aal et al., 2008; Abdel‐Aal et al., 2016; Ficco et al., 2014; Lachman et al., 2017).
Apart from other non‐communicable disease conditions, coloured wheat grains may also hold significant potential for addressing wheat‐related diseases like Celiac Disease, given their antioxidant and anti‐inflammatory properties that may protect the intestinal epithelium from gluten‐induced cell damage (Dias et al., 2021; Sabença et al., 2021). However, current research primarily focuses on the genetic differences and overall phenolic content of these flours. As such, there is still a lack of detailed studies on gluten protein content and composition, as well as immunoreactivity of gluten in these coloured wheat flours, which are all factors crucial for evaluating their safety for those with Celiac Disease (Cebolla et al., 2018). In line with such needs, this study was intended to (1) compare the phenolic and metabolic profile of different varieties of coloured wheat using modern metabolomic methods based on ultra‐performance liquid chromatography–electrospray ionisation–tandem mass spectrometry (LC–MS/MS), (2) provide an in‐depth qualitative and quantitative profiling of gliadin proteins and peptides from pigmented wheat grains by bottom‐up proteomics, (3) investigate the changes in gliadin digestibility and immunogenicity in relation to the upregulation of anthocyanins and carotenoids, and (4) access the ability of coloured wheat gastrointestinal digests to mechanistically modify intestinal epithelial cell inflammation in Celiac Disease.
2. DATA AND METHODOLOGIES
2.1. Study setting
A total of five coloured wheat genotypes – grown at the Agricultural Research Institute in Kroměříž, Czech Republic – were selected for this study: Bohemia (red grain), Bona Vita (yellow endosperm), AF Jumiko (purple pericarp), AF Oxana (blue aleurone) and AF Zora (black grain). Table 1 provides an overview of the main characteristics of the selected varieties. The experimental site is situated at an elevation of 235 meters above sea level, featuring Luvic Chernozem (Loamic) soil. The region experiences an average annual temperature of 9.2°C, with mild winters and an average yearly precipitation of 576 mm. The wheat genotypes were planted in experimental plots of 10 m2 each, following conventional agricultural practices. Each genotype was grown on five separate plots, harvested individually and 1 kg of grain from each plot was collected to create composite samples. The grains were stored in cloth bags at 22°C in a dark environment until further processing. Before milling, grains were cleaned using a 2 mm sieve, with any remaining impurities manually removed. Wholemeal flours were then produced using an Ultra Centrifugal Mill ZM 200 (Retsch Inc., Germany), operating at a rotor speed of 15,000 rpm and a screen aperture size of 250 μm. To preserve the bioactive components, the flours were promptly cooled to −20°C and stored at this temperature until analysis.
TABLE 1.
Description of wheat (Triticum aestivum L.) genotypes. CZE – Czech Republic, SVK – Slovakia.
| Cultivar | Growth type | Country of origin | Cultivar status | Genotype | Grain colour |
|---|---|---|---|---|---|
| Bohemia | Winter | CZE | Released | (540i‐92 × 6192a‐92) × (540i‐92 × Kontrast) | Red |
| Bona Vita | Winter | SVK | Released | (SO‐690 × Arida) × Arida | Yellow endosperm |
| AF Jumiko | Winter | CZE | Released (2018) | ANK‐28A × Meritto | Purple pericarp |
| AF Oxana | Winter | CZE | Released (2019) | RU 440–6 × Ludwig | Blue aleurone |
| AF Zora | Winter | CZE | Released (2021) | (Skorpion × Bohemia) × (Indigo × Bohemia) | Black |
Collection of intestinal mucosal explants from Celiac Disease patients for the establishment and culture of human organoids and gluten‐specific CD4+ T‐cell lines (the two in vitro cell models used herein) was approved by the Ethical Committee of the School of Medicine, University ‘Federico II’ of Naples (UNINA) (ethical approval 115/09/ESPROT and 343/17/ES01, respectively). Written informed consent was obtained from all patients or from next of kin, caretakers or guardians on behalf of children under 12 years of age involved in this study.
2.2. Methodologies
The extraction of proline and glutamine‐rich gliadin proteins from petroleum‐ether defatted flours was carried out in accordance with previous methods (da Silva et al., 2023). Experiments involving the digestion of gliadin proteins in vitro, on the other hand, followed a recently established protocol as outlined in Brodkorb et al.'s work from 2019 (Brodkorb et al., 2019).
Extraction of free and conjugated phenolic compounds was performed using 80% ethanol and 0.5% hydrochloric acid, followed by centrifugation and repetition of the process with acidified ethanol and acetone. The combined supernatants were evaporated, underwent protein removal and were filtered before solid‐phase extraction. LC–MS/MS analysis were conducted on a Dionex UltiMate 3000 RSLC‐nano system coupled to an Orbitrap Q‐Exactive HF mass spectrometer (for proteomics) and on a Vanquish HPLC system, coupled to an Orbitrap Exploris 120 mass spectrometer (for metabolomics). Parameters such as temperature, solvents, flow rate and injection volume were set accordingly, and MS data acquisition included full scan and data‐dependent MS/MS analyses with specific settings for confirmation. Parallel reaction monitoring (PRM) and single reaction monitoring (SRM) experiments targeting specific gluten peptides (α‐gliadin p31‐43 and p58‐89) and anthocyanin compounds were performed in positive ion mode, monitoring specific transitions for quantification. Samples and MS/MS data were processed according to standard protocols and workflows on both Proteome Discoverer (v. 2.5.0.400) and Compound Discoverer (v. 3.3.1.111) search engines (Dias et al., 2024).
One to two duodenal biopsies from individual Celiac Disease patients and from controls (needed for comparison purposes in organoid‐related studies) were taken with standard endoscopic EGDS during routine gastroduodenoscopy. For organoid experiments, biopsies were rinsed with ice‐cold isolation buffer and placed in 10 mL of isolation buffer with the addition of 2 mmol/L ethylenediaminetetraacetic acid and 0.5 mmol/L dithiothreitol. After 1 h of agitation at 4°C, the biopsy samples were washed with the isolation buffer. The resulting digest was filtered through a 70 μm strainer and rinsed with washing buffer. Crypts were then collected by centrifugation and resuspended in 40 μL of ice‐cold Matrigel, allowing them to grow in 3D in 48‐well plates. Organoid‐derived monolayers were established by seeding cells in L‐WRN/ISC medium on uncoated polyester membrane transwell inserts with a 0.4 μm pore size. The inflammatory response triggered by gastrointestinal digests of coloured wheat flours and gliadin preparations has been monitored by Western Blot (Furone et al., 2023). Gliadin‐reactive CD4+ T‐cell lines were established from gut biopsies of Celiac Disease patients as previously described (Camarca et al., 2017). Briefly, intestinal explants were rinsed and enzymatically digested with collagenase. Isolated intestinal mononuclear cells were stimulated with irradiated autologous PBMC, deamidated gliadin digests and IL‐2 as growth factor and cryopreserved in liquid nitrogen at Dra. Gianfrani's laboratory. Six have been used for accessing responses to coloured wheat flour digests and gluten peptides by the detection of IFN‐γ by ELISA. Cytotoxicity assays were performed on peripheral blood mononuclear cells from healthy donors by Ficoll‐Paque gradient, to assess any residual toxicity that might be present in samples due to extraction or digestion procedures. PBMCs were seeded at 2 × 105 in a 96‐well plate and incubated with mitogen phytohaemagglutinin in presence of absence of coloured wheat digests.
3. RESULTS
Disclaimer: Detailed results obtained from the metabolomics analyses and in vitro cell assays are not fully included in this report, as the study is still blinded and parts of the analyses are yet ongoing. Further collaboration between the fellow's sending institute and hosting sites is planned after the end of the programme. As before, the EU‐FORA fellowship and funding will be acknowledged in later manuscripts submitted for peer‐reviewed scientific journals.
In Dias et al., 2024 (Dias et al., 2024), a comprehensive proteomic‐based assessment on the immunogenicity potential of gliadin proteins and peptides (the main environmental triggers of Celiac Disease) from novel anthocyanin‐ and carotenoid‐rich wheat varieties by (semi)‐quantitative mass spectrometry has been performed. It has been found that gliadin expression and peptide release following a simulated gastrointestinal digestion – particularly those containing CD‐immunostimulatory 𝛾‐gliadin epitopes – is differential and grain‐dependent, and that anthocyanin accumulation, as opposed to carotenoids, correlated with a lower immunogenicity and toxicity of gliadins at both protein and peptide levels (Figure 1). Considering that the amount of immunogenic peptides is the primary factor necessary for the achievement of the threshold for the inflammatory response mediated by T‐cells in subjects genetically predisposed to have CD, the consumption of pigmented cereals with a reduced load of gluten epitopes, could be a way to maintain the pathogenic T‐cells below the threshold of the inflammatory cascade, thus preventing or delaying, the detrimental autoimmune response in genetically at‐risk individuals (Andrén Aronsson et al., 2016; Pisapia et al., 2016; Størdal et al., 2013). Metabolomic analyses, on the other hand, revealed distinctive patterns of flavonoids and phenolic acids across the different wheat varieties. Flavonoid glycosides, including luteolin and apigenin derivatives for instance, showed significant variation, with the black wheat (Zora) exhibiting the highest levels (data not shown). SRM analysis highlighted significant variation in anthocyanin content, particularly in cyanidin‐, delphinidin‐ and petunidin‐3‐O‐glucosides, between coloured and conventional wheat grains (Figure 2).
FIGURE 1.
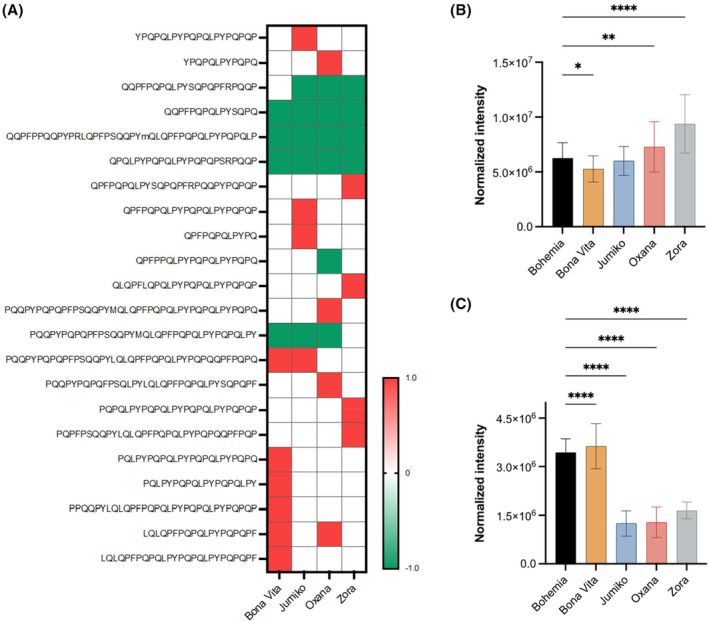
Heatmap analysis (A) and normalised XIC intensities (B) of peptides containing at least one copy of PFPQPQLPY (DQ2.5‐glia‐α1a), PYPQPQLPY (DQ2.5‐glia‐α1b) or PQPQLPYPQ (DQ2.5‐glia‐α2) T‐cell epitopes in pigmented wheat grains. Only sequences showing statistically different changes to Bohemia are shown in (A) (p < 0.05). Red colour means ‘overproduction’ while green colour means ‘underproduction’ or absence. (C) Normalised XIC intensities of peptides containing at least one copy of DQ2.5‐glia‐𝛾1–5 T‐cell epitopes. Bars represent mean values ± standard deviation. *p < 0.05, **p < 0.01, ****p < 0.0001. Ion intensities have been normalised for the peptide load. From Dias et al. (2024).
FIGURE 2.
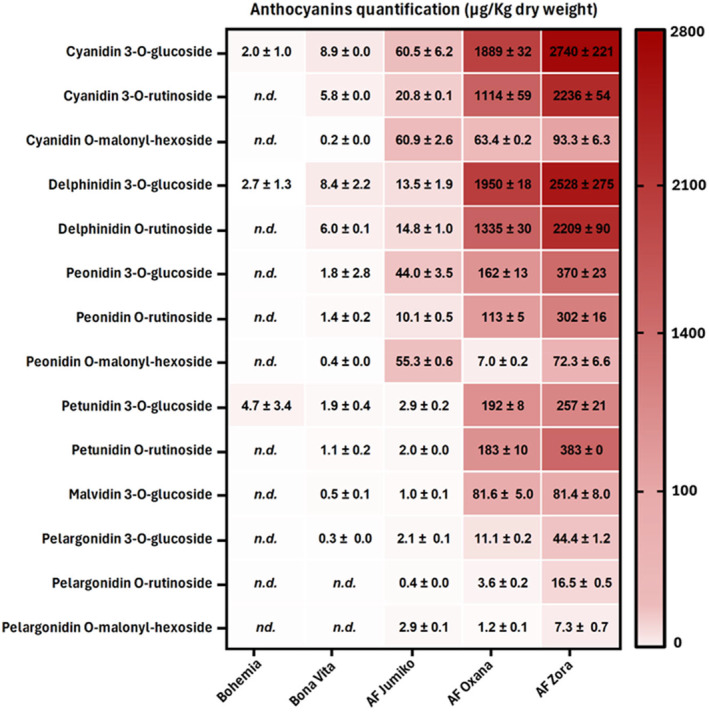
Quantification of 14 different cyanidin, delphinidin, peonidin, petunidin, malvidin and pelargonidin glucosyl derivatives (expressed in μg of anthocyanin per kilogram of dry weight matter) in coloured wheat flours. Data is presented as mean values ± standard deviation. Linear correlation: XIC area = 1.62 × 108 × [cyanidin 3‐O‐glucoside] (0.001–10 μg/mL), R 2 = 0.9994, LOD = 0.42 μg/mL, LOQ = 1.26 μg/mL.
While non‐targeted mass spectrometry can provide valuable insights into the immunoreactivity of gliadin digests, several methodological factors and potential biases must be considered. First, it is often assumed that all identified immunoreactive peptide sequences trigger gliadin‐specific T‐cell responses of the same intensity and in a dose‐dependent manner. However, intestinal T‐cell responses to gluten peptides are known to be heterogeneous, hierarchical and dependent on deamidation (Camarca et al., 2009). Additionally, the distribution and abundance of peptides containing T‐cell nonamers vary by grain (Figure 1).
Another assumption is that the ion current intensity measured by mass spectrometry accurately reflects the relative abundance of peptides across samples. This may not be reliable due to factors such as charge competition or ionisation inefficiency in complex matrices, which can affect the linear dynamic range and detection limit of the same analyte (Dias et al., 2024). Moreover, the food matrix itself can alter the functionality and behaviour of compounds compared to their isolated or free states (Aguilera, 2019).
In vitro studies have shown that deamidated gliadin protein digests from Bohemia, Bona Vita, Jumiko, Oxana and Zora activate gluten‐specific CD4+ T‐cell lines from Celiac Disease patients (n = 6), without causing cellular toxicity or showing significant differences between genotypes. However, when wholegrain wheat digests were tested, no IFN‐γ production by CD4+ T‐cell lines was elicited (data not shown). Preliminary experiments on patient‐derived intestinal organoids showed that wholegrain wheat digests induced activation of inflammatory markers in a dose–response manner, when compared to controls (data not shown). Further metabolomic studies are then required to understand how naturally present anthocyanin and carotenoid compound (among other components) may impact gliadin catabolism, as well as the bioavailability, propensity to deamidation and bioactivity of immunogenic peptides throughout the gastrointestinal tract.
4. CONCLUSION
At this stage, it is too early to determine if coloured wheat varieties are safe for Celiac Disease patients. The complexity of wheat, with its diverse gluten proteins, means that immunoreactivity can be affected by various factors like food processing, digestion and the food matrix. However, ongoing research using advanced bioanalytical techniques, including high‐resolution mass spectrometry and disease‐specific cellular models, shows promise. These studies could pave the way for developing coloured wheat varieties that not only minimise gluten immunotoxicity but also offer nutritional benefits, potentially providing a safer alternative for Celiac Disease patients. As this research progresses, it may lead to innovative dietary solutions that align with both health needs and consumer preferences.
ABBREVIATIONS
- EGDS
esophagogastroduodenoscopy
- ELISA
enzyme‐linked immunosorbent assay
- HPLC
high‐performance liquid chromatography
- LC–MS/MS
liquid chromatography–tandem mass spectrometry
- LOD
limit of detection
- LOD
limit of quantification
- PRM
parallel reaction monitoring
- SRM
single reaction monitoring
- XIC
extracted ion chromatogram
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
ACKNOWLEDGEMENTS
This report is funded by EFSA as part of the EU‐FORA programme.
Dias, R. , Mottola, I. , Bellomo, C. , da Silva, S. , Milheiro, D. , Barone, M. V. , Martinek, P. , de Freitas, V. , & Gianfrani, C. (2024). Putting gluten back on menu – Safety assessment of polyphenol‐rich wheat varieties in Celiac Disease. EFSA Journal, 22 (S1), e221115. 10.2903/j.efsa.2024.e221115
Approved: 23 September 2024
REFERENCES
- Abdel‐Aal, E.‐S. M. , Abou‐Arab, A. A. , Gamel, T. H. , Hucl, P. , Young, J. C. , & Rabalski, I. (2008). Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties. Journal of Agricultural and Food Chemistry, 56(23), 11171–11177. 10.1021/jf802168c [DOI] [PubMed] [Google Scholar]
- Abdel‐Aal, E.‐S. M. , Hucl, P. , Shipp, J. , & Rabalski, I. (2016). Compositional differences in anthocyanins from blue‐ and purple‐grained spring wheat grown in four environments in Central Saskatchewan. Cereal Chemistry, 93(1), 32–38. 10.1094/CCHEM-03-15-0058-R [DOI] [Google Scholar]
- Aguilera, J. M. (2019). The food matrix: Implications in processing, nutrition and health. Critical Reviews in Food Science and Nutrition, 59(22), 3612–3629. 10.1080/10408398.2018.1502743 [DOI] [PubMed] [Google Scholar]
- Andrén Aronsson, C. , Lee, H. S. , Koletzko, S. , Uusitalo, U. , Yang, J. , Virtanen, S. M. , Liu, E. , Lernmark, Å. , Norris, J. M. , & Agardh, D. (2016). Effects of gluten intake on risk of celiac disease: A case‐control study on a Swedish birth cohort. Clinical Gastroenterology and Hepatology, 14(3), 403–409.e403. 10.1016/j.cgh.2015.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awika, J. M. (2011). Major cereal grains production and use around the world. In Advances in cereal science: Implications to food processing and health promotion (Vol. 1089, pp. 1–13). American Chemical Society. 10.1021/bk-2011-1089.ch001 [DOI] [Google Scholar]
- Brodkorb, A. , Egger, L. , Alminger, M. , Alvito, P. , Assunção, R. , Ballance, S. , Bohn, T. , Bourlieu‐Lacanal, C. , Boutrou, R. , Carrière, F. , Clemente, A. , Corredig, M. , Dupont, D. , Dufour, C. , Edwards, C. , Golding, M. , Karakaya, S. , Kirkhus, B. , Le Feunteun, S. , … Recio, I. (2019). INFOGEST static in vitro simulation of gastrointestinal food digestion. Nature Protocols, 14(4), 991–1014. 10.1038/s41596-018-0119-1 [DOI] [PubMed] [Google Scholar]
- Camarca, A. , Anderson, R. P. , Mamone, G. , Fierro, O. , Facchiano, A. , Costantini, S. , Zanzi, D. , Sidney, J. , Auricchio, S. , Sette, A. , Troncone, R. , & Gianfrani, C. (2009). Intestinal T cell responses to gluten peptides are largely heterogeneous: Implications for a peptide‐based therapy in celiac disease. Journal of Immunology, 182(7), 4158–4166. 10.4049/jimmunol.0803181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarca, A. , Auricchio, R. , Picascia, S. , Fierro, O. , Maglio, M. , Miele, E. , Malamisura, B. , Greco, L. , Troncone, R. , & Gianfrani, C. (2017). Gliadin‐reactive T cells in Italian children from preventCD cohort at high risk of celiac disease. Pediatric Allergy and Immunology, 28(4), 362–369. 10.1111/pai.12720 [DOI] [PubMed] [Google Scholar]
- Cappelli, A. , & Cini, E. (2021). Challenges and opportunities in wheat flour, pasta, bread, and bakery product production chains: A systematic review of innovations and improvement strategies to increase sustainability, productivity, and product quality. Sustainability, 13(5), 2608 https://www.mdpi.com/2071‐1050/13/5/2608 [Google Scholar]
- Cebolla, Á. , Moreno, M. D. L. , Coto, L. , & Sousa, C. (2018). Gluten immunogenic peptides as standard for the evaluation of potential harmful prolamin content in food and human specimen. Nutrients, 10(12), 1927 https://www.mdpi.com/2072‐6643/10/12/1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva, S. , Pérez‐Gregorio, R. , Mateus, N. , Freitas, V. , & Dias, R. (2023). Evidence of increased gluten‐induced perturbations in the nucleophilic tone and detoxifying defences of intestinal epithelial cells impaired by gastric disfunction. Food Research International, 173, 113317. 10.1016/j.foodres.2023.113317 [DOI] [PubMed] [Google Scholar]
- Dias, R. , da Silva, S. , Monteiro, B. , Pérez‐Gregorio, R. , Mateus, N. , Gianfrani, C. , Barone, M. V. , Martinek, P. , & Freitas, V. (2024). Mass spectrometry‐based quantification of immunostimulatory gliadin proteins and peptides in coloured wheat varieties: Implications for celiac disease. Food Research International, 178, 114008. 10.1016/j.foodres.2024.114008 [DOI] [PubMed] [Google Scholar]
- Dias, R. , Pereira, C. B. , Pérez‐Gregorio, R. , Mateus, N. , & Freitas, V. (2021). Recent advances on dietary polyphenol's potential roles in celiac disease. Trends in Food Science & Technology, 107, 213–225. 10.1016/j.tifs.2020.10.033 [DOI] [Google Scholar]
- Efremova, T. T. , Morozov, S. V. , Chernyak, E. I. , & Chumanova, E. V. (2023). Combining the genes of blue aleurone and purple pericarp in the genotype of spring bread wheat Saratovskaya 29 to increase anthocyanins in grain. Journal of Cereal Science, 109, 103616. 10.1016/j.jcs.2022.103616 [DOI] [Google Scholar]
- Erenstein, O. , Jaleta, M. , Mottaleb, K. A. , Sonder, K. , Donovan, J. , & Braun, H.‐J. (2022). Global trends in wheat production, consumption and trade. In Reynolds M. P. & Braun H.‐J. (Eds.), Wheat improvement: Food security in a changing climate (pp. 47–66). Springer International Publishing. 10.1007/978-3-030-90673-3_4 [DOI] [Google Scholar]
- Ficco, D. B. M. , De Simone, V. , Colecchia, S. A. , Pecorella, I. , Platani, C. , Nigro, F. , Finocchiaro, F. , Papa, R. , & De Vita, P. (2014). Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (Triticum turgidum L. ssp. turgidum convar. Durum) wheats. Journal of Agricultural and Food Chemistry, 62(34), 8686–8695. 10.1021/jf5003683 [DOI] [PubMed] [Google Scholar]
- Furone, F. , Bellomo, C. , Carpinelli, M. , Nicoletti, M. , Hewa‐Munasinghege, F. N. , Mordaa, M. , Mandile, R. , Barone, M. V. , & Nanayakkara, M. (2023). The protective role of lactobacillus rhamnosus GG postbiotic on the alteration of autophagy and inflammation pathways induced by gliadin in intestinal models. Frontiers in Medicine, 10, 1085578. 10.3389/fmed.2023.1085578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg, M. , Kaur, S. , Sharma, A. , Kumari, A. , Tiwari, V. , Sharma, S. , Kapoor, P. , Sheoran, B. , Goyal, A. , & Krishania, M. (2022). Rising demand for healthy foods‐anthocyanin biofortified colored wheat is a new Research Trend. Frontiers in Nutrition, 9. 10.3389/fnut.2022.878221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman, J. , Martinek, P. , Kotíková, Z. , Orsák, M. , & Šulc, M. (2017). Genetics and chemistry of pigments in wheat grain—A review. Journal of Cereal Science, 74, 145–154. 10.1016/j.jcs.2017.02.007 [DOI] [Google Scholar]
- Paznocht, L. , Burešová, B. , Kotíková, Z. , & Martinek, P. (2021). Carotenoid content of extruded and puffed products made of colored‐grain wheats. Food Chemistry, 340, 127951. 10.1016/j.foodchem.2020.127951 [DOI] [PubMed] [Google Scholar]
- Pisapia, L. , Camarca, A. , Picascia, S. , Bassi, V. , Barba, P. , Del Pozzo, G. , & Gianfrani, C. (2016). HLA‐DQ2.5 genes associated with celiac disease risk are preferentially expressed with respect to non‐predisposing HLA genes: Implication for anti‐gluten T cell response. Journal of Autoimmunity, 70, 63–72. 10.1016/j.jaut.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Reynolds, A. N. , Akerman, A. P. , & Mann, J. (2020). Dietary fibre and whole grains in diabetes management: Systematic review and meta‐analyses. PLoS Medicine, 17(3), e1003053. 10.1371/journal.pmed.1003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabença, C. , Ribeiro, M. , Sousa, T. D. , Poeta, P. , Bagulho, A. S. , & Igrejas, G. (2021). Wheat/gluten‐related disorders and gluten‐free diet misconceptions: A review. Food, 10(8), 1765. https://www.mdpi.com/2304‐8158/10/8/1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini, P. , Kumar, N. , Kumar, S. , Mwaurah, P. W. , Panghal, A. , Attkan, A. K. , Singh, V. K. , Garg, M. K. , & Singh, V. (2021). Bioactive compounds, nutritional benefits and food applications of colored wheat: A comprehensive review. Critical Reviews in Food Science and Nutrition, 61(19), 3197–3210. 10.1080/10408398.2020.1793727 [DOI] [PubMed] [Google Scholar]
- Shewry, P. R. , & Hey, S. J. (2015). The contribution of wheat to human diet and health. Food and Energy Security, 4(3), 178–202. 10.1002/fes3.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Størdal, K. , White, R. A. , & Eggesbø, M. (2013). Early feeding and risk of celiac disease in a prospective birth cohort. Pediatrics, 132(5), e1202–e1209. 10.1542/peds.2013-1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Zhao, Q. , Guo, W. , Bao, W. , & Wang, X. (2018). Association of whole grain intake with all‐cause, cardiovascular, and cancer mortality: A systematic review and dose–response meta‐analysis from prospective cohort studies. European Journal of Clinical Nutrition, 72(1), 57–65. 10.1038/ejcn.2017.149 [DOI] [PubMed] [Google Scholar]


