Abstract
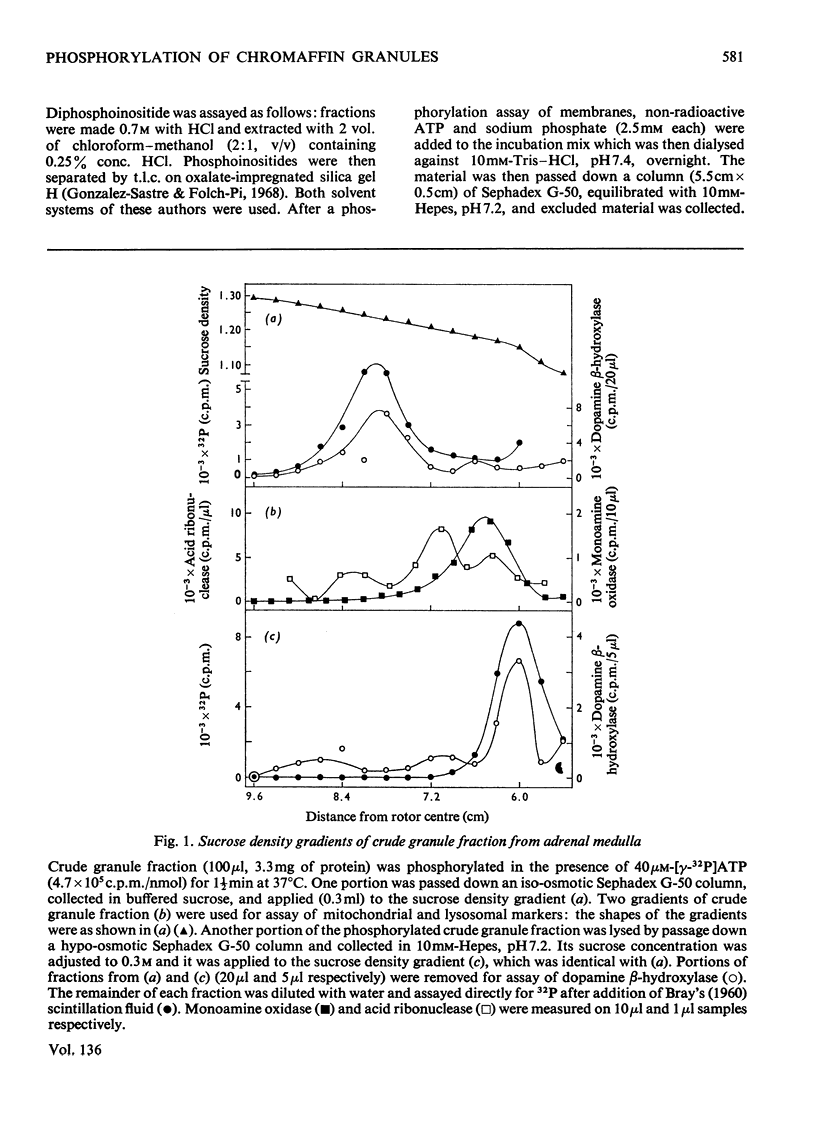
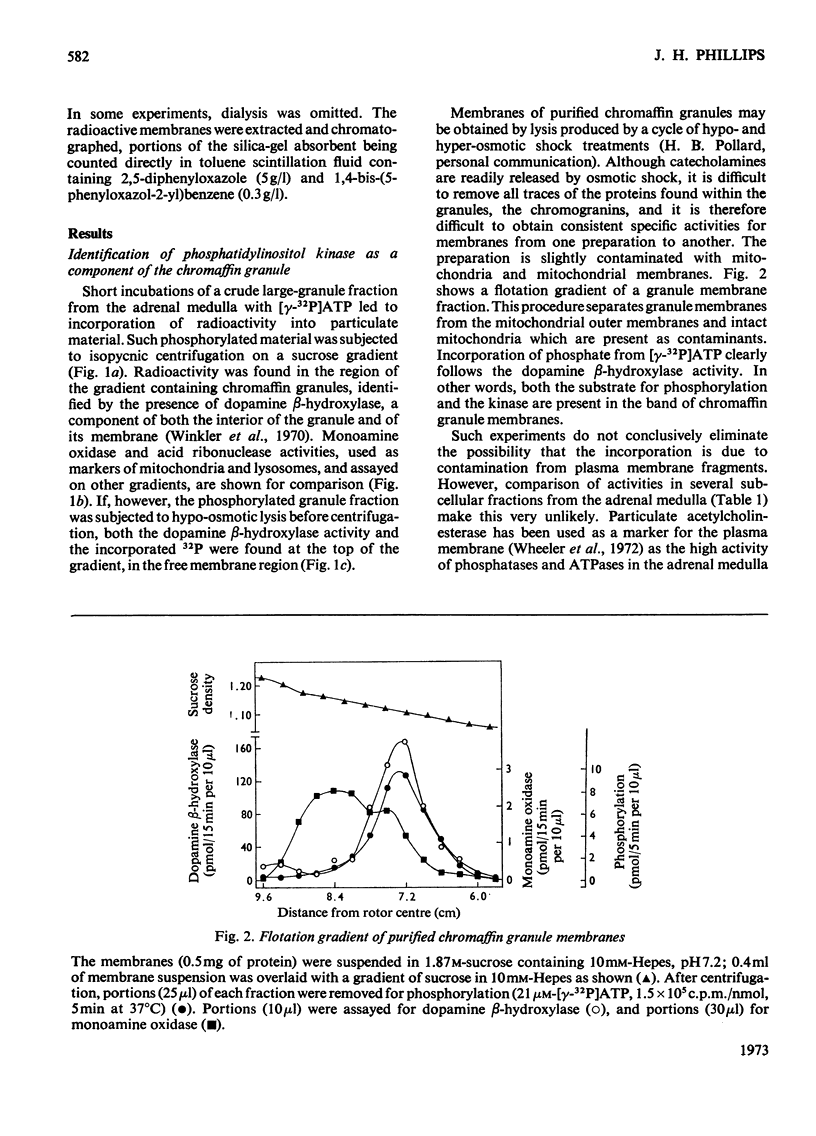
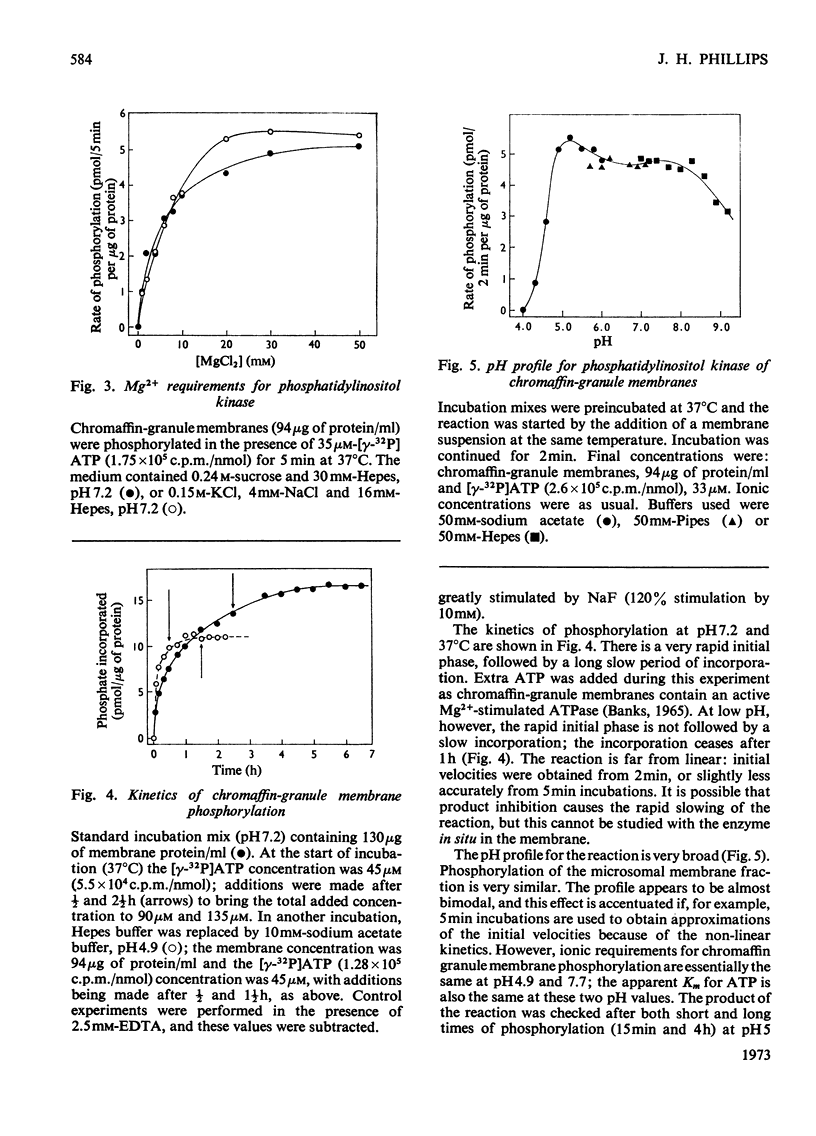
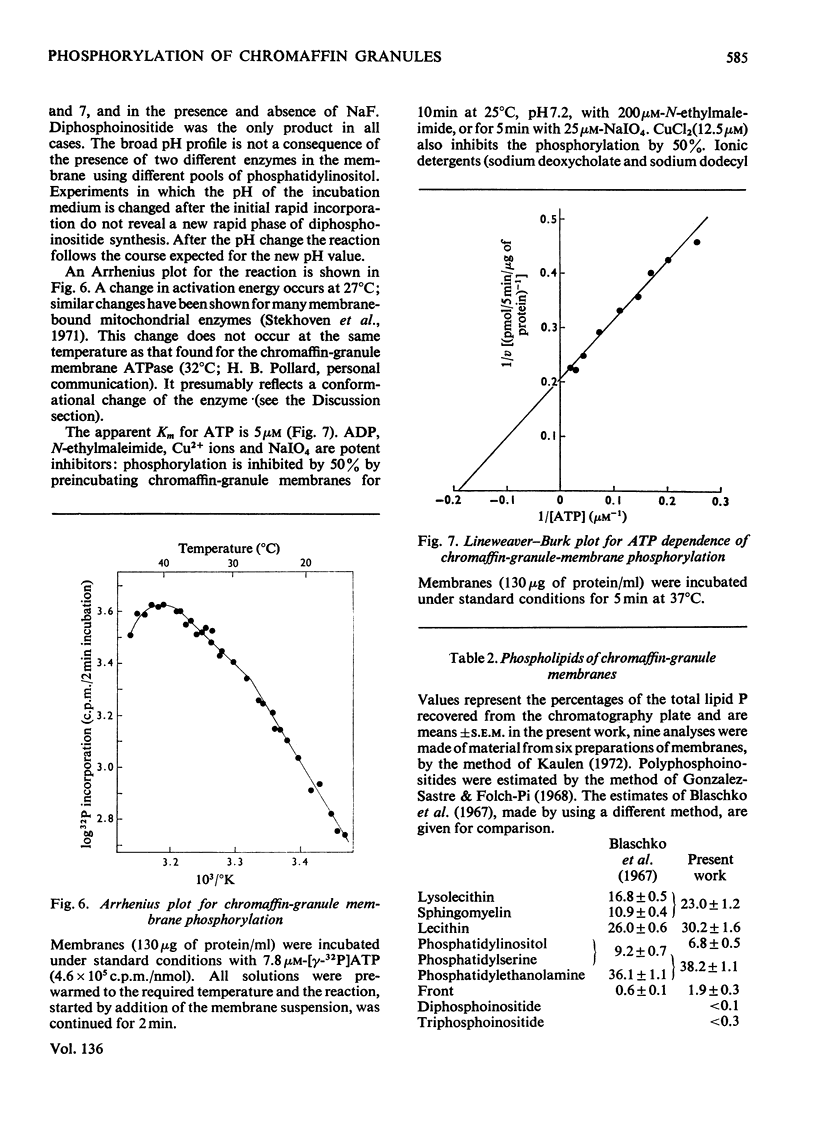
Phosphorylation of bovine chromaffin granules by ATP leads to the formation of diphosphoinositide in the granule membrane. Both phosphatidylinositol kinase and its substrate are components of this membrane, and triphosphoinositide is not formed under the conditions of the assay. The reaction is Mg2+-dependent and is stimulated by Mn2+ and F− ions. The initial reaction is rapid, with a broad pH profile and a `transition' temperature for its activation energy at 27°C. The apparent Km for ATP is 5μm. ATP, N-ethylmaleimide, Cu2+ ions and NaIO4 are inhibitory. The phospholipids of chromaffin-granule membranes have been analysed: 6.8% of the lipid P is found in phosphatidylinositol, and only 2–3% in phosphatidylserine. Comparison of the rate of phosphorylation of intact and lysed granules suggests that the sites for phosphorylation are on the outer (cytoplasmic) surface of the granules, and diphosphoinositide may therefore make an important contribution to the charge of the chromaffin granule in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANKS P. THE ADENOSINE-TRIPHOSPHATASE ACTIVITY OF ADRENAL CHROMAFFIN GRANULES. Biochem J. 1965 May;95:490–496. doi: 10.1042/bj0950490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H., Firemark H., Smith A. D., Winkler H. Lipids of the adrenal medulla. Lysolecithin, a characteristic constituent of chromaffin granules. Biochem J. 1967 Aug;104(2):545–549. doi: 10.1042/bj1040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- Buckley J. T., Lefebvre Y. A., Hawthorne J. N. Identification of an actively phosphorylated component of adrenal medulla chromaffin granules. Biochim Biophys Acta. 1971 Sep 1;239(3):517–519. doi: 10.1016/0005-2760(71)90047-6. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Eichberg J. Diphosphoinositide and triphosphoinositide in animal tissues. Extraction, estimation and changes post mortem. Biochem J. 1965 Sep;96(3):634–643. doi: 10.1042/bj0960634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Friedman S., Kaufman S. 3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic acttivity. J Biol Chem. 1965 Dec;240(12):4763–4773. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sastre F., Folch-Pi J. Thin-layer chromatography of the phosphoinositides. J Lipid Res. 1968 Jul;9(4):532–533. [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Harwood J. L., Hawthorne J. N. The properties and subcellular distribution of phosphatidylinositol kinase in mammalian tissues. Biochim Biophys Acta. 1969 Jan 7;171(1):75–88. doi: 10.1016/0005-2744(69)90107-7. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S., Fullington J. G. Stabilities of metal complexes of phospholipids: Ca(II), Mg(II), and Ni(II) complexes of phosphatidylserine and triphosphoinositide. Biochemistry. 1965 Aug;4(8):1599–1605. doi: 10.1021/bi00884a021. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S., Reinertsen J. L. Comparison of metal-binding properties of trans-1,2-cyclohexanediol diphosphate and deacylated phosphoinositides. Biochemistry. 1969 Dec;8(12):4855–4858. doi: 10.1021/bi00840a031. [DOI] [PubMed] [Google Scholar]
- KIRSHNER N. Uptake of catecholamines by a particulate fraction of the adrenal medulla. J Biol Chem. 1962 Jul;237:2311–2317. [PubMed] [Google Scholar]
- Kai M., White G. L., Hawthorne J. N. The phosphatidylinositol kinase of rat brain. Biochem J. 1966 Nov;101(2):328–337. doi: 10.1042/bj1010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen H. D. Separation of phosphatidylserine and -inositol by one-dimensional thin-layer chromatography of lipid extracts. Anal Biochem. 1972 Feb;45(2):664–667. doi: 10.1016/0003-2697(72)90230-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthews E. K., Evans R. J., Dean P. M. The ionogenic nature of the secretory-granule membrane. Electrokinetic properties of isolated chromaffin granules. Biochem J. 1972 Dec;130(3):825–832. doi: 10.1042/bj1300825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClare C. W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971 Feb;39(2):527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Jamieson J. D., Palade G. E. Composition of cellular membranes in the pancreas of the guinea pig. I. Isolation of membrane fractions. J Cell Biol. 1971 Apr;49(1):109–129. doi: 10.1083/jcb.49.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Harwood J. L., Coleman R., Hawthorne J. N. Characteristics of rat liver phosphatidylinositol kinase and its presence in the plasma membrane. Biochim Biophys Acta. 1967 Dec 5;144(3):649–658. doi: 10.1016/0005-2760(67)90053-7. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Trifaró J. M. The role of ATP and ATPase in the release of catecholamines from the adrenal medulla. I. ATP-evoked release of catecholamines, ATP, and protein from isolated chromaffin granules. Mol Pharmacol. 1967 Nov;3(6):561–571. [PubMed] [Google Scholar]
- Potter L. T. A radiometric microassay of acetylcholinesterase. J Pharmacol Exp Ther. 1967 Jun;156(3):500–506. [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Mehlhorn R. J., Keith A. D. Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem. 1971 Jun 25;246(12):4036–4040. [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekhoven F. M., Sani B. P., Sanadi D. R. Activation energies for the ATP-driven reversal of oxidative phosphorylation in submitochondrial particles. Biochim Biophys Acta. 1971 Jan 12;226(1):20–32. doi: 10.1016/0005-2728(71)90174-5. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Dworkind J. Phosphorylation of membrane components of adrenal chromaffin granules by adenosine triphosphate. Mol Pharmacol. 1971 Jan;7(1):52–65. [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Weihing R. R., Manganiello V. C., Chiu R., Phillips A. H. Purification of hepatic microsomal membranes. Biochemistry. 1972 Aug 1;11(16):3128–3135. doi: 10.1021/bi00766a028. [DOI] [PubMed] [Google Scholar]
- Wheeler G. E., Coleman R., Finean J. B. Cholinesterase activities in subcellular fractions of rat liver. Association of acetylcholinesterase with the surface membrane and other properties of the enzyme. Biochim Biophys Acta. 1972 Mar 17;255(3):917–930. doi: 10.1016/0005-2736(72)90403-8. [DOI] [PubMed] [Google Scholar]
- Winkler H., Hörtnagl H., Smith A. D. Membranes of the adrenal medulla. Behaviour of insoluble proteins of chromaffin granules on gel electrophoresis. Biochem J. 1970 Jun;118(2):303–310. doi: 10.1042/bj1180303. [DOI] [PMC free article] [PubMed] [Google Scholar]



