Abstract
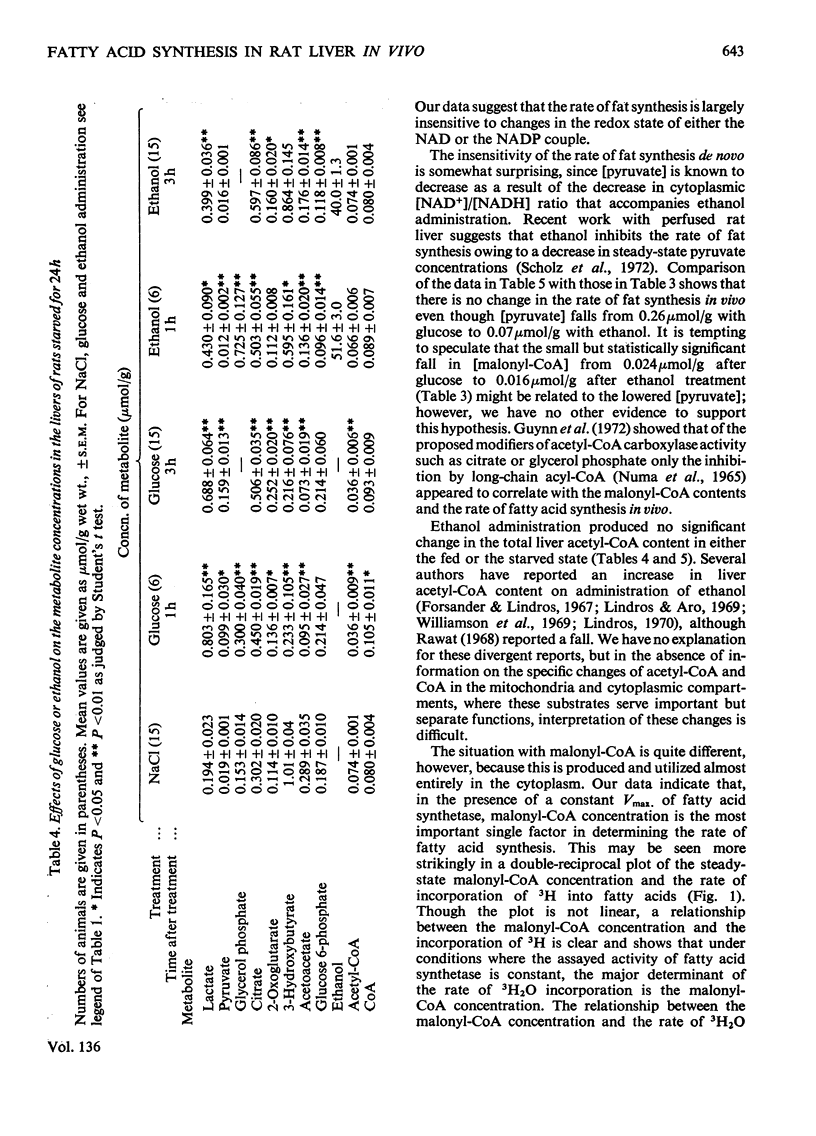
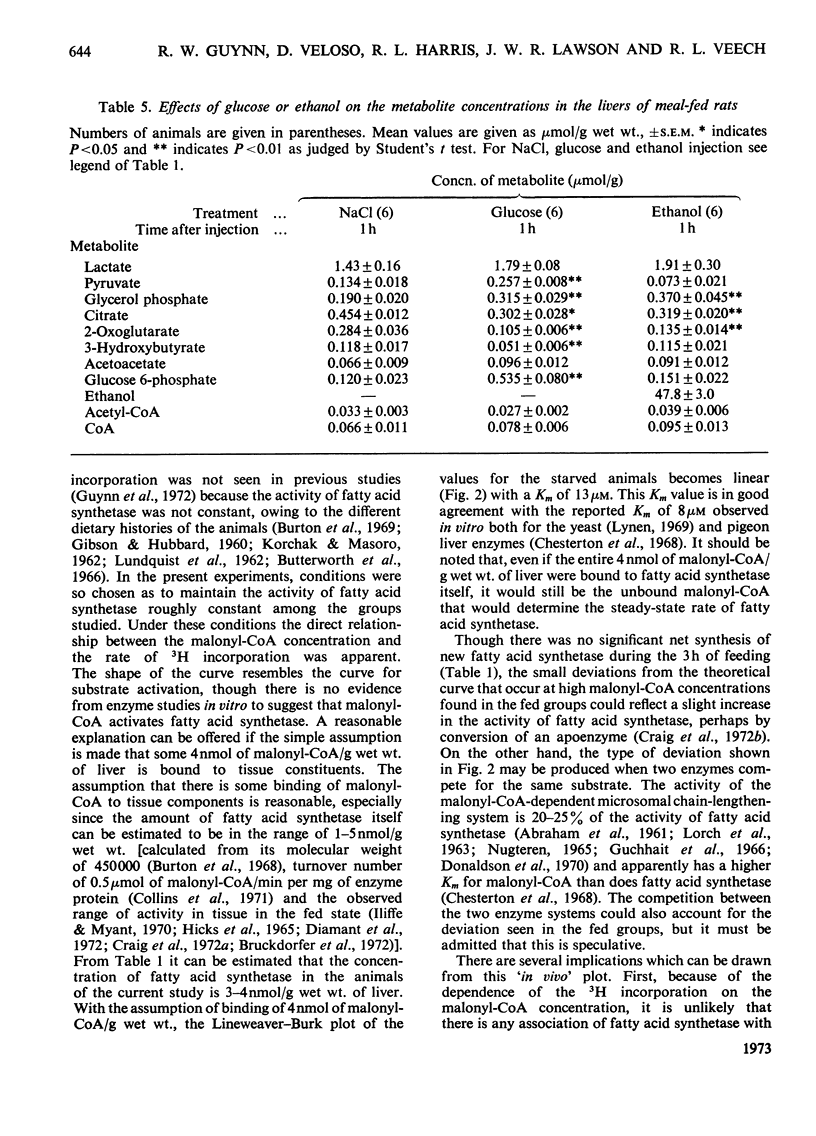
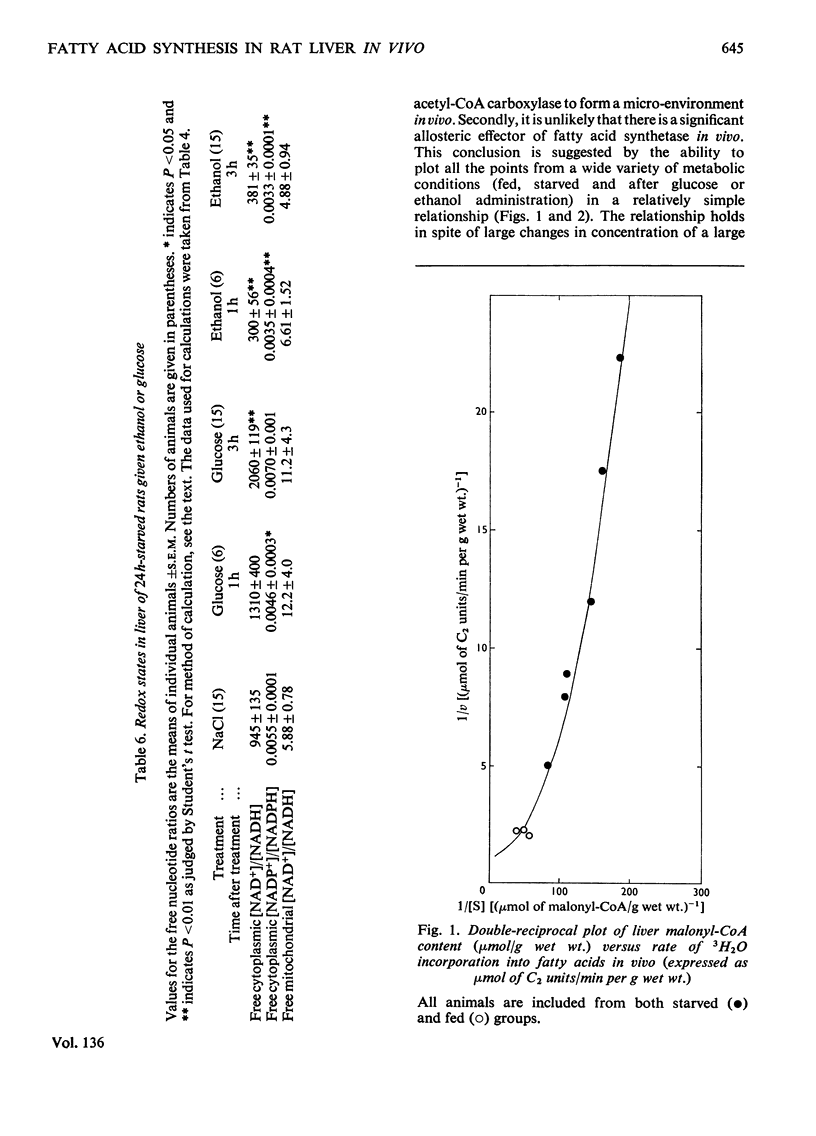
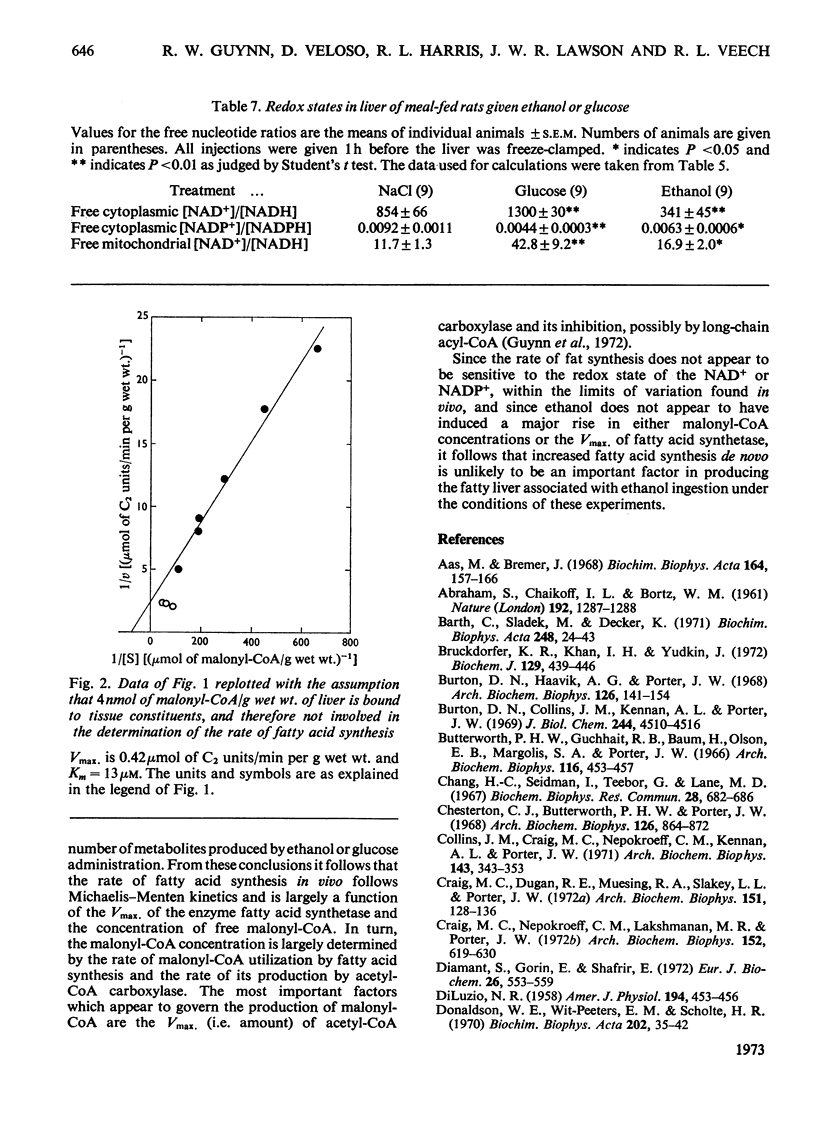
1. The effect of ethanol on liver fatty acid synthesis was studied in vivo in 24h-starved and `meal-fed' rats (i.e. fed for 3h per day and not ad libitum). 2. In the fed animal 3H2O was incorporated into fat at a rate of 0.46μmol of C2 units/min per g wet wt. of liver. Administration of either ethanol (3.2g/kg) or equicaloric amounts of glucose had no effect on the rate of 3H2O incorporation into lipid. 3. In the 24h-starved animal, administration of the same dose of ethanol produced an increase in the rate of 3H2O incorporation from 0.06 to 0.12μmol of C2 units/min per g fresh wt. after 3h whereas [malonyl-CoA] increased from 0.006 to 0.009μmol/g. Glucose given in amounts equicaloric to ethanol was significantly more lipogenic, increasing both the 3H2O incorporation from 0.06 to 0.20μmol of C2 units/min per g and the malonyl-CoA content from 0.006 to 0.013 μmol/g wet wt. at 3h. 4. The decrease in the redox state of free cytoplasm NAD or NADP couples or the changes in content of citrate, glucose 6-phosphate and pyruvate of liver after ethanol administration had no measurable effect on the rate of fatty acid synthesis in vivo. 5. Under the conditions of the experiments there was no significant difference, among any of the groups, in the activity of liver fatty acid synthetase measured in vitro. A double-reciprocal plot of the rate of 3H2O incorporation and the total tissue malonyl-CoA concentrations showed a striking relationship. It has been concluded that the rate of fatty acid synthesis in vivo is determined principally by the Vmax. of fatty acid synthetase and the concentration of free malonyl-CoA. 6. It has also been concluded that under the conditions of the present study, the synthesis of fatty acids de novo is unlikely to be an important factor in the increased liver lipid content associated with ethanol administration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., CHAIKOFF I. L., BORTZ W. M., KLEIN H. P., DEN H. Particle involvement in fatty acid synthesis in liver and yeast systems. Nature. 1961 Dec 30;192:1287–1288. doi: 10.1038/1921287a0. [DOI] [PubMed] [Google Scholar]
- Aas M., Bremer J. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim Biophys Acta. 1968 Oct 22;164(2):157–166. doi: 10.1016/0005-2760(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Barth C., Sladek M., Decker K. The subcellular distribution of short-chain fatty acyl-CoA synthetase activity in rat tissues. Biochim Biophys Acta. 1971 Oct 5;248(1):24–33. doi: 10.1016/0005-2760(71)90071-3. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Khan I. H., Yudkin J. Fatty acid synthetase activity in the liver and adipose tissue of rats fed with various carbohydrates. Biochem J. 1972 Sep;129(2):439–446. doi: 10.1042/bj1290439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. N., Collins J. M., Kennan A. L., Porter J. W. The effects of nutritional and hormonal factors on the fatty acid synthetase level of rat liver. J Biol Chem. 1969 Aug 25;244(16):4510–4516. [PubMed] [Google Scholar]
- Burton D. N., Haavik A. G., Porter J. W. Comparative studies of the rat and pigeon liver fatty acid synthetases. Arch Biochem Biophys. 1968 Jul;126(1):141–154. doi: 10.1016/0003-9861(68)90568-7. [DOI] [PubMed] [Google Scholar]
- Butterworth P. H., Guchhait R. B., Baum H., Olson E. B., Margolis S. A., Porter J. W. Relationship between nutritional status and fatty acid synthesis by microsomal and soluble enzymes of pigeon liver. Arch Biochem Biophys. 1966 Sep 26;116(1):453–457. doi: 10.1016/0003-9861(66)90052-x. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Seidman I., Teebor G., Lane M. D. Liver acetyl CoA carboxylase and fatty acid synthetase: relative activities in the normal state and in hereditary obesity. Biochem Biophys Res Commun. 1967 Sep 7;28(5):682–686. doi: 10.1016/0006-291x(67)90369-5. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Butterworth P. H., Porter J. W. Sites of binding of acetate, malonate and acetoacetate to the pigeon liver fatty acid synthetase. Arch Biochem Biophys. 1968 Sep 10;126(3):864–872. doi: 10.1016/0003-9861(68)90480-3. [DOI] [PubMed] [Google Scholar]
- Collins J. M., Craig M. C., Nepokroeff C. M., Kennan A. L., Porter J. W. Studies on a protein isolated from livers of diabetic and fasted rats. Arch Biochem Biophys. 1971 Apr;143(2):343–353. doi: 10.1016/0003-9861(71)90220-7. [DOI] [PubMed] [Google Scholar]
- Craig M. C., Dugan R. E., Muesing R. A., Slakey L. L., Porter J. W. Comparative effects of dietary regimens on the levels of enzymes regulating the synthesis of fatty acids and cholesterol in rat liver. Arch Biochem Biophys. 1972 Jul;151(1):128–136. doi: 10.1016/0003-9861(72)90481-x. [DOI] [PubMed] [Google Scholar]
- DI LUZIO N. R. Effect of acute ethanol intoxication on liver and plasma lipid fractions of the rat. Am J Physiol. 1958 Sep;194(3):453–456. doi: 10.1152/ajplegacy.1958.194.3.453. [DOI] [PubMed] [Google Scholar]
- Diamant S., Gorin E., Shafrir E. Enzyme activities related to fatty-acid synthesis in liver and adipose tissue of rats treated with triiodothyronine. Eur J Biochem. 1972 Apr 24;26(4):553–559. doi: 10.1111/j.1432-1033.1972.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Forsander O. A., Lindros K. O. Influence of ethanol on the acetyl-coenzyme A level of intact rat liver. Acta Chem Scand. 1967;21(9):2568–2568. doi: 10.3891/acta.chem.scand.21-2568. [DOI] [PubMed] [Google Scholar]
- GIBSON D. M., HUBBARD D. D. Incorporation of malonyl CoA into fatty acids by liver in starvation and alloxan-diabetes. Biochem Biophys Res Commun. 1960 Nov;3:531–535. doi: 10.1016/0006-291x(60)90169-8. [DOI] [PubMed] [Google Scholar]
- Gordon E. R. Effect of an intoxicating dose of ethanol on lipid metabolism in an isolated perfused rat liver. Biochem Pharmacol. 1972 Nov 15;21(22):2991–3004. doi: 10.1016/0006-2952(72)90192-x. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- Hicks S. E., Allmann D. W., Gibson D. M. Inhibition of hyperlipogenesis with puromycin or actinomycin D. Biochim Biophys Acta. 1965 Oct 4;106(2):441–444. doi: 10.1016/0005-2760(65)90060-3. [DOI] [PubMed] [Google Scholar]
- Iliffe J., Myant N. B. The sensitivity of acetyl-coenzyme A carboxylase to citrate stimulation in a homogenate of rat liver containing subcellular particles. Biochem J. 1970 Apr;117(2):385–395. doi: 10.1042/bj1170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORCHAK H. M., MASORO E. J. Changes in the level of the fatty acid synthesizing enzymes during starvation. Biochim Biophys Acta. 1962 Apr 9;58:354–356. doi: 10.1016/0006-3002(62)91022-3. [DOI] [PubMed] [Google Scholar]
- KORCHAK H. M., MASORO E. J. Changes in the level of the fatty acid synthesizing enzymes during starvation. Biochim Biophys Acta. 1962 Apr 9;58:354–356. doi: 10.1016/0006-3002(62)91022-3. [DOI] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDATE INTO FAT. ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF NORMAL AND DIABETIC RATS. Biochem J. 1965 Jun;95:832–837. doi: 10.1042/bj0950832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Gascoyne T. The redox state of the nicotinamide-adenine dinucleotides in rat liver homogenates. Biochem J. 1968 Jul;108(4):513–520. doi: 10.1042/bj1080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBER C. S., SCHMID R. The effect of ethanol on fatty acid metabolism; stimulation of hepatic fatty acid synthesis in vitro. J Clin Invest. 1961 Feb;40:394–399. doi: 10.1172/JCI104266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORCH E., ABRAHAM S., CHAIKOFF I. L. FATTY ACID SYNTHESIS BY COMPLEX SYSTEMS. THE POSSIBILITY OF REGULATION BY MICROSOMES. Biochim Biophys Acta. 1963 Dec 27;70:627–641. doi: 10.1016/0006-3002(63)90807-2. [DOI] [PubMed] [Google Scholar]
- LUNDQUIST F., TYGSTRUP N., WINKLER K., MELLEMGAARD K., MUNCK-PETERSEN S. Ethanol metabolism and production of free acetate in the human liver. J Clin Invest. 1962 May;41:955–961. doi: 10.1172/JCI104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S. Liver adaptation and injury in alcoholism. N Engl J Med. 1973 Feb 15;288(7):356–362. doi: 10.1056/NEJM197302152880710. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., Spritz N. Effects of prolonged ethanol intake in man: role of dietary adipose, and endogenously synthesized fatty acids in the pathogenesis of the alcoholic fatty liver. J Clin Invest. 1966 Sep;45(9):1400–1411. doi: 10.1172/JCI105448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindros K. O., Aro H. Ethanol-induced changes in levels of metabolites related to the redox state and ketogenesis in rat liver. Ann Med Exp Biol Fenn. 1969;47(1):39–42. [PubMed] [Google Scholar]
- Lindros K. O. Interference of ethanol and sorbitol with hepatic ketone body metabolism in normal, hyper- and hypothyroid rats. Eur J Biochem. 1970 Mar 1;13(1):111–116. doi: 10.1111/j.1432-1033.1970.tb00905.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. Biochem Soc Symp. 1968;27:61–86. [PubMed] [Google Scholar]
- Lowenstein J. M. Effect of (-)-hydroxycitrate on fatty acid synthesis by rat liver in vivo. J Biol Chem. 1971 Feb 10;246(3):629–632. [PubMed] [Google Scholar]
- MALLOV S., BLOCH J. L. Role of hypophysis and adrenals in fatty infiltration of liver resulting from acute ethanol intoxication. Am J Physiol. 1956 Jan;184(1):29–34. doi: 10.1152/ajplegacy.1955.184.1.29. [DOI] [PubMed] [Google Scholar]
- MALLOV S. Effect of adrenalectomy on ethanol and fat metabolism in the rat. Am J Physiol. 1957 Jun;189(3):428–432. doi: 10.1152/ajplegacy.1957.189.3.428. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Kilburn E. Acetyl coenzyme A carboxylase. The roles of synthesis and degradation in regulation of enzyme levels in rat liver. J Biol Chem. 1969 Nov 25;244(22):6254–6262. [PubMed] [Google Scholar]
- Moss J., Yamagishi M., Kleinschmidt A. K., Lane M. D. Acetyl coenzyme A carboxylase. Purification and properties of the bovine adipose tissue enzyme. Biochemistry. 1972 Sep 26;11(20):3779–3786. doi: 10.1021/bi00770a017. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Numa S. Purification of rat liver acetyl coenzyme A carboxylase and immunochemical studies on its synthesis and degradation. Eur J Biochem. 1970 Sep;16(1):161–173. doi: 10.1111/j.1432-1033.1970.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. The enzymic chain elongation of fatty acids by rat-liver microsomes. Biochim Biophys Acta. 1965 Oct 4;106(2):280–290. doi: 10.1016/0005-2760(65)90036-6. [DOI] [PubMed] [Google Scholar]
- REBOUCAS G., ISSELBACHER K. J. Studies on the pathogenesis of the ethanol-induced fatty liver. I. Synthesis and oxidation of fatty acids by the liver. J Clin Invest. 1961 Aug;40:1355–1362. doi: 10.1172/JCI104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat A. K. Effects of ethanol infusion on the redox state and metabolite levels in rat liver in vivo. Eur J Biochem. 1968 Dec 5;6(4):585–592. doi: 10.1111/j.1432-1033.1968.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Srere P. A., Foster D. W. On the proposed relation of citrate enzymes to fatty acid synthesis and ketosis in starvation. Biochem Biophys Res Commun. 1967 Mar 9;26(5):556–561. doi: 10.1016/0006-291x(67)90101-5. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Guynn R., Veloso D. The time-course of the effects of ethanol on the redox and phosphorylation states of rat liver. Biochem J. 1972 Apr;127(2):387–397. doi: 10.1042/bj1270387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T., Thurman R. G., Fukami M. H. Metabolic effects of ethanol in perfused rat liver. J Biol Chem. 1969 Sep 25;244(18):5044–5054. [PubMed] [Google Scholar]


