Abstract
Crohn’s disease (CD) is a chronic, complex inflammatory disorder of the gastrointestinal tract that presents significant therapeutic challenges. Despite the availability of a wide range of treatments, many patients experience primary non-response, secondary loss of response, or adverse events, limiting the overall effectiveness of current therapies. Clinical trials often report response rates below 60%, partly due to stringent inclusion criteria. Emerging therapies that target novel pathways offer promise in overcoming these limitations. This review explores the latest investigational drugs in phases I, II, and III clinical trials for treating both luminal and perianal CD. We highlight promising therapies that target known mechanisms, including selective Janus kinase inhibitors, anti-adhesion molecules, tumor necrosis factor inhibitors, and IL-23 selective inhibitors. In addition, we delve into novel therapeutic strategies such as sphingosine-1-phosphate receptor modulators, miR-124 upregulators, anti-fractalkine (CX3CL1), anti-TL1A, peroxisome proliferator-activated receptor gamma agonists, TGFBRI/ALK5 inhibitors, anti-CCR9 agents, and other innovative small molecules, as well as combination therapies. These emerging approaches, by addressing new pathways and mechanisms of action, have the potential to surpass the limitations of existing treatments and significantly improve CD management. However, the path to developing new therapies for inflammatory bowel disease (IBD) is fraught with challenges, including complex trial designs, ethical concerns regarding placebo use, recruitment difficulties, and escalating costs. The landscape of IBD clinical trials is shifting toward greater inclusivity, improved patient diversity, and innovative trial designs, such as adaptive and Bayesian approaches, to address these challenges. By overcoming these obstacles, the drug development pipeline can advance more effective, accessible, and timely treatments for CD.
Keywords: biologic therapies, challenges, clinical trials, Crohn’s disease, mechanism of action, perianal Crohn’s disease, placebo, small molecules, trial design
Plain language summary
Crohn’s disease: hope on the horizon with new therapies in development
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) that affects millions of people worldwide. Many people with CD do not respond well to current treatments, and researchers are looking for new options. Clinical trials are research studies that test new drugs and treatments. They are carefully designed to protect the safety of participants. Several new approaches to treating CD are currently undergoing clinical trials. These include drug candidates in various stages of development, from early research to large-scale phase III trials. Cellular therapies are also being tested, involving the injection of cells locally or intravenously to promote healing. Crohn’s disease (CD) is a long-term condition that causes inflammation in the digestive tract. It can be difficult to treat, as current medications don’t always work for everyone, and some people experience side effects or stop responding to treatment over time. Even in clinical trials, where new treatments are tested, less than 60% of patients show positive responses. Researchers are working on new treatments that target different pathways involved in Crohn’s disease. This review looks at drugs being tested in early to late-stage clinical trials. Some of these drugs target well-known pathways, like JAK inhibitors and IL-23 blockers, while others focus on newer areas, such as specific receptors or molecules involved in inflammation. These emerging therapies aim to provide better, longer-lasting relief for patients. However, developing new treatments isn’t easy. Clinical trials for Crohn’s disease face many challenges, including complicated trial designs, ethical concerns about using placebos, difficulties in recruiting enough patients, and high costs. To overcome these issues, researchers are exploring more flexible and inclusive trial methods, which could help bring new treatments to patients more quickly and efficiently.
Introduction
Crohn’s disease (CD) is a chronic, relapsing inflammatory condition associated with significant complications due to persistent inflammation, leading to increased risks of comorbidity, hospitalization, surgery, and disability, all of which profoundly impact patients’ mental health and quality of life.1 –4 Effective management of CD, ideally initiated in the early stages of the disease, is crucial to prevent relapses and complications, thereby reducing the need for corticosteroids, surgeries, and hospital admissions, and improving overall patient well-being. 5
The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program emphasizes a treat-to-target strategy, recommending clinical and endoscopic healing targets, along with the normalization of serum and fecal biomarkers, restoration of quality of life, prevention of disability, and ensuring normal growth in children.6,7 To date, the therapeutic arsenal for inflammatory bowel disease (IBD) has included mesalamine, immunosuppressants such as methotrexate and thiopurine, cyclosporin, anti-tumor necrosis factor alpha (TNFα) agents, anti-integrin therapies, anti-IL-12 and IL-23 monoclonal antibodies, and a few small molecules.8 –11
However, randomized clinical trials (RCTs) reveal that only about half of the patients achieved clinical remission, with efficacy declining within a year. 12 Thus, the goal of complete and sustained remission remains elusive. Achieving this target requires comprehensive advancements in early diagnosis, personalized treatment, patient monitoring, and the development of innovative therapies and clinical trial designs.
This review aims to provide a comprehensive overview of the current pipeline for novel and emerging therapies specifically targeting luminal and perianal CD. A timeline of drugs approved by the US Food and Drug Administration (FDA) is seen in Figure 1.
Figure 1.
Timeline of FDA approvals for Crohn’s disease, and new drugs entering the scene.
ALK5, activin receptor-like kinase 5, also known as the transforming growth factor (TGF-β) type 1 receptor; CCR9, C-C motif chemokine receptor 9; FKN, fractalkine; JAK, Janus kinase; miR-124, microRNA-124; PPARγ, peroxisome proliferator-activated receptor gamma; MOA, mechanism of action; S1PR, sphingosine-1-phosphate receptor; TL1A, TNF-like ligand 1A; TNF, tumor necrosis factor.
Source: Created with Biorender.com (accessed 21 September 2024).
New therapeutic agents, including antagonists of the TNF-like ligand 1A (TL1A), modulators of receptor-interacting serine/threonine kinase 2 (RIPK2), interleukins, peroxisome proliferator-activated receptor gamma (PPARγ) agonists, and other innovative strategies are reported and discussed.
Pipeline of clinical trials for luminal CD
Phase II and III clinical trials
This section provides a glimpse into the diverse pipeline of phase II and III clinical trials exploring novel therapeutic modalities for managing CD, as represented in Table 1. Targeted therapies for fibrostenosing CD are discussed in a separate section.
Table 1.
Drugs in phase II and III clinical trials for the treatment of luminal Crohn’s disease.
| Class | Name | Target | Route of administration | Phase of the trial | State of the trial | NCT | Estimated/actual completion | Primary endpoint | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Biochemical | Endoscopical | Histological | Radiological | Safety | ||||||||
| Selective Inhibitors of IL-23 | Guselkumab | IL23/p19 subunit | IV and SC | Phase III | Active | NCT03466411 | 2030-06-30 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ |
| SC | Phase III | Active | NCT05197049 | 2025-03-31 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | |||
| IV and SC | Phase III | Recruiting | NCT06408935 | 2028-08-30 | ✘ | ✘ | ✘ | ✘ | ✔ | ✘ | |||
| Risankizumab | IL23/p19 subunit | SC | Phase III | Recruiting | NCT06063967 | 2028-02-23 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | |
| Mirikizumab | IL23/p19 subunit | IV or SC | Phase III | Completed | NCT03926130 | 2023-10-02 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | |
| IV or SC | Phase III | Recruiting | NCT04232553 | 2026-12-20 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | |||
| TNF inhibitors | V565 | TNFα | Oral | Phase II | Completed | NCT02976129 | 2019-03-08 | ✔ | ✔ | ✘ | ✘ | ✘ | ✘ |
| SAR441566 | TNFα | Oral | Phase II | Not yet recruiting | NCT06637631 | 2029-05-22 | ✘ | ✘ | ✔ | ✘ | ✘ | ✘ | |
| SP1R modulators | Etrasimod | S1P1, S1P4, and S1P5 receptors | Oral | Phase II/III | Recruiting | NCT04173273 | 2029-08-08 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ |
| JAK-inhibitors | Zasocitinib | TYK2 | Oral | Phase II | Recruiting | NCT06233461 | 2027-07-23 | ✘ | ✘ | ✔ | ✘ | ✘ | ✘ |
| Brepocitinib | TYK2/JAK1 | Oral | Phase II | Completed | NCT03395184 | 2023-10-19 | ✘ | ✘ | ✔ | ✘ | ✘ | ✔ | |
| Ritlecitinib | JAK1 | Oral | Phase II | Completed | NCT03395184 | 2023-10-19 | ✘ | ✘ | ✔ | ✘ | ✘ | ✔ | |
| VTX958 | TYK2 | Oral | Phase II | Active | NCT05688852 | 2027-05 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | |
| Anti-TL1A | Tulisokibart | TL1A | IV/SC | Phase IIa | Recruiting | NCT06430801 | 2029-09-11 | ✔ | ✘ | ✔ | ✘ | ✘ | ✔ |
| SC | Phase III | Recruiting | NCT06651281 | 2037-12-17 | ✘ | ✘ | ✘ | ✘ | ✘ | ✔ | |||
| RVT-3101 | TL1A | SC | Phase II | Active | NCT05910528 | 2025-12-15 | ✔ | ✘ | ✘ | ✘ | ✘ | ✘ | |
| Duvakitug | TL1A | SC | Phase IIb | Active | NCT05499130 | 2025-01-15 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ | |
| Interleukins | LD SC IL-2 | Regulatory T-cells | SC | Phase Ib/IIa | Recruiting | NCT04263831 | 2025-12-31 | ✘ | ✘ | ✘ | ✘ | ✘ | ✔ |
| MiR-124 upregulators | ABX464 (obefazimod) | MiR-124 | Oral | Phase IIb | Not yet recruiting | NCT06456593 | 2028-04 | ✔ | ✘ | ✔ | ✘ | ✘ | ✔ |
| PPARγ agonists | MBF-118 | PPARγ nuclear receptor | Oral | Phase IIa | Completed | NCT05940558 | 2024-11 | ✘ | ✘ | ✘ | ✘ | ✘ | ✔ |
| Anti-fractalkine (CX3CL1) | E6011 (quetmolimab) | FKN | IV | Phase II | Completed | NCT03733314 | 2024-04-03 | ✔ | ✘ | ✘ | ✘ | ✘ | ✘ |
| TGFβRI/ALK5 selective inhibitors | AGMB-129 | TGFβRI/ALK5 | Oral | Phase IIa | Recruiting | NCT05843578 | 2025-12 | ✘ | ✘ | ✘ | ✘ | ✘ | ✔ |
| Anti-integrins | MORF-057 | α4β7 receptor | Oral | Phase II | Recruiting | NCT06226883 | 2028-04 | ✘ | ✘ | ✔ | ✘ | ✘ | ✘ |
| Anti-CCR9 | AZD7798 | CCR9 receptor | IV, SC | Phase IIa | Recruiting | NCT06450197 | 2027-05-21 | ✔ | ✘ | ✘ | ✘ | ✘ | ✘ |
| Combination therapy | Guselkumab and Golimumab | P19 IL23/TNF | IV, SC | Phase II | Active | NCT05242471 | 2029-03-27 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ |
| Combination therapy | Vedolizumab and upadacitinib | α4β7/JAK | IV, Oral | Phase III | Not yet recruiting | NCT06227910 | 2028-08-01 | ✔ | ✘ | ✔ | ✘ | ✘ | ✘ |
| Combination therapy | Vorinostat and ustekinumab | HDAC/p40 IL12 and IL23 | Oral/IV and SC | Phase I/II | Recruiting | NCT03167437 | 2026-06-30 | ✘ | ✘ | ✘ | ✘ | ✘ | ✔ |
ALK5, activin receptor-like kinase 5; CCR9, C-C chemokine receptor 9; CX3CL1, chemokine (C-X3-C motif) ligand 1; FKN, fractalkine; HDAC, histone deacetylase; IL-23, interleukin-23; IV, intravenous; JAK, Janus kinase; LD SC IL-2, low-dose subcutaneous interleukin-2; MiR-124, microRNA-124; NCT, National Clinical Trial (Identifier); PPARγ, peroxisome proliferator-activated receptor gamma; SC, subcutaneous; S1P1, S1P4, and S1P5, sphingosine-1-phosphate receptors; TGFβRI/ALK5, transforming growth factor beta receptor I/activin receptor-like kinase 5; TL1A, tumor necrosis factor-like cytokine 1A; TNF, tumor necrosis factor; TYK2, tyrosine kinase 2; α4β7, alpha4 beta7 integrin receptor.
The main mechanisms of action are schematized in Figure 2.
Figure 2.
Mechanisms of action of emerging therapies for Crohn’s disease.
ALK5, activin receptor-like kinase 5; CCR9, C-C chemokine receptor 9; CX3CL1, chemokine (C-X3-C motif) ligand 1; IL-23, interleukin-23; JAK, Janus kinase; MiR-124, microRNA-124; NCT, National Clinical Trial (Identifier); PPARγ, peroxisome proliferator-activated receptor gamma; SC, subcutaneous; S1P, sphingosine-1-phosphate; TL1A, tumor necrosis factor-like cytokine 1A; TNF, tumor necrosis factor; α4β7, alpha4 beta7 integrin receptor.
Source: Created with Biorender.com (accessed 12 November 2024).
Selective inhibitors of IL-23
IL-23, belonging to the IL-12 cytokine family, consists of p40 and p19 subunits, with p19 being exclusive to IL-23. It plays a vital role in regulating and enhancing T-helper 17 cells and activating various innate immune cells, which holds significance in developing chronic inflammatory disorders like CD.13,14 Risankizumab, a monoclonal IgG1 antibody strategically designed to bind to IL-23, thereby inhibiting its activity, marks the first FDA-approved selective IL-23p19 treatment for individuals with moderate-to-severe CD.15,16 In The Lancet, D’Haens et al. detailed the ADVANCE and MOTIVATE phase III trials, which compared the efficacy and safety of risankizumab to a placebo during the induction period. 15 Similarly, Ferrante et al. reported on the FORTIFY phase III trial, assessing risankizumab against a placebo during the maintenance period. 16 The co-primary endpoints for the induction trials were clinical remission and endoscopic response at week 12, and for the maintenance trial, the same endpoints were measured at week 52. Results from both trials favored risankizumab over placebo for the primary endpoints. Real-world data have confirmed risankizumab’s efficacy and favorable safety profile even in highly refractory cohorts and patients who had received ustekinumab therapy.17 –19 The APRISE study (NCT05841537) is an observational cohort study that aims to gather real-world data on risankizumab in the treatment of CD.
A phase III clinical trial assesses the safety and efficacy of risankizumab subcutaneous (SC) induction treatment for adults with moderately to severely active CD is now recruiting (NCT06063967). Participants will receive risankizumab or placebo during Period A, followed by adjusted doses based on their response in Period B. The trial aims to evaluate clinical remission, endoscopic response, and overall disease activity over approximately 49 weeks.
Brazikumab (MEDI2070) is a human IgG2 monoclonal antibody that binds exclusively to the p19 subunit of IL-23. In a phase IIa trial in patients with moderate-to-severe active that had failed or were intolerant to ⩾1 anti-TNFα agent, brazikumab demonstrated significant clinical response rates at week 8, with a notable composite outcome including fecal calprotectin or C-reactive protein (CRP) reduction. An open-label extension (OLE) period showed sustained clinical response and remission. 20 However, the trial lacked endoscopic assessments. Despite initial optimism, AstraZeneca halted the development of brazikumab for Crohn’s disease in June 2023, terminating a phase IIb trial (NCT02574637) and the phase IIb/III INTREPID trial (NCT03759288 and NCT03961815), due to a strategic decision to discontinue the development of brazikumab in IBD after a review of its development timeline and the evolving competitive landscape.
Guselkumab
Guselkumab’s efficacy, a monoclonal human IgG1 antibody specifically targeting p19 IL-23, was analyzed in a program consisting in three separate studies: a 48-week phase II dose-ranging study (GALAXI 1) and two 48-week phase III confirmatory studies (GALAXI 2 and GALAXI 3). GALAXI-1 trial was a phase II, double-blind study with five arms designed to assess its efficacy and safety in patients with moderately to severely active CD who had inadequate response or intolerance to steroids and biologics.21,22 GALAXI-1 employed a treat-through design, enrolling 309 patients randomized in a 1:1:1:1:1 ratio to receive guselkumab at dosages of 200, 600, or 1200 mg, or placebo at weeks 0, 4, and 8, or ustekinumab. The patients underwent an intravenous (IV) induction phase followed by an SC maintenance phase. At week 48, 57%–73% of the patients in the guselkumab dose groups achieved clinical remission based on Crohn’s Disease Activity Index (CDAI), 44%–46% had an endoscopic response, and 17%–33% had endoscopic remission. In the long-term extension (LTE) study of the GALAXI-1 trial, patients receiving maintenance therapy with guselkumab were followed for 3 years, with efficacy endpoints assessed at week 144. Key outcomes included clinical remission, patient-reported outcome (PRO2) remission, and endoscopic response. The study included 220 patients in the combined guselkumab group (referring to the various dosages of 200, 600, and 1200 mg) and 114 in the ustekinumab group. At week 144, 54.1% of patients in the combined guselkumab group were in clinical remission, 51.4% were in PRO2 remission, and 34.7% showed an endoscopic response. Clinical remission based on PRO2 is defined as average daily liquid or very soft stool frequency (SF) score ⩽2.8 and not worse than baseline and average daily abdominal pain (AP) score ⩽1 and not worse than baseline.
Guselkumab exhibited a favorable safety profile, with comparable adverse event incidences across groups, and everyday events included headache and nasopharyngitis. No cases of active tuberculosis, opportunistic infections, anaphylactic/serum sickness reactions, major adverse cardiovascular events, or deaths occurred during the LTE. 23 Phase III double-blind, double-dummy multicenter trials (GALAXI-2 and GALAXI-3), recently presented at Digestive Disease Week 2024, were conducted to assess further guselkumab’s efficacy in adult patients with CD (NCT03466411). 24 These trials compared guselkumab against placebo and ustekinumab, involving a total of 1021 participants. Eligible patients had moderate-to-severe CD and inadequate response or intolerance to previous therapies. They were randomized 2:2:2:1 to receive guselkumab 200 mg IV followed by either 200 mg SC every 4 weeks or by 100 mg SC every 8 weeks (Q8W), ustekinumab induction followed by maintenance, or placebo. The primary endpoints for guselkumab versus placebo were clinical response at week 12 and clinical remission at week 48, and clinical response at week 12 and endoscopic response at week 48. Both dosages of guselkumab met these composite co-primary endpoints compared to placebo in each individual study. Secondary endpoints included the comparison of efficacy between guselkumab and ustekinumab in terms of clinical remission at week 48, endoscopic response and remission the same endpoint as well as clinical remission combined with endoscopic response at week 48 and deep remission at week 48. Guselkumab exhibited superior efficacy over ustekinumab across all prespecified pooled endoscopic endpoints at the week 48 assessment. Specifically, patients receiving guselkumab showed significantly higher rates of endoscopic response, endoscopic remission, clinical remission combined with endoscopic response, deep remission, and clinical remission alone compared to those receiving ustekinumab. These findings underscore guselkumab’s potential as a highly effective treatment option for patients with moderate-to-severe CD. A new phase IIIb, open-label, multicenter trial named REASON aims to assess the effectiveness of treatment in CD using various outcome measures, including the evaluation of transmural healing, over a 96-week period and is now recruiting (NCT06408935). Inclusion criteria comprehend segmental Magnetic Resonance Index of Activity (MaRIA) of at least 11. The primary outcome includes achieving a MaRIA < 11 in all intestinal segments at 48 weeks. Secondary outcomes include achieving a MaRIA score inferior to 11 in all intestinal segments at weeks 16 and 96, endoscopic responses, transmural responses via intestinal ultrasound, and PROs such as PRO2 and inflammatory bowel disease questionnaire (IBDQ) scores. Additionally, changes in CDAI, CRP, and fecal calprotectin levels will be monitored. Safety outcomes include reporting treatment-emergent adverse events (TEAEs) up to week 48.
A phase III trial double-blind and placebo-controlled named GRAVITI (NCT05197049) aims to assess the efficacy and safety of guselkumab solely SC therapy in individuals with moderately to severely active CD. The study involves multiple arms, with participants receiving one of three doses of SC guselkumab or a placebo. The results are unpublished, however, Johnson & Johnson already announced that their phase III GRAVITI met all primary and secondary endpoints, achieving significant clinical remission and endoscopic response at week 12. The study supports guselkumab’s potential to be the only IL-23 inhibitor with both SC and IV induction options. Results were consistent with previous GALAXI studies; therefore, the pharmaceutical company has submitted application for FDA approval of guselkumab for this indication. Of note, the pharmacokinetic (PK) findings indicate that there was no clear relationship between systemic exposure to guselkumab and clinical outcomes such as CDAI improvement, clinical remission, or endoscopic response at week 12. 21
Mirikizumab
Mirikizumab, a humanized IgG4 monoclonal antibody targeting the p19 component of IL-23, offers both IV and SC administration options. In the recently concluded VIVID-1 phase III study, adult patients were randomized in a ratio of 6:3:2 to receive mirikizumab 900 mg IV every 4 weeks until week 12, followed by 300 mg SC every 4 weeks until week 52, ustekinumab or placebo. 25 The co-primary endpoints, assessing the superiority of mirikizumab over placebo, were composite measures: (1) clinical response by PRO based on SF and AP at week 12 and endoscopic response at week 52, and (2) clinical response by PRO based on SF and AP response at week 12 and clinical remission by CDAI at week 52. Both co-primary endpoints were achieved. Particularly notable was the achievement of the first endpoint, where a significantly higher proportion of patients in the mirikizumab arm achieved clinical response at week 12 and endoscopic response at week 52 compared to those receiving placebo. Additionally, patients treated with mirikizumab achieved all critical major secondary endpoints compared to placebo. 25 Mirikizumab demonstrated non-inferiority to ustekinumab in clinical remission by CDAI but not in endoscopic response. 25 VIVID-2 is a phase III, multicenter, open-label, LTE study of patients who underwent SERENITY, the phase II trial of mirikizumab in CD (NCT02891226), or VIVID-1. It is currently recruiting (NCT04232553). The primary outcomes include endoscopic response based on the Simple Endoscopic Score for Crohn’s Disease (SES-CD) and clinical remission, assessed by the CDAI, at week 52. Secondary outcomes include endoscopic remission based on the SES-CD total score at week 52, clinical response based on PRO at week 52, changes in biochemical parameters at week 12 (CRP and fecal calprotectin), and changes in the IBDQ from baseline to week 52.
In the AMAG trial, a phase II study of mirikizumab for CD, PKs showed that drug clearance and distribution were influenced by factors like disease severity and body weight, though these effects were not clinically significant. 26 A dose-dependent response was observed during the induction phase, with higher doses yielding better endoscopic outcomes. However, during the maintenance phase, efficacy was consistent regardless of initial dose, with 300 mg SC providing adequate disease control. No patient factors required dose adjustments.
TNF inhibitors
Biologic agents targeting TNFα have become a well-established therapy, after authorization by the European Medicines Agency (EMA) of infliximab, adalimumab, and certolizumab.27 –30 Thereafter, additional compounds have been investigated.
V565
As innovative treatment, oral V565 represents a novel antibody specifically targeting TNFα, engineered to resist degradation by intestinal proteases. A concluded phase II study (ClinicalTrials.gov identifier: NCT02976129), employing a double-blind, placebo-controlled, parallel-group design, aimed to evaluate the efficacy of V565 in treating patients with active CD over 6 weeks. Involving 125 patients, the study randomized participants in a 2:1 ratio to receive either V565 or a placebo three times daily, with monitoring extending for 28 days. The primary objective was to assess clinical response, defined as at least a 70% reduction in the CDAI score alongside more than a 40% decrease in inflammatory markers (such as CRP or fecal calprotectin) by day 42. While the rate of clinical response did not significantly differ between the V565 and placebo groups (35.4% vs 37.2%), the treatment group exhibited higher rates of improvement in endoscopic findings (56.3% compared to 30.0% in the placebo group). The frequency of serious adverse events (SAEs) was comparable in both cohorts, with no reported fatalities. There are currently no ongoing phase III trials involving this drug.
SAR441566
The SPECIFI-CD trial (NCT06637631) is a phase II, multinational, multicenter, randomized, double-blind, placebo-controlled study evaluating SAR441566 in adults with moderate-to-severe CD. 31 The trial aims to assess the efficacy and safety of different doses of SAR441566 compared to a placebo. It involves a 12-week induction phase and a 40-week maintenance period, followed by up to 92 weeks of open-label treatment. Primary outcomes include endoscopic response and clinical remission. The study will enroll 260 participants, with results expected by May 2029.
SP1R modulators
Sphingosine-1 phosphate (S1P) is a highly bioactive molecule originating from cell membranes, primarily exerting its effects by activating five G protein-coupled type 1 and type 5 receptors on the cell surface (S1P1–S1P5). 32 The S1P gradient between blood and tissues facilitates immune cell recruitment and inflammation. Upon activation, this initiates a cascade of signaling events that regulate various biological processes, including lymphocyte migration, endothelial cell permeability, angiogenesis, cell differentiation, proliferation, survival, and apoptosis. 32 S1P receptors regulate the influx of leukocytes from primary and secondary lymphoid organs into the intestinal wall, thereby contributing to the maintenance of intestinal inflammation.33,34 Modulating this pathway holds the potential to impede lymphocyte migration into the gut, a critical factor in chronic inflammation, thereby reducing inflammation and minimizing tissue damage. 35 Oral small molecules that can modulate S1P receptors have been developed, offering advantages over biologic agents such as ease of administration and rapid action. However, in a phase II, multicenter, randomized, double-blind, placebo-controlled trial, amiselimod (MT-1303) was not superior to placebo for the induction of clinical response in patients with CD. 36 Similarly, ozanimod, a selective sphingosine-1-phosphate receptor (S1PR) modulator, explicitly targeting the S1P1 and S1P5 receptors on endothelial cells and oligodendrocytes, was involved in the YELLOWSTONE program which consisted of phase III, randomized, double-blind, placebo-controlled induction (NCT03440372 and NCT03440385) and maintenance (NCT03464097) trials and an OLE study (NCT03467958). The trial NCT03440385 missed its primary endpoint, and the program was therefore terminated.
A clinical trial regarding the efficacy and safety of etrasimod, discussed below, is recruiting.
Etrasimod
Etrasimod exhibits high affinity for S1P1,4,5 receptors with minimal impact on S1P3 and no activity on S1P2. It partially and reversibly inhibits lymphocyte migration from lymphoid tissues, leading to a dose-dependent reduction in peripheral blood lymphocyte count. 37 The efficacy of etrasimod in treating adults CD patients is under evaluation in a seamless phase II/III trial named CULTIVATE (NCT04173273), which consists of five sub studies still currently recruiting. 38 The program includes a phase II, placebo-controlled, dose-ranging study (substudy 1); a phase III induction therapy study (substudy 2); a phase III maintenance therapy study (substudy 3); and an LTE (substudy 4), whose results are eagerly awaited.
Initial data from the induction period of the first substudy, named substudy 1, suggested that once-daily dose of etrasimod is associated with endoscopic and clinical improvement. The randomized, double-blind phase II CULTIVATE substudy 1 spanned a 14-week induction period followed by a 52-week extension phase. Eligible patients were adults with a moderately to severely active CD and a history of inadequate response, loss of response, or intolerance to at least one treatment. Eighty-three patients were randomized in a 1:1 ratio to receive oral etrasimod 2 mg or etrasimod 3 mg once daily. At the end of the 14-week induction period, 21% of etrasimod 2 mg recipients and 10% of etrasimod 3 mg recipients achieved an endoscopic response (primary endpoint), while 14% and 7%, respectively, achieved endoscopic remission. Additionally, 31% and 44% achieved clinical remission. 38
JAK inhibitors
New small molecules act on the Janus kinase (JAK) pathway, inhibiting critical cytokines involved in various rheumatic diseases. In recent years, numerous studies have focused on the JAK-STAT pathway, providing novel therapeutic strategies to enhance the treatment of IBD.39 –41 JAK, a family of intracellular tyrosine kinases consisting of JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), plays a crucial role in transmitting cytokine-mediated signals through the STAT pathway. 42 These kinases are activated by various cytokine receptors, leading to inflammation through T-cell proliferation and differentiation, as well as B-cell activation. In the context of IBD, IL-6, IL-12, and IL-23 serve as critical drivers of disease activity, and their activation occurs via the Janus Kinase-Signal Transducer and activator of transcription (JAK-STAT) pathway. 43 Blocking the activation of this pathway halts the activity of several chemokines involved in mediating inflammation. Several pan-JAK inhibitors and selective JAK inhibitors, including the newer TYK2 inhibitors, are in various stages of development for IBD.
Tofacitinib, a pan-JAK inhibitor, and filgotinib, a JAK1 selective inhibitor, have not received approval for CD. Initial testing of tofacitinib in patients with CD was conducted in a phase IIa clinical trial (NCT00615199), with results reported by Sandborn et al. in 2014. 44 The study, which focused on a 4-week induction period for moderate-to-severe CD, had a primary endpoint of clinical response at week 4. The findings showed no significant differences in clinical response or remission between the placebo and treatment groups. Further investigation into tofacitinib for CD was carried out in two placebo-controlled, randomized, multicenter IIb trials (NCT01393626 and NCT01393899), reported by Panés et al. in 2017. 45 At the end of the 8-week induction phase, there were no significant differences between the placebo and treatment groups. The high placebo response rate in both trials was noteworthy. The maintenance study, with a primary endpoint of clinical remission or response at week 26, also showed no significant differences in clinical remission or response between the groups.
Filgotinib, a selective JAK1 inhibitor, despite favorable results in a phase II CD study (FITZROY), did not meet the primary endpoints in the induction cohorts of the phase III study (DIVERSITY), which included 1374 patients with moderate to severely active CD until week 10. 46 Additionally, a phase II trial (DIVERGENCE 1) evaluating the safety and efficacy of filgotinib in small bowel CD did not show statistically significant differences compared to placebo. 47 In contrast, the data presented in the DIVERGENCE 2 trial offers promising prospects for the potential application of filgotinib in treating perianal fistulizing CD, as described below. 48
Upadacitinib, a selective JAK1 inhibitor, received marketing authorization for CD based on results from 12-week induction studies (U-EXCEL and U-EXCEED) involving 1021 patients and a 52-week maintenance study (U-ENDURE) of 502 patients. 49 In U-EXCEL and U-EXCEED, patients receiving upadacitinib showed significantly higher rates of clinical remission and endoscopic response compared to placebo after 12 weeks. Similarly, in U-ENDURE, patients on maintenance therapy with upadacitinib sustained higher rates of clinical remission and endoscopic response at 52 weeks compared to placebo. Adverse events included higher incidences of herpes zoster infections and hepatic disorders in the upadacitinib groups. A trial evaluating the efficacy and safety of dual-target therapy (ustekinumab + upadacitinib) versus intensified ustekinumab monotherapy in patients with CD who did not respond adequately to standard ustekinumab doses is currently registered (NCT06520397). Participants will be randomly assigned to either treatment, with the primary outcome being endoscopic remission at 16 weeks. Secondary outcomes will include clinical response, deep remission, and safety evaluations. The study aims to determine if dual-target therapy is more effective and as safe as intensified ustekinumab. Expected completion is in 2027.
Deucravacitinib (BMS-986165), a TYK2 inhibitor, phase II trial was terminated after a planned review of the Independent Data Monitoring Committee (NCT03635112). The phase II trial of Izencitinib (TD-1473), a pan-JAK inhibitor, was terminated due to lack of efficacy in the short-term acute phase (NCT03599622).
Ivarmacitinib (SHR0302), a selective JAK1 inhibitor, was subject to investigation in a phase II randomized, double-blind, placebo-controlled, multicenter study assessing its safety and efficacy in 112 patients with moderate-to-severe active CD (ClinicalTrials.gov identifier: NCT03677648). Participants who completed the initial 12-week treatment entered a blind active-arm extension phase for an additional 12 weeks. The primary endpoint under evaluation was clinical remission at week 12, defined as a CDAI score <150. The study’s actual completion date was 2021-12-09 and currently, results from this study are pending.
Zasocitinib
A phase IIb, randomized, double-blind, placebo-controlled, dose-ranging phase II trial of Zasocitinib (formerly TAK-279) is currently recruiting (NCT06233461). Zasocitinib is a highly selective, oral allosteric TYK2 inhibitor. The aim of the phase II study is to evaluate if zasocitinib is effective and safe for treating CD: participants will be randomly assigned to receive zasocitinib at one of three doses or a placebo for 52 weeks. Participants must have previously undergone treatments for CD and shown inadequate response, loss of response, or intolerance to therapies such as 6-mercaptopurine, azathioprine, corticosteroids, or biologic agents like TNF antagonists. There will be a 12-week induction period followed by a 40-week maintenance period. The study’s primary outcome is the endoscopic response at week 12, measured by a significant decrease in SES-CD score. Secondary outcomes include clinical remission and response based on CDAI and PRO2 scores, endoscopic remission, absence of bowel urgency, IBDQ total score indicating quality of life, and changes in fatigue as measured by FACIT-Fatigue score.
Brepocitinib and ritlecitinib
Brepocitinib (PF-06700841), a JAK1/TYK2 inhibitor and ritlecitinib (PF-06651600), a JAK3 inhibitor, underwent a phase II trial (NCT03395184), which is completed. This phase IIa trial was a double-blind, randomized, placebo-controlled study to assess the efficacy and safety of both drugs in patients with moderate-to-severe CD. The study involves two experimental groups and respective placebo treatments. Participants in the first group receive PF-06700841 or a placebo for 12 weeks, followed by PF-06700841 at 30 mg daily for 52 weeks. Participants in the second group receive PF-06651600 or a placebo for 12 weeks, followed by PF-06651600 at 50 mg daily for 52 weeks. The primary objective was to evaluate the proportion of subjects achieving a reduction of more than 50% in SES-CD from baseline at week 12, safety measures such as adverse events, SAE, electrocardiogram findings, laboratory test findings, withdrawals due to adverse events, and alteration of vital signs findings from baseline to week 68.
The results of this trial are not been published yet.
VTX958
An ongoing phase II open multicenter, randomized, double-blind, placebo-controlled, parallel-group study called Harmony-CD (NCT05688852) recruited individuals with moderately to severely active CD to investigate the efficacy of VTX958, a TYK2 inhibitor. Approximately 132 eligible patients are randomized, with stratification based on prior biologic use for CD treatment. The study comprises distinct phases, including a 30-day screening period, a 12-week double-blind induction treatment period, a 40-week double-blind maintenance treatment period, an OLE lasting up to 144 weeks, and a 30-day safety follow-up period. The overall treatment duration, covering induction, maintenance, and OLE periods, is limited to 36 months. Primary objectives include evaluating VTX958’s efficacy in reducing the CDAI score and achieving endoscopic response at the end of the induction period. Secondary objectives involve assessing VTX958’s effectiveness in inducing clinical and symptomatic response and remission and evaluating endoscopic and clinical remission after the induction period. Furthermore, a follow-up visit for all participants will occur 30 days after their last dose of the study drug (ClinicalTrials.gov identifier: NCT05688852).
Anti-TL1A
The TNF and TNFR superfamilies, TNFSF and TNFRSF respectively, encompass a diverse array of proteins critical to various biological processes, particularly important in IBD. 50 TL1A, a TNF-like cytokine encoded by the TNFSF15 gene is a type II transmembrane protein which is capable of influencing a wide range of immune responses mediates both in its membrane-bound and soluble forms.51 –53 It is secreted by antigen-presenting cells and plays a role in pro-inflammatory effects that contribute to chronic inflammation through T-cell activation by interacting with its receptor, death domain receptor 3 (DR3).51,54 Genome-wide association studies have identified a correlation between a genetic variant in the TNF superfamily member 15 (TNFSF15) locus and IBD. 55 Additionally, TL1A is highly expressed in the tissue of patients with CD and is linked to profibrotic and severe diseases.56 –58 Several human trials are underway to test the safety and efficacy of anti-TL1A antibodies for treating ulcerative colitis (UC) and CD. Two phase II trials, named TUSCANY and ARTEMIS-UC, investigated an anti-TL1A antibody and demonstrated advancements in broadening therapeutic choices for UC.59,60 A phase IIa double-blind placebo-controlled RCT to assess the efficacy of PF-06480605, a fully human IgG1 monoclonal antibody, in CD patients was recently stopped due to a change in sponsor (NCT05471492). The study was designed as a trial with parallel assignment. The primary outcome measure was the proportion of participants with an endoscopic response at week 14, defined as a reduction from baseline in the SES-CD of at least 50%. A new trial is expected to be registered by the new sponsor. A new TL1-antibody, FG-M701, is expected to enter phase I this year.
Tulisokibart
Tulisokibart (formerly MK-7240), a humanized IgG1 monoclonal antibody targeting to TNFSF15/TL1A, underwent a recent phase IIa multicenter open-label study that included 55 patients with moderate-to-severe CD to evaluate its safety and efficacy. 61 Patients received 1000 mg IV on day 1, followed by 500 mg doses at weeks 2, 6, and 10. The primary endpoint was endoscopic response (reduction in SES-CD score of ⩾50%) at week 12; and an exploratory endpoint efficacy in a subpopulation identified by a genetics-based companion diagnostic test (CDx). The study cohort featured a substantial proportion of individuals with prior exposure to biologic therapies (70.9%) and an average disease duration of 10.3 years. Compared to historical placebo estimates, a significantly higher proportion of patients treated with MK-7240 achieved endoscopic response (26% vs 12%, p = 0.002) and clinical remission (49% vs 16%, p < 0.001). Efficacy was also observed in biologic-exposed patients. Tulisokibart demonstrated good tolerability, with no reported severe adverse events. Following the 12-week induction period, all participants can continue in the OLE for another 38 weeks, and the study named APOLLO-CD is currently recruiting (NCT06430801).
RVT-3101
RVT-3101 is a fully human IgG1 monoclonal antibody targeting TL1A, administered SC once a month. The TAHOE study (NCT05910528) is a phase II multicenter double-blind two-arm evaluating two doses of RVT-3101 once in patients with moderate to severely active CD. Two treatment sequences (A and B) involving induction and maintenance doses of RVT-3101 are compared across various outcome measures. Primary outcomes include the proportion of participants achieving clinical remission by CDAI at week 14. Secondary measures encompass endoscopic responses, clinical responses by CDAI, safety profiles, PRO2, and PK parameters (Ctrough, ADA, NAb) over a longer period (up to week 64). This rigorous design aims to comprehensively assess both therapeutic efficacy and tolerability of RVT-3101 in clinical practice. It is now active (ClinicalTrials.gov identifier: NCT05910528).
Duvakitug
Duvakitug (previously TEV-48574) is a fully human IgG1 monoclonal antibody that targets TL1A. A phase IIb randomized, double-blind, dose-ranging study named RELIEVE UCCD with the aim of evaluating duvakitug in adult patients with UC or CD through a basket trial design (NCT05499130) is currently recruiting.62,63 It employs a parallel assignment intervention model with quadruple masking. TEV-48574 is administered via SC infusion in different dose regimens for UC and CD, with matching placebos for each condition. The primary outcome measures include the number of participants achieving clinical remission for UC and endoscopic response for CD at week 14. The estimated completion date is January 15, 2025.
Anti-fractalkine (CX3CL1)
Fractalkine (FKN) is a CX3C chemokine with a unique structure featuring a combination of chemokine and mucin characteristics and a transmembrane domain.64,65 It is primarily expressed in vascular endothelial cells. It exists in two functional forms: as an adhesion molecule in its membrane-bound state and as a chemoattractant in its soluble form, which occurs after shedding by metalloproteases, ADAM 10 or ADAM 17. 66 The membrane-bound FKN efficiently promotes the firm adhesion of cells expressing its receptor, CX3C chemokine receptor 1 (CX3CR1), through mechanisms involving integrin-independent and integrin-dependent pathways.67,68 Additionally, soluble FKN triggers calcium mobilization, integrin activation, and cell migration in CX3CR1-expressing cells, akin to other soluble chemokines. FKN expression is not limited to ECs but is also found on dendritic cells, neurons, and activated astrocytes. Anti-FKN monoclonal antibodies are under study for different inflammatory diseases, such as CD, rheumatoid arthritis, and primary biliary cholangitis.
E6011 (quetmolimab)
A 12-week double-blind, placebo-controlled phase II trial evaluating the efficacy and safety of E6011 (quetmolimab), a humanized IgG2 monoclonal antibody against human FKN, in participants with active CD, is completed (NCT03733314). Results are awaited. Participants were randomized to receive either IV E6011 or a placebo. The study includes a screening period, induction period (double-blind), rescue period (open-label), extension period (open-label), post-observation period, and follow-up period. At the end of the induction period, participants showed a reduction in CDAI score of 70 points or more compared to baseline and proceeded to the OLE period. Those with less than a 70-point reduction enter the rescue period. After the rescue period, participants with a 70-point or more significant decrease in CDAI score move to the OLE period, while those with less are discontinued. Primary outcome measures include the percentage of participants with clinical response at week 12, defined as a decrease of ⩾100 points in CDAI score from baseline. Secondary outcomes include various measures related to CDAI scores, PRO, endoscopic response, and steroid-free remission.
Interleukins
IL-2 is a versatile cytokine crucial in averting chronic inflammation within the gastrointestinal tract. Its protective actions encompass regulatory T (Treg) cells’ generation, sustenance, and functionality (Tregs).69 –71 The utilization of a low dose of IL-2 has emerged as a prospective therapeutic approach for individuals grappling with IBD. 72
LD SC IL-2
A phase Ib/IIa clinical trial is currently recruiting (NCT04263831) to explore the safety and effectiveness of low-dose SC interleukin-2 (LD SC IL-2) in patients with CD. LD IL-2, known for its ability to increase Treg cells at low doses selectively, has shown safety in several clinical trials.73,74 This trial involves the daily SC administration of LD IL-2 over 8 weeks in patients with CD to determine the maximum effective dose, establish safety parameters, and evaluate initial signs of effectiveness. Additionally, the study aims to analyze the modulation of Treg cells in peripheral blood and lamina propria, correlating these changes with clinical outcomes. Deep immunophenotyping will be conducted to comprehensively assess the impact of LD IL-2 on various immune cells in both peripheral and mucosal compartments, establishing a connection between changes in immune phenotype and clinical response.
MiR-124 upregulators
MiR-124 plays a key role in modulating inflammation and innate immunity, potentially helping to restore physiological pathways disrupted by inflammatory diseases. 75 A novel compound, ABX464 (obefazimod), which selectively upregulates miR-124 in immune cells such as human peripheral blood mononuclear cells, purified CD4+ cells, and macrophages, is currently being tested. 76 In CD patients, miR-124 has been identified as a pro-inflammatory molecule that targets the aryl hydrocarbon receptor (AhR), which is downregulated in the intestines of IBD patients. An AhR ligand, TCDD (2,3,7,8-Tetrachlorodibezo-p-dioxin), activates miR-124, leading to improvement in dextran sodium sulfate (DSS)-induced colitis by regulating Th17 and Treg differentiation or inducing the secretion of the anti-inflammatory cytokine IL-22.77,78 An inverse relationship between miR-124 and AhR levels has been observed in intestinal epithelial cells and colon tissues of patients with active CD. However, studies have shown that anti-miR-124 treatment alleviates intestinal inflammation by inhibiting AhR in TNBS-induced colitis. 79
ABX464 (obefazimod)
ABX464 (obefazimod) is a first-in-class, once-daily, small molecule that is being explored as a new treatment option for patients with CD. 80
Obefazimod showed both effectiveness and safety in patients with UC during phase IIa and phase IIb trials, which were double-blind and placebo-controlled for induction, followed by open-label maintenance studies.80 –82
The ENHANCE-CD trial (NCT06456593) is a randomized, double-blind, placebo-controlled phase IIb clinical trial that aims to evaluate the efficacy and safety of obefazimod in patients with moderately to severely active CD who have not responded adequately to conventional or advanced therapies. The study includes a 12-week induction phase, a 40-week maintenance phase, and a 48-week extension phase, and aims to involve 212 participants. This study employs a randomized, parallel assignment design with quadruple masking to evaluate the efficacy and safety of obefazimod across different doses (50, 25, 12.5 mg) compared to placebo in participants with moderately to severely active CD, Primary outcomes include changes in CDAI and SES-CD at weeks 12 and 52, focusing on clinical remission, endoscopic response, and safety profiles. Secondary measures assess early responses at week 12 and extend safety evaluations up to week 100. Recruitment is expected to start in September 2024, with primary completion anticipated by December 2026 and study completion by April 2028.
Anti-adhesion molecules
One effective therapeutic strategy involves targeting the adhesion molecules involved in intestinal cell trafficking, exemplified by vedolizumab, a monoclonal antibody targeting gut-homing integrin α4β7. 83 Notably, the pathways regulating cell trafficking in the gut exhibit organ-specific characteristics. 84 α4β7 integrin is selectively upregulated on T-cells primed in gut-associated lymphoid tissue, while its ligand mucosal addressin cell adhesion molecule (MAdCAM)-1 is expressed explicitly on intestinal endothelial cells. This interaction facilitates the preferential migration of gut-primed T-cells to the intestinal tissues. Therefore, treatments for IBD that interfered with cell trafficking were developed, even though with mixed results. 85 Indeed, natalizumab, an α4β1-binding monoclonal antibody, is approved for treating CD by the US FDA. 86 On the other hand, in the phase III BERGAMOT trial for CD, etrolizumab, which targets the β7 integrin subunit of integrin heterodimers α4β7 and αEβ7, demonstrated effectiveness only during the maintenance phase, with no significant efficacy observed during induction. 87 The OLE phase III JUNIPER trial was terminated due to mixed results in the parent studies (NCT02403323).
Furthermore, two monoclonal antibodies IgG2 targeting MAdCAM-1 were tested in CD. However, preliminary results from a phase II trial of PF-00547659 in CD did not show superiority over the placebo, suggesting a limited impact on CD outcomes (NCT01276509). 88 Three studies were initiated or scheduled involving patients with moderate-to-severe CD receiving ontamalimab (induction studies NCT03559517 and NCT03566823; maintenance study NCT03627091). 89 However, due to the premature discontinuation of the program, the CD studies involved only a limited number of patients, and no definitive conclusions have been drawn regarding its effectiveness. 89 Takeda terminated the trials for reasons unrelated to the safety or efficacy of the drug.
MORF-057
MORF-057 is a small molecule specifically designed to target the α4β7 receptor. 90 The GARNET study is randomized, double-blind, placebo-controlled phase II trial (NCT06226883) and is currently recruiting participants to investigate the safety and effectiveness of MORF-057 in adults diagnosed with moderately to severely active CD. The study will assess the efficacy of two different dose regimens of MORF-057 during a 14-week induction period, followed by an open-label maintenance period of 38 weeks. Participants who complete this phase have the option to continue into a maintenance extension for an additional 52 weeks. Participants in the third group receive a placebo during the induction period and open-label MORF-057 during the maintenance period. The primary endpoint will be the evaluation of endoscopic response using SES-CD at week 14. The secondary outcomes will be the clinical response and remission at week 14 measured with the CDAI.
Anti-CCR9
CCR9/CCL25 interactions are known to enhance the migration of memory T-cells to the gut due to the high expression of CCL25 in the intestinal lining. 91 Consequently, CCR9 and CCL25 have become key targets for balancing pro-inflammatory and anti-inflammatory responses in the gut. The process of leukocyte extravasation from blood vessels and their migration to effector sites comprises several steps, which include the interaction of adhesion molecules like a4b7 integrins and the signaling of chemoattractant receptors such as CCR9 through chemokines. Vercirnon, an oral inhibitor of CCR9, underwent a phase III randomized, double-blind, placebo-controlled clinical trial that investigated its’ efficacy and safety and did not show statistically significant improvement in the primary endpoint, which was clinical response, or the key secondary endpoint, that was clinical remission, compared to placebo at week 12. No significant differences were observed in changes of CRP or fecal calprotectin levels between treatment groups. These results were in contrast with the results of two phase II placebo-controlled studies, including the PROTECT1 trial. 92
AZD7798
AZD7798, a humanized anti-CCR9 monoclonal antibody, has been developed to targets T-cells subset and deplete CCR9-expressing lymphocytes, both circulating and tissue-resident. 93 A randomized, double-blind, placebo-controlled trial phase I clinical trial (NCT05452304) is currently recruiting participants to evaluate the safety, tolerability, and PK of single ascending and repeat dose administrations of AZD7798 in healthy subjects and patients with CD. The first part of the trial involves healthy subjects in a first-in-human single ascending dose study with up to seven dose levels, followed by planned doses on days 1 and 15 after the completion of part Ia. Results of this part of the trial were presented at the recent American Gastroenterology Association congress. Six single ascending dose cohorts were evaluated with follow-up to day 85 including a total of 48 healthy subjects aged 19–48 years participated. AZD7798 was generally well-tolerated with no major safety concerns. Mild cytokine release syndrome (Common Terminology Criteria for Adverse Events [CTCAE] Grade 1) was observed. Rapid and sustained depletion of CCR9+ cells was observed across all doses. The second part will include CD patients, who will be divided into three strata receiving varying doses or a placebo. The third part will involve healthy Japanese and Chinese subjects randomized to receive either AZD7798 or a placebo. Key outcomes will include the number of participants experiencing adverse events and various PK measures.
AMALTHEA is a randomized, double-blind, placebo-controlled phase IIa trial is currently recruiting (NCT06450197). This clinical trial aims to assess the efficacy and safety of AZD7798, compared to a placebo, in treating CD following a parallel assignment design. Participants in the experimental arm receive AZD7798, while those in the placebo arm receive an inert placebo. The primary aim is to assess the remission of CDAI by week 12. Secondary outcomes include endoscopic responses and remission as measured by SES-CD, changes in CDAI scores, symptomatic remission based on SF and AP. Additionally, the study monitors PK via serum concentrations of AZD7798 and evaluates immunogenicity through the incidence and titer of anti-drug antibodies over a 36-week period.
The CALLISTO trial (NCT06681324), sponsored by AstraZeneca, is a Phase II study examining the safety, tolerability, and effects of AZD7798 on mucosal repair in patients with active ileal CD and an ileostomy. This randomized, placebo-controlled trial includes a 12-week blinded induction phase followed by a 40-week open-label maintenance period for eligible participants. Approximately 30 participants will be recruited globally, with the study’s estimated start in December 2024 and completion by early 2027. Primary measures focus on adverse events, vital signs, and lab results during the induction period.
Microbiome-directed therapies
Anti-mycobacterium paratuberculosis (MAP) therapy
There is an ongoing debate regarding the potential role of Mycobacterium avium subspecies paratuberculosis (MAP) in CD. 94 While MAP is known to cause Johne’s disease in ruminants, its involvement in CD remains uncertain. MAP is prevalent in dairy herds and can be transmitted to humans via contaminated products. Studies have detected MAP in CD patients at a higher rate than in non-CD patients.94 –96 The mechanisms by which MAP could contribute to CD are not fully understood but may involve immune response dysregulation and inflammatory pathways. Clinical trials investigating anti-MAP therapy have yielded conflicting results.97 –106 While some studies suggest potential benefits, others show no significant improvement in CD outcomes. A phase III RCT was conducted to investigate anti-MAP therapy in CD using a new oral formulation of combination antibiotic therapy named RHB-104. The MAP US study (NCT01951326), involving 331 patients with moderate-to-severe CD, was a double-blinded, placebo-controlled trial that assessed the safety and efficacy of adding RHB-104 to standard CD treatment. RHB-104 consists of higher doses of clarithromycin (95 mg), clofazimine (10 mg), and rifabutin (45 mg) compared to previous studies. At both week 16 and week 24, the primary endpoint of achieving clinical remission was completed by a more significant proportion of patients in the RHB-104 add-on group compared to placebo. Additionally, there was a significant decrease in the antibiotic arm’s biochemical markers of disease activity. Some patients who did not achieve clinical remission by week 26 were eligible to participate in the open-label MAP US2 study with RHB-104, which is completed (NCT03009396). The primary outcome measure of MAP US2 was clinical remission at week 16. Key secondary outcomes included MAP measurement from the blood by PCR at baseline and, during treatment, data unavailable in the initial MAP US study. An OLE study (MAP US2) examining open-label RHB-104 among participants who did not respond to treatment by week 26 of 38 patients who switched from placebo to RHB-104, 14 (36.8%) attained clinical remission based on CDAI. In contrast, among those who initially did not respond to RHB-104 and completed an additional 16 weeks of therapy, only 3 achieved clinical remission.
Selective inhibitors of IL-6
Targeting IL-6 has emerged as a promising strategy for reducing inflammation and associated carcinogenesis risk; therefore, various approaches have been investigated to inhibit IL-6 signaling for treating IBD. 107 Notably, targeting the IL-6 receptor with tocilizumab, a humanized IgG1 monoclonal antibody, has shown efficacy in some inflammatory diseases. 108 Tocilizumab was evaluated in a placebo-controlled phase II RCT involving patients with CD and a CDAI score ⩾150. The primary endpoint, which aimed for a reduction of the CDAI score of ⩾70 points, was achieved by 80% of patients receiving bi-weekly tocilizumab, compared to 31% of those on placebo, underscoring the significant efficacy of tocilizumab. 109 However, further development of tocilizumab for CD was halted due to rare reports of gastrointestinal perforations observed in concurrent arthritis clinical trials and an enhanced understanding of IL-6’s homeostatic role in the intestinal epithelium. 110
Olokizumab is a humanized monoclonal IgG2 antibody that inhibits the IL-6 pathway by binding to the third attachment site of IL-6, thereby preventing its interaction with gp130. 111 In a phase II clinical trial involving patients with CD, olokizumab was administered to 247 CD patients who had an inadequate response to anti-TNFα therapy. These patients were randomly assigned to one of three groups in a double-blinded manner: placebo, 10 mg, or 50 mg of olokizumab. 112 The study successfully achieved its predefined endpoints related to improved CD activity parameters. Moreover, the rates of drug response and remission were notably higher in the 50 mg treatment group compared to the other groups. However, it is important to note that some patients treated with the antibody experienced gastrointestinal abscesses and perforations, consistent with previous reports in rheumatoid arthritis patients treated with tocilizumab. 110
A recent approach explicitly targets the IL-6 trans-signaling axis to mitigate pathogenic chronic inflammation in IBD. A phase I study evaluated the safety, tolerability, PK, and P) of olamkicept, a decoy protein that targets IL-6 trans-signaling, in healthy subjects and patients with CD. 113 In the single ascending dose trial, olamkicept was administered IV or SC across various doses, showing no severe adverse events. Common side effects included headache and nasopharyngitis. PKs were dose-independent, with complete target engagement observed at serum concentrations of 1–5 μg/mL. The multiple ascending dose trial confirmed safety and tolerability with weekly IV doses, supporting further development of olamkicept for IBD. Olamkicept showed promising results in a phase IIa clinical trial for patients with active IBD. Olamkicept demonstrated clinical response and remission rates without significant adverse effects related to immunosuppression. 114 However, further studies only examined its efficacy and safety in patients with UC.115,116
Combination treatments
BI 706321 and ustekinumab
RIPK2 is a dual-specific kinase situated downstream of the nucleotide-binding oligomerization domain (NOD) 1 and NOD2 signaling pathways, which are crucial for regulating innate immunity. 117 Additionally, RIPK2 contributes to adaptive immunity by influencing CD4+ T-cell proliferation and T-helper cell development. 118 Dysregulation of NOD/RIPK2 signaling is associated with the onset of CD.119,120 Consequently, modulating the NODs/RIPK2 signaling pathway holds promise for developing innovative immunotherapies.
A phase IIa, randomized, double-blind, placebo-controlled trial (NCT04978493) was underway to assess the safety, efficacy, PK, and pharmacodynamic (PD) of orally administered BI 706321 over 12 weeks in patients with CD undergoing ustekinumab induction treatment. The trial has been recently terminated due to the company’s decision.
Guselkumab and golimumab
The DUET-CD study (NCT05242471) is a phase II randomized, double-blind trial currently active. It aims to investigate the efficacy and safety of combination therapy with guselkumab and golimumab compared to guselkumab monotherapy, golimumab monotherapy, and placebo in patients with moderate-to-severe active CD. The anticipated primary completion date is in 2025. The trial includes a placebo comparator and multiple experimental arms. Participants in the placebo SC arm may switch to an active treatment if there is no clinical response. Participants will either receive guselkumab SC, golimumab SC, or JNJ-78934804 (a combination of guselkumab and golimumab) in low-, mid-, or high-dose regimens. Participants also have escalation and extension opportunities. This clinical trial aims to evaluate the efficacy and safety of these treatments for CD, focusing on achieving clinical remission based on the CDAI and endoscopic response measured by a reduction in the SES-CD at week 48. Secondary outcomes will include the percentage of participants with PRO2 remission at week 48, endoscopic remission at week 48, clinical remission at week 24, endoscopic response at week 24, and adverse events. PK measures will also be collected, including serum concentrations of guselkumab and golimumab over time, the percentage of participants with antibodies to guselkumab and golimumab and their titers, as well as the percentage of participants with neutralizing antibodies to guselkumab and golimumab.
Vedolizumab and upadacitinib
The VICTRIVA trial (NCT06227910) exploring the efficacy and safety of combining vedolizumab and upadacitinib aims to enroll 396 patients, who will be assigned to treatment with either vedolizumab and placebo or vedolizumab and upadacitinib. The study is a randomized, triple-blind, phase II clinical trial designed to evaluate the treatment of moderate-to-severe CD. The trial will be divided into an induction phase and a maintenance phase, with a follow-up to 70 weeks. The trial will soon start recruiting. Participants will be randomly assigned to one of two groups during the induction period. The first group will receive vedolizumab 300 mg IV infusion at weeks 0, 2, 6, and 10, along with upadacitinib 45 mg orally, once daily for 12 weeks. The second group will receive vedolizumab 300 mg IV infusion at the same intervals, but instead of upadacitinib, they will receive placebo capsules orally, once daily for 12 weeks.
In the maintenance period, participants who achieve a CDAI reduction of ⩾70 points from baseline at week 12 will continue to receive vedolizumab 300 mg IV infusion Q8W for 40 weeks. This dose may be escalated based on protocol-specified criteria.
Primary outcome measures will include clinical remission based on CDAI at week 12 and endoscopic response based on SES-CD at week 12. Secondary outcomes will include clinical remission based on PRO2 at week 12, endoscopic remission based on SES-CD at week 12, corticosteroid-free clinical remission at week 12, clinical response based on CDAI at week 12, clinical remission based on CDAI at week 52, endoscopic response based on SES-CD at week 52, clinical remission based on PRO2 at week 52, endoscopic remission based on SES-CD at week 52, corticosteroid-free clinical remission at week 52, and clinical response based on CDAI at week 52.
This study seeks to determine the effectiveness of the combination of vedolizumab and upadacitinib compared to vedolizumab alone and a placebo in achieving clinical remission and endoscopic response in CD patients.
Vorinostat and ustekinumab
Previous research has demonstrated that epigenetic changes are linked to and may contribute to the development of various diseases. 121 One type of epigenetic change involves the addition and removal of acetyl groups on histone proteins, which are mediated by enzymes called histone acetyltransferases and histone deacetylases (HDACs).122,123 Of particular interest is evidence suggesting that HDAC inhibitor (HDACi), such as butyrate, can potentially mitigate gastrointestinal inflammation. 124 Studies have shown that treatment with butyrate reduces the production of inflammatory molecules like nitric oxide, IL-6, and IL-12 in dendritic cells and macrophages. 124 Moreover, butyrate promotes the development of Treg cells in the intestine, which modulate gastrointestinal inflammation and maintain mucosal balance. 124 Similarly, another HDACi, vorinostat, has shown promise in alleviating graft-versus-host disease (GVHD) affecting the gastrointestinal tract in patients receiving bone marrow transplants. 125 This anti-inflammatory effect was attributed to the increased activity of Tregs, suggesting that vorinostat, like butyrate, reduces inflammation by enhancing the function of cells capable of dampening immune responses. 126
An open-label phase I/II trial that aims to investigate the safety and effectiveness of vorinostat in patients with moderate-to-severe CD is now recruiting (NCT03167437). The study also evaluates the potential for maintenance therapy with ustekinumab after successful treatment with vorinostat. During the treatment phase with vorinostat, participants will take the drug orally twice daily for 12 weeks, with regular monitoring of symptoms and blood tests. Those who respond well to vorinostat may continue with an extension phase for 6 months. Following the vorinostat treatment, participants may receive maintenance therapy with ustekinumab for 2 years. The trial's primary objective is to assess the safety and tolerability of vorinostat in CD patients. At the same time, secondary outcomes include evaluating the efficacy of both vorinostat and ustekinumab in achieving clinical improvements and mucosal healing.
Local cell administration
A recent global consensus by the Stenosis Therapy Anti-Fibrotic Research (STAR) Consortium has addressed the definitions, diagnosis, and management of fibrostenosing small bowel CD in clinical practice. 127 The authors did not recommend the intralesional injection of corticosteroids or anti-TNF agents in cases of successful endoscopic dilation therapy. 127 Similarly, a previous consensus by the Global Interventional Inflammatory Bowel Disease Group on the endoscopic treatment of strictures associated with CD also did not endorse these interventions. 128 The evidence supporting the intralesional injection of corticosteroids is unclear, with conflicting results from two RCTs and generally low-quality remaining evidence.129 –138 While no RCTs have been published on the intralesional injection of anti-TNF agents, several case series have reported its efficacy.139 –142
Mesenchymal stem cells
A phase I/II clinical trial assesses the safety and efficacy of laparoscopically administered adipose-derived allogenic mesenchymal stem cells (adAMSC) in treating CD patients with a single inflammatory stenosis (NCT05521672). Conducted across multiple centers in Spain, the study aims to evaluate if this intervention can induce an anti-inflammatory effect, potentially reducing the need for surgical resections. It includes screening, treatment, and follow-up periods over 36 months. Participants undergo a single dose of 120 million cells directly into perilesional adipose tissue during a laparoscopic intervention, followed by rigorous follow-up assessments over a 2-year period. The primary outcomes include assessing the percentage of complications related to surgery and treatment administration, changes in stenosis dimensions measured by magnetic resonance imaging (MRI) over 52 weeks, and alterations in CDAI and IBDQ32 scores throughout the study period. Secondary endpoints focus on identifying patients requiring surgical resection due to obstructive episodes.
A phase I/II clinical trial (NCT03901235), sponsored by the University of Liege and conducted at CHU de Liege, investigates the efficacy of mesenchymal stem cell (MSC) injections in healing refractory intestinal and perianal lesions in CD. The study targets adult patients with long-standing disease and persistent lesions unresponsive to standard and biologic therapies. Using a non-randomized, open-label design, it aims to assess the healing of deep ulcers, strictures, and perianal fistulas as primary outcomes. Safety evaluations include monitoring adverse events associated with the MSC treatment. Secondary outcomes measure disease activity, quality of life, and cumulative intestinal damage using established clinical indices.
A phase I/II prospective, single-arm, open-label study (NCT04164849) aims to evaluate the effectiveness and safety of human umbilical cord MSCs in treating refractory moderate-to-severe CD. Participants receive either MSC injection into the diseased intestinal mucosa or combined IV MSC injection followed by mucosal injection. The outcomes will include the evaluation of clinical and endoscopic response or remission at weeks 4, 8, 12, and 24 post-treatment using CDAI and SES-CD.
Platelet-rich plasma injections
A single-center, randomized, non-controlled open-label trial aims to assess the effectiveness and safety of combining endoscopic balloon dilatation with autologous platelet-rich plasma (PRP) injection in treating colonic stenosis <5 cm in length in CD patients (NCT06165289). Patients will undergo either high-concentration or low-concentration PRP injections following endoscopic balloon dilatation. Outcomes will be evaluated including the evaluation of restenosis rate 3 months after treatment, defined as the inability to pass a 10.5 mm endoscope through the stricture, changes in intestinal ultrasound measurements and proportion of patients requiring surgery due to stenosis or perforation one year after treatment.
Drugs in phases II and III of clinical trials are depicted in Figure 3.
Figure 3.
Drugs in phase II and III clinical trials for the treatment of luminal Crohn’s disease.
ALK5, activin receptor-like kinase 5, also known as the transforming growth factor (TGF-β) type 1 receptor; CCR9, C-C motif chemokine receptor 9; FKN, fractalkine; JAK, Janus kinase; miR-124, microRNA-124; PPARγ, peroxisome proliferator-activated receptor gamma; MOA, mechanism of action; S1PR, sphingosine-1-phosphate receptor; TL1A, TNF-like ligand 1A; TNF, tumor necrosis factor.
Source: Created with Biorender.com (accessed 12 November 2024).
Phase I trials
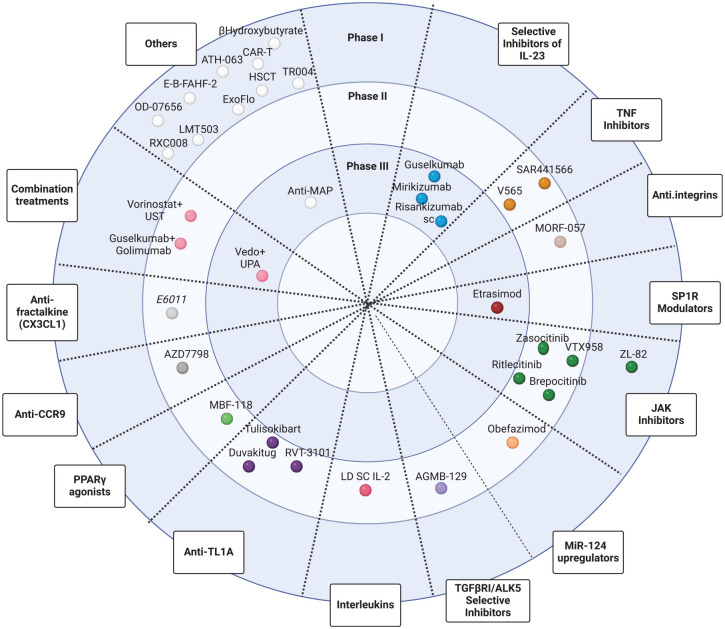
An overview of the drugs under study for luminal CD is represented in the disc in Figures 3 and 4.
Figure 4.
Drugs and cellular therapies in phase I, II, and III clinical trials for the treatment of luminal Crohn’s disease.
ALK5, activin receptor-like kinase 5, also known as the transforming growth factor (TGF-β) type 1 receptor; CAR-T, chimeric antigen receptor T-cell therapy; CCR9, C-C motif chemokine receptor 9; FKN, fractalkine; HSCT, hematopoietic stem cell transplantation; JAK, Janus kinase; miR-124, microRNA-124; MOA, mechanism of action; PPARγ, peroxisome proliferator-activated receptor gamma; S1PR, sphingosine-1-phosphate receptor; TL1A, TNF-like ligand 1A; TNF, tumor necrosis factor; UPA, upadacitinib; UST, ustekinumab; Vedo, vedolizumab.
Source: Created with Biorender.com (accessed 12 November 2024).
Beta-hydroxybutyrate
Beta-hydroxybutyrate (BHB), a ketone body supplement, is under study for patients with CD (NCT06351124). The primary aim of this trial is to determine whether BHB supplementation is not only feasible and acceptable to patients but also effective in reducing systemic inflammation through a prospective, open-label, randomized, two-arm pilot design phase I/II trial. Participants will be adults diagnosed with CD, who are about to begin a new therapy such a small molecule or a biologic drug. The trial will randomly assign participants to one of two groups: a control group receiving standard care and an intervention group receiving standard care plus BHB supplementation which will consist in three capsules of BHB, three times a day, for 4 weeks. Participants will be carefully monitored throughout the study. Data collection will include a 24-h food recall questionnaire to document food consumption, alongside blood and fecal samples taken at the start and end of the 4-week period to evaluate microbiome changes. These samples will help researchers measure changes in microbial diversity and BHB serum levels. Clinical response will be measured by a significant reduction in fecal calprotectin and CRP levels.
ZL-82
New small molecules targeting the JAK pathway inhibit critical cytokines in rheumatic diseases and IBD. JAK kinases (JAK1, JAK2, JAK3, TYK2) transmit cytokine signals, crucial for inflammation via T-cell proliferation and B-cell activation. 143 Ongoing phase II trials for VTX958 and zasocitinib, a TYK2 inhibitor, and recently completed trials for brepocitinib (JAK1/TYK2 inhibitor) and ritlecitinib (JAK3) inhibitor have been previously described in this article.
A phase I trial will explore the tolerability, safety, and PK characteristics of ZL-82, an oral selective JAK3 inhibitor, in healthy adult subjects (NCT06055023). The study will also investigate the effects of food on the PK of ZL-82 and assess the PK profile after multiple doses. ZL-82 will be administered in escalating doses to different groups of participants. Each dosage group will include eight participants, and the doses range from 12.5 to 600 mg.
E-B-FAHF-2
The Icahn School of Medicine at Mount Sinai is sponsoring a clinical trial to evaluate the safety, tolerability, and efficacy of E-B-FAHF-2, a butanol purified form of the Food Allergy Herbal Formula-2 (FAHF-2) in treating mild-to-moderate CD (NCT03992469). FAHF-2 is composed of nine Chinese herbal medications that are originally part of the traditional Chinese herbal formula, Wu Mei Wan. 144 The trial aims to fill the therapeutic void for patients with mild-to-moderate CD by potentially offering a new maintenance therapy, after induction of remission with budesonide (Entocort® EC Capsules).
The study is interventional and comprises two main phases. The first is a safety and tolerability assessment with an 8-week, double-blind, RCT to evaluate the safety and tolerability of E-B-FAHF-2 compared to a placebo. Patient in the experimental arm will take low-dose of E-B-FAHF-2 (29 mg/kg/day) for 2 weeks, followed by a full dose (71 mg/kg/day) for 6 weeks. The second part is an exploratory efficacy extension phase with a 6-month, open-label trial to assess the long-term safety and preliminary efficacy of E-B-FAHF-2 in maintaining remission. Adverse events will be assessed as well as therapy escalation, fecal calprotectin levels, PROs (PROMIS Profile-29), IBD Self-Efficacy Scale (IBD-SES), and immunologic changes in Peripheral Blood Mononuclear Cells (PBMC) cytokine levels.
OD-07656
A phase I study to evaluate the safety, tolerability, PK, and PD of OD-07656 in healthy adult participants is now recruiting (NCT06206811). OD-07656 is a small molecule that targets the IL-1 receptor-associated kinase 4 (IRAK4). IRAK-4 is a key component of the toll-like receptor (TLR) signaling pathway, crucial for innate immunity and mediates the TLRs/IL-1β pathway. 145 Studies have shown that IRAK4 is a crucial kinases downstream of the TLR pathway, playing a positive regulatory role in signaling. 145 When stimulated by pathogen-associated molecular patterns, TLRs activate IRAK4 by recruiting MyD88 to bind to the Toll/IL-1 receptor (TIR) domain. Activated IRAK4 then activates IRAK1 and undergoes autophosphorylation, leading to NF-κB activation, which increases the expression of inflammatory factors and promotes inflammatory responses. 145
OD-07656 is being investigated as a potential treatment for IBD, including CD, as well as for Blau syndrome and spondylarthritis. It is a first-in-human study involving single and multiple ascending oral doses of OD-07656 in healthy adults. The study involves multiple arms to evaluate different aspects of OD-07656. In the first arm, single ascending doses will be used to assess safety and tolerability. In the second arm, multiple ascending doses will be administered to further evaluate safety. A third arm will investigate how food affects OD-07656’s bioavailability across different dose forms. In the last arm, researchers will examine potential PK interactions between OD-07656 and midazolam, assessing safety when these drugs were taken together. By administering midazolam alongside OD-07656, researchers can determine whether OD-07656 alters CYP3A4 activity, either by inhibiting or inducing the enzyme, which could affect the metabolism of other drugs.
ATH-063
A recently completed trial evaluated ATH-063 in healthy subjects to assess its safety, tolerability, PK, and PD (NCT05807971). ATH-063 is a small molecule that is believed to act as a genomic regulator of a gene target identified using artificial intelligence and machine learning applied to clinical and molecular data from institutions such as the Cleveland Clinic and The Crohn’s & Colitis Foundation. The trial was a phase I, randomized, double-blind, placebo-controlled study, including single ascending dose and multiple ascending dose cohorts, as well as a food-effect arm. Participants received ATH-063 orally under fasting conditions, with doses adjusted based on safety and PK data. The study aimed to gather data on adverse events, drug effects on the body, and PK parameters like absorption and elimination rates. Results are pending.
LMT503
LMT503 is a small molecule designed to treat IBD by modulating macrophage polarization. 146 Macrophages play a crucial role in immune response and tissue homeostasis by switching between pro-inflammatory (M1) and anti-inflammatory (M2) states. LMT503 reprograms macrophage metabolism, shifting them from an M1-like inflammatory state to an M2-like anti-inflammatory state. In vitro studies showed that LMT503 suppresses M1 markers (e.g., iNOS) and enhances M2 markers (e.g., Arg-1).
A phase I, double-blind, randomized, placebo-controlled study aims to evaluate the safety, tolerability, and PK of LMT503 in healthy subjects and will soon start recruiting (NCT05659953). The study includes single ascending dose and multiple ascending dose parts, along with an integrated food-effect arm. Participants will be randomly assigned to receive either LMT503 or a placebo.
Stem cell transplantation
Hematopoietic stem cell transplantation (HSCT) is used to treat various blood-related diseases by harvesting stem cells from peripheral blood, bone marrow, or umbilical cord units. 147 There are two types: autologous and allogeneic. Autologous HSCT has recently gained attention for treating refractory autoimmune diseases like CD by regenerating the immune system and establishing tolerance. The ASTIC trial (2007–2011) assessed HSCT in CD patients who had failed multiple treatments. 148 Although adverse events were common, including one fatality, 38% of patients achieved remission after 1 year in a follow-up analysis.
The ASTIClite trial, aiming to reduce toxicity, was halted due to SAEs. 149 The trial compared the safety and efficacy of autologous HSCT with a lower dose of cyclophosphamide during stem cell mobilization and conditioning against the standard of care.
Currently, four ongoing trials are exploring autologous HSCT in CD patients, with one examining vedolizumab as maintenance therapy. The critical issue with HSCT is its safety, as the procedure is linked to febrile neutropenia, bacteremia, septic shock, and acute GVHD.148,150,151 Given the significant risk of mortality and morbidity associated with HSCT in CD patients, it is essential to carefully weigh the risks and benefits before proceeding with this high-risk treatment. More research is needed to establish standard protocols.
Currently, five ongoing trials are enrolling patients for autologous HSCT in CD (NCT02580617, NCT04224558, NCT00692939, NCT03000296, NCT03219359). The fifth trial specifically examines the use of vedolizumab as maintenance therapy following autologous HSCT.
The first trial (NCT02580617) is a phase I open-label study and focuses on assessing the safety and efficacy of allogenic adipose-derived MSCs with three groups with different dosages. The primary objective of this trial is to evaluate the safety profile of HSCT in this patient population, with a secondary focus on clinical outcomes measured by the CDAI.
The second trial is a phase I/II open-label trial targets patients with refractory CD (NCT04224558). The trial protocol includes a regimen of high-dose immunoablation using drugs such as mesna, cyclophosphamide, and fludarabine, followed by autologous HSCT. The primary outcomes in this trial encompass various clinical parameters crucial for assessing disease severity and response to treatment, including mucosal healing measured by the SES-CD score, inflammatory markers like erythrocyte sedimentation rate, fecal calprotectin concentration, and CRP levels. Additionally, the trial evaluates safety outcomes such as TEAEs related to HSCT procedures.
The third trial is a phase I/II trial (NCT00692939) and investigates a comprehensive regimen involving multiple immunosuppressive agents preceding autologous stem cell transplantation. The drugs utilized include alemtuzumab, Anti-Thymocite Globulin (ATG), melphalan, thiotepa, rituximab, and cyclophosphamide. Primary outcomes here focus on tracking treatment-related toxicities, incidence of life-threatening infections, and changes in disease activity indices such as HBI, CDAI, and Pediatric Crohn’s Disease Activity Index. Secondary outcomes aim to monitor recovery milestones post-transplant, including absolute neutrophil count and platelet count, as well as long-term complications affecting cardiac, endocrine, and immune systems.
The fourth trial (NCT03000296) evaluates the safety and clinical benefits of autologous HSCT using a specific conditioning regimen. The regimen involves high-dose cyclophosphamide, ATG, followed by HSCT. The primary outcomes include safety assessments through treatment-related adverse events over 12 months. Secondary outcomes focus on disease activity indices, quality-of-life measures, and endoscopic evaluations up to 24 months post-transplantation.
The fifth trial (NCT03219359) explores the combination of autologous stem cell transplantation with vedolizumab. The treatment protocol involves stem cell mobilization followed by high-dose chemotherapy using agents like cyclophosphamide and ATG, leading to stem cell transplant. Post-transplant, participants receive vedolizumab immediately upon discharge to evaluate its potential role in maintaining remission. Primary outcomes in this trial include assessing clinical remission rates at one-year post-transplant using the CDAI. Secondary outcomes encompass endoscopic activity indices, changes in quality-of-life measures, and overall disease burden.
The outcomes of these trials will provide critical insights into the potential benefits and risks associated with HSCT patients with CD.
CAR-T
Chimeric antigen receptor (CAR)-T cell therapy has established itself as a cornerstone in treating various hematologic malignancies. 152 While its primary application has been in oncology, CAR-T cells have also shown potential in targeting and eliminating autoreactive cells implicated in autoimmune and immune-mediated diseases. 152 However, CAR-T cell therapy has been linked to different side effects including cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. 153 A form of IBD-like colitis has also been reported following CAR-T cell therapy. 153
Research has explored extending CAR engineering, originally designed for directing effector T-cells against tumors, toward targeting Treg cells and immune components. Despite promising results in murine colitis models using targets like CEA or IL23R, identifying suitable antigens for directing CAR-T cells to the intestines of patients with IBD remains an ongoing challenge. 154
An early phase I open-label clinical trial (NCT05239702) is currently underway to investigate the safety and efficacy of CD7 CAR-T cells in treating refractory autoimmune diseases, including CD as well as others. The primary objectives include evaluating dose-limiting toxicity (DLT) within 28 days post-infusion and monitoring TEAEs over a 2-year follow-up period. Secondary outcomes aim to assess overall response rates, best overall response within ⩽3 months, CAR-T cell concentrations in blood and bone marrow, duration of remission, and overall survival up to 24 months post-infusion.
ExoFlo™
ExoFlo™ 48 is an extracellular vesicle (EV) product made from a single-donor bone marrow MSC (BM-MSC) culture under Current Good Manifacturing Practice (CGMP) regulations. It delivers the immunomodulatory and regenerative benefits of BM-MSCs without the limitations associated with cellular therapy.155,156 A phase I clinical trial (NCT05130983) to evaluate the safety and feasibility of ExoFlo™, an allogeneic stem cell-derived product, for patients with medically refractory CD. Participants will receive IV doses of ExoFlo™: 15 mL on days 0, 2 and 4, followed by 30 mL at weeks 2 and 6, and then every 4 weeks until week 46, totaling 15 doses.
Primary objectives focus on safety and feasibility, while secondary objectives include efficacy in inducing clinical remission, response, and improvements in quality of life. The study began in January 2023 and is expected to complete by September 2024.
Treg immunotherapy
The therapeutic promise of a distinct subset of immunosuppressive Treg cells, characterized by FOXP3 expression, has been validated across diverse preclinical models including IBD.157,158 These findings have spurred advancements in clinical applications of adoptive cell therapy involving Treg cells.
The TRIBUTE study (NCT03185000) is a phase I trial that aims to TR004, derived from autologous Treg cells expanded in vitro, for CD. TR004 will be administered intravenously as a single dose. The study will assess safety, feasibility, and exploratory efficacy endpoints over a 24-month period, including disease activity scores, biomarker analyses, and immunological responses.
The initial findings of the TRIBUTE feasibility study were presented at the recent European Crohn’s and Colitis Organization (ECCO) congress. 159 Out of five screened patients, four successfully underwent Treg expansion. A patient was withdrawn prior to dosing due to pregnancy after contraceptive failure. No dose-limiting toxicities were observed. One patient, who had long-standing colonic CD and PSC, was found to have dysplasia during the week 8 colonoscopy and underwent a colectomy by week 20. Another patient began oral prednisolone treatment at week 6 due to active disease. Among the three patients who received the treatment, the median CDAI lowered, although changes should be cautiously interpreted due to the small sample size.
Targeted therapies for fibrostenosing Crohn’s disease
Fibrostenosing CD presents a unique challenge in the management of IBD due to its complex nature and varied clinical manifestations. 127 Diagnosis of fibrostenosing CD requires precise imaging modalities, including CT, MRI, and intestinal ultrasonography and even when a diagnosis is made, there is a lack of available therapies with proven anti-fibrotic effects, necessitating a multidisciplinary approach to treatment.
The clinical trial for spesolimab, IL-36 receptor antagonist, in treating fibrostenotic CD was a phase II study discontinued by the sponsor Boehringer Ingelheim in mid-2023 due to limited efficacy (NCT05013385). The drug, originally used for generalized pustular psoriasis, was being tested in patients with CD who had already failed two different biological treatments.
The subsequent classes of drugs are being tested, with the hope of having a role in this context.
PPARγ agonists
PPAR, a member of the nuclear hormone receptor superfamily, consists of three isoforms: PPARα, PPARγ, and PPARβ/δ (also known as PPARβ). 160 Recent studies have highlighted the extensive regulatory effects of PPAR, making it a key focus of attention. 161 PPAR plays a crucial role in various physiological processes, including the transport and catabolism of fatty acids, glucose metabolism, lipogenesis, cholesterol transport and biosynthesis, and anti-inflammatory responses. 162 PPARα is primarily expressed in the liver, heart, muscle, brown adipose tissue, intestine, and kidney, contributing to energy dissipation and modulating fatty acid functionality by influencing transport, esterification, and oxidation processes. For instance, it enhances the expression of fatty acid transporter proteins, facilitating fatty acid activation and breakdown. 163 In contrast, ubiquitously expressed PPARβ/δ mainly participates in fatty acid oxidation and regulates blood glucose levels. Meanwhile, PPARγ, most highly expressed in white adipose tissue and present in the liver, skeletal muscle, intestines, and immune cells, affects energy storage through adipogenesis and lipid synthesis. 163 Notably, the acetylation of PPARγ leads to decreased expression, inhibits the adaptability of adipocytes, and disrupts the metabolic equilibrium within adipose tissue. 164 Previous studies have shown that PPAR-γ agonists, such as the thiazolidinedione (TZD) class of anti-diabetic drugs, have anti-fibrogenic effects in several body tissues, including the lungs, skin, kidneys, eyes, and heart.165 –174 The PPAR-γ agonists inhibit fibrogenesis by regulating the Smad-dependent or Smad-independent TGF-β signaling pathways. It has also been showed to be influenced by the 5-Aminosalycilates (5-ASA) compounds. 175 The rationale for IBD would be mainly for regulating intestinal fibrosis, as well as others recently described. 176
MBF-118
MBF-118 is a small molecule designed as a partial agonist of PPARγ. A phase IIa (NCT05940558) study with the aim of assessing the safety, tolerability, and exploratory efficacy of a daily oral regimen of 100 or 200 mg MBF-118 over 28 days in patients with CD exhibiting stenosis was recently completed. The study is structured as a single-center, open-label group comprising two cohorts: one receiving a daily dose of 100 mg MBF-118 for 28 days and the other receiving a daily dose of 200 mg MBF-118 for the same duration, with approximately five subjects per cohort and no placebo control. The trial does not involve randomization, and the initial five patients entering the study will be administered 100 mg, followed by the subsequent five patients who will receive 200 mg. Each participant's involvement spans approximately 14–16 weeks, encompassing a 2- to 4-week screening period, a 4-week treatment period, and an 8-week follow-up period. The objective is to gather robust safety, tolerability, and PK data to inform dosage and therapeutic regimen decisions in subsequent clinical investigations. Results are awaited.
TGFβRI/ALK5 selective inhibitors
Transforming growth factor β (TGF-β) is crucial in the development of intestinal fibrosis, although the exact mechanisms are not fully understood. 177 TGF-β signals through receptors such as activin receptor-like kinase 5 (ALK5), also known as the TGF-β type 1 receptor, found in various cell types. 178 ALK5 phosphorylates Smad2 and Smad3 proteins to transmit signals to the nucleus.
Specifically, the TGF-β1 isoform promotes collagen synthesis and fibroblast contraction in the mucosa of patients with fibrostenosing CD, utilizing the Smad2-Smad3 pathway and affecting tissue inhibitor of metalloproteinases regulation. TGF-β is pivotal in fibrosis regulation, with initial clinical data supporting its targeting for fibrotic conditions. 179 Currently, no approved therapies specifically target fibrostenosing CD, 127 but a new compound with this mechanism of action is under investigation for this purpose.
AGMB-129
AGMB-129 is an oral small molecule GI-restricted inhibitor of ALK5 (TGFβR1) designed for treating fibrostenosing CD. The STENOVA clinical trial (NCT05843578) is actively recruiting participants. This is a phase IIa study designed to be randomized, placebo-controlled, double-blind, that aims to assess the safety, tolerability, the PK, and PD of the investigational drug AGMB-129 in patients with fibrostenotic CD. In the screening period, patients will undergo ileocolonoscopy and magnetic resonance enterography to confirm the presence of intestinal strictures, along with evaluations of obstructive symptoms such as AP, cramping, and vomiting. Eligible participants will be randomly assigned in equal proportions (1:1:1) to receive either a high dose of AGMB-129, a low dose of AGMB-129, or a placebo.
ROCK inhibitors
ROCK (Rho-associated coiled-coil kinases 1 and 2) serves as a central component in the fibrotic signaling pathway. Inhibition of ROCK1 and ROCK2 has shown strong anti-fibrotic effects in preclinical studies.180 –182 However, systemic inhibition of ROCK1 and ROCK2 is associated with hypotension. 183
RXC008
RXC008 is a gastrointestinal-targeted ROCK inhibitor, designed to selectively inhibit ROCK 1 and 2 enzymes. By acting locally in the GI tract, RXC008 aims to address fibrosis associated with fibrostenotic CD without the cardiovascular side effects seen with pan-ROCK inhibitors. 183 The drug RXC008 exhibits a unique profile in its ability to restrict systemic exposure, primarily due to several interrelated mechanisms, the most important being its’ quick metabolization by paraoxonase enzymes in the plasma, ensuring minimal systemic exposure. A phase 1 clinical trial testing RXC008 is currently undergoing and consists of two parts. The first part involves healthy volunteers, focusing on single ascending doses and multiple ascending doses over 14 days, primarily assessing safety and PK. The second part will include patients with fibrostenotic CD, administering the drug over a month with placebo controls. Initial results from the healthy volunteer cohorts are expected by the end of 2024.
Pipeline of clinical trials for perianal CD
Most ongoing perianal Crohn’s disease trials focus on administering stem cells locally, sourced from various origins. 184 The rationale behind these therapies resides in the unique properties of mesenchymal cells, including their potential to differentiate into various mesodermal cell lineages and their ability to modulate immune cell activation, proliferation, differentiation, and maturation, making them promising candidates for therapeutic use. 185 These cells can be sourced from adipose tissue or bone marrow or be allogeneic or autologous. Studies have shown promising results with local injection of stem cells directly into fistulas, resulting in higher healing rates compared to standard therapies. However, challenges remain in terms of optimizing dosages, delivery methods, and ensuring long-term efficacy. One of the complexities in investigating medical management for perianal CD is the difficulty in controlling for surgical interventions, as surgery is often necessary as part of the treatment of perianal disease and is frequently used alongside medical treatments. Other challenges reside in heterogeneity in surgical approaches. As a result, trials investigating treatments for perianal CD need to carefully account for the surgical component and consider designing studies with clear protocols for the timing and type of surgery.
Stem cells include autologous stromal vascular fraction (SVF) cells, mesenchymal cells from human adipose tissue like darvadstrocel or those obtained from the Lipogems® International system, allogeneic MSCs derived from bone marrow, exosomes derived from MSCs of human placenta origin, and allogeneic MSCs sourced from human umbilical cord tissue (umbilical cord mesenchymal stem cells, UC-MSCs). Additionally, the results of a phase II trial evaluating Filgotinib’s use in perianal CD have been recently presented at the ECCO congress. Furthermore, a phase III trial assessing the efficacy of guselkumab is now recruiting.
Local treatments
Adipose-derived stem cells
SVF cells are autologous adipose-derived stromal vascular fraction (ADSVF) and comprise various cell types, including adipose-derived stem cells, mesenchymal and endothelial progenitor cells, leukocyte subtypes, lymphatic cells, pericytes, and vascular smooth muscle cells. 186 A phase II trial evaluating ADSVF with microfat compared to placebo for treating refractory fistulas is recruiting (NCT04010526). It aims to enroll 84 participants. The experimental arm receives ADSVF locally, while the control gets a placebo.
Through a phase II design incorporating randomized allocation and masked outcomes assessment, the STOMP-II trial aims to evaluate the safety and efficacy of AVB-114 alongside standard care (seton placement) in patients with CD (NCT04847739). AVB-114 utilizes autologous adipose-derived MSCs on a specialized bioabsorbable matrix to enhance tissue healing within fistula tracts. The trial’s dual-phase structure allows for optional crossover, and key outcomes include assessing the proportion of subjects achieving combined remission of treated perianal fistula over 36 weeks, as well as clinical remission rates, durability and time to remission, fistula relapse rates, radiologic response, disease activity indices (PDAI, sCDAI), quality-of-life assessments (EQ-5D-5L, IBDQ), impact on daily functionality, healthcare resource utilization, and incidence of adverse events.
The Lipogems International system allows for the harvesting, processing, and autologous transplantation of human adipose tissue mesenchymal cells. This sterile and disposable device employs mechanical microfragmentation of adipose tissue to create a fluid product enriched with mesenchymal cells and pericytes. A recent study assessed the efficacy and safety of local injections of microfragmented adipose tissue in patients with complex perianal CD fistulas. 187 Out of 15 patients treated, 10 achieved combined remission after a single injection, with confirmed improvements via MRI. No significant complications were reported, suggesting that this approach could be a promising therapy for refractory cases. The ATTIC study aims to investigate this treatment’s effectiveness further in a larger cohort of CD patients with complex perianal fistulas unresponsive to biologic therapies (NCT05594862). The intervention group will receive surgical drainage with cone fistulectomy followed by microfractured autologous adipose tissue infiltration. In contrast, the control group will undergo the same procedure with infiltration of physiological solution. This RCT will evaluate its impact on quality of life and the need for ostomy; it is now active.
Bone marrow-derived MSCs
A phase IB/IIA placebo-controlled interventional trial aims to assess the safety and efficacy of adult allogeneic bone marrow-derived MSCs to treat medically refractory perianal fistulizing CD (NCT04519671). The study aims to enroll 20 participants with CD and medically and surgically refractory perianal fistulizing disease. The primary outcome measure is the number of treatment-related adverse events at month 6. In contrast, secondary outcome measures include complete and partial clinical healing, lack of response, and worsening of disease at months 6 and 12.
Human placenta MSCs-derived exosomes
A phase I/II trial that aims to evaluate the safety and efficacy of human placenta MSCs-derived exosomes for treating refractory anal fistula in patients with CD is registered (NCT05499156). 188 The research also investigates changes in fistula appearance on MRI scans and assesses the patient’s quality of life before and after treatment. The intervention involves injecting MSC-derived exosomes into the fistula tract for three consecutive weeks. The design consists of two groups, with 40 participants randomly assigned to either the treatment or control group. The patients’ fistulas are evaluated clinically and through MRI scans before and 12 weeks after treatment. The primary outcome includes safety, clinical efficacy, and change in inflammatory parameters.
Human UC-MSCs
A phase I trial (NCT05039411) is now recruiting to evaluate the safety of locally injected allogeneic UC-MSCs in five patients. Each participant will receive 60 million UC-MSCs per visit (PF2020-CELL) for five consecutive visits, spaced 4 weeks apart. The primary outcome measure is the absence of TEAEs, with secondary measures focusing on clinical healing. This non-randomized study aims to determine the safety and efficacy of UC-MSC injections.
A recent pilot study evaluated the efficacy and safety of allogeneic UC-MSCs (TH-SC01) for treating complex perianal fistulas in patients with CD. 189 Ten adult patients with complex treatment-refractory CD perianal fistulas received a single intralesional injection of 120 million TH-SC01 cells. Combined remission at 24 weeks after cell administration was the primary endpoint. Results showed that 60.0% of patients achieved combined remission at 24 weeks, defined as an absence of suppuration through an external orifice, complete re-epithelization, and absence of collections larger than 2 cm measured by MRI. No SAE occurred, and the probability of remaining recurrence-free was 70% at week 52. The study concluded that local injection of TH-SC01 cells might be an effective and safe treatment for complex treatment-refractory perianal fistulizing Crohn’s disease (pfCD) after conventional and/or biological therapies fail. A phase I interventional trial (NCT05626023) is underway to evaluate the safety and tolerability of human TH-SC01 cell injection for perianal fistulas in CD. Nine participants will receive a single injection of TH-SC01 cells, with different doses based on weight. Primary outcomes include assessing the severity and incidence of adverse events within 28 days post-administration, exploring DLT, and determining the maximum tolerated dose within 84 days. A phase I clinical study aims to investigate the distribution and dynamic behavior of nuclide labeled TH-SC01 cells in vivo among patients suffering from perianal fistula and is now recruiting (NCT06429241). The MSCs are labeled with a nuclide to track their distribution and behavior within the body using PET/CT imaging. The study employs an open-label, single-group assignment model, where all participants receive the same treatment. Key primary outcomes are standardized uptake values in organs and tissues, measured at several intervals following the injection. Secondary outcomes include the proportion of participants experiencing significant clinical improvement at week 24, changes in pain scores, and the incidence of treatment-related adverse events over a 24-month period. Additionally, the study monitors radiation exposure metrics to assess the safety and distribution of the labeled cells.
BM-MSC-derived EVs
ExoFlo as explained previously in this article is an EV product manufactured from a single-donor BM-MSC culture.190 –194 A phase IB/IIA clinical trial is investigating ExoFlo for treating perianal fistulizing CD (NCT05836883). This trial utilizes a multicenter, single-blind, placebo-controlled design with a dose-escalation approach across three cohorts. The intervention involves administering ExoFlo, derived from adult allogeneic BM-MSC-derived EVs, via local injection. The study aims to evaluate safety, feasibility, and healing outcomes in subjects with perianal fistulas associated with CD. The primary outcomes include assessing the safety profile and feasibility of ExoFlo treatments, while secondary outcomes focus on evaluating healing progress over a 12-month period.
Systemic treatments
Ustekinumab
The Ustekinumab in Fistulizing Perianal CD (USTAP) trial, sponsored by Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives (GETAID), represents the sole RCT that is investigating ustekinumab in this patient population (NCT04496063) and is now active. This study is designed as a multicenter, randomized, double-blind, placebo-controlled phase IV trial that investigates patients with moderate-to-severe CD and at least one active perianal fistula track. Participants will be divided into two groups. Patients will receive either ustekinumab or placebo, initially IV and subsequently SC. Each participant will be involved in the study for a total duration of 48 weeks. The primary goal of the study is to evaluate the combined remission rate at week 12, defined by two criteria: the complete absence of drainage from all fistula tracts and the absence of abscess collections greater than 2 cm in diameter, as confirmed by a masked central MRI. In addition to the primary objective, the study aims to achieve several secondary objectives such as clinical and radiological remission.
Guselkumab
Guselkumab is a monoclonal IgG1 antibody targeting the p19 subunit of IL-23. It has shown good efficacy in luminal CD, however its’ role in perianal CD is yet to be defined. FUZION CD is a phase III, randomized, placebo-controlled, parallel-group, multicenter trial with the goal of evaluating the efficacy and safety of guselkumab in patients with fistulizing perianal CD (NCT05347095). It is now recruiting. The trial aims to enroll 280 participants and consists of three phases: a 6-week screening phase, a 48-week treatment phase, and a 16-week follow-up phase, with a potential LTE for participants who complete the treatment period. The primary outcome measure is the percentage of participants achieving combined fistula remission at week 24. Secondary outcome measures include various assessments of fistula remission, CDAI, quality of life, and safety evaluations. Participants will be randomized into three groups receiving guselkumab or placebo, with different dosing regimens. The study is expected to be completed by May 2027.
Filgotinib
A recent phase II RCT named DIVERGENCE 2 assessed the efficacy and safety of filgotinib in treating perianal fistulizing CD in patients with prior treatment failures. 48 In this trial, participants received filgotinib, 200 mg of filgotinib, 100 mg, or placebo orally once daily for up to 24 weeks, with the primary endpoint being a combined fistula response at week 24. Filgotinib 200 mg demonstrated numerical reductions in draining perianal fistulas compared to placebo, and it was generally well-tolerated. However, SAE were more frequent in the filgotinib 200 mg group. 48
Challenges in the drug pipeline for IBD trials
The development of new treatments for IBD faces significant hurdles, primarily due to complex trial designs, ethical dilemmas surrounding placebo use, difficulties in patient recruitment, and the escalating costs and timelines of drug development. 195 These challenges not only slow down the drug pipeline but also impact the accessibility and affordability of new treatments. The landscape of clinical trials in IBD has evolved significantly in recent years, with an increasing focus on inclusivity, diverse patient representation, and the specific challenges of conducting trials in complex patient populations. 196 Below is a detailed discussion of the key issues affecting IBD clinical trials today.
Placebo use in clinical trials
One of the most contentious issues in IBD clinical trials is the use of placebo groups. The argument in favor of continuing placebo-controlled trials stems from several factors. First, regulatory agencies such as the FDA and the EMA often require placebo-controlled trials for drug approval, especially for conditions where the disease fluctuates naturally, such as IBD. 197 Placebos can help demonstrate the true efficacy of a new drug by providing a clear comparison against no treatment.
Additionally, the use of placebo allows for smaller sample sizes because the expected effect size of the new drug compared to placebo is often larger than when comparing two active treatments. Moreover, placebo-controlled trials are considered more straightforward when it comes to demonstrating superiority, which is often a higher bar to meet. These practical and financial benefits encourage pharmaceutical companies to design trials with placebo groups. 198
However, there are strong ethical concerns against the continued use of placebos. Once there is moderate or high certainty about the efficacy of a treatment, it becomes questionable whether it is ethical to withhold that treatment from patients in a placebo group. In many cases, patients assigned to placebo are exposed to unnecessary risks, such as worsening symptoms, increased morbidity, and even mortality, which could have been prevented with active treatment.199,200 This concern is particularly acute in IBD, where placebo patients may suffer from severe disease exacerbations or complications like venous thromboembolism, as shown by recent meta-analyses.199,200 In CD in particular, early treatment has been associated with better outcomes. 7
In addition to the ethical concerns, placebo-controlled trials may no longer provide the most useful information for clinicians and patients. As the number of treatment options for IBD grows, doctors and patients are more interested in knowing how new treatments compare directly to existing therapies rather than to placebo. Head-to-head trials, which compare two or more active treatments, provide more relevant data for clinical decision-making. Furthermore, advances in clinical trial methodologies now allow for more accurate and unbiased comparisons between active treatments, making placebo groups less necessary.
Complexities in trial design
The design of clinical trials for IBD has evolved significantly over the past few decades. Initially, trials focused on short-term induction treatments to quickly assess a drug’s efficacy in controlling active disease. 201 Over time, the focus expanded to include randomized maintenance therapies, where patients who responded to the initial treatment are re-randomized to either continue the treatment or switch to placebo. 201 More recently, integrated induction and maintenance designs have become popular. These trials attempt to answer both short-term and long-term efficacy questions in a single study, which can make them more efficient overall.
However, these designs are not without limitations. In many cases, patients who respond well to induction therapy and are then switched to placebo during the maintenance phase continue to show improvements due to a carry-over effects from the initial treatment. 16 This phenomenon is particularly pronounced with biologic therapies that have long half-lives and sustained PD effects, such as ustekinumab and risankizumab. As a result, efficacy rates at the end of the maintenance period may be skewed, reflecting the initial induction response rather than the true long-term efficacy of the drug.
Responder re-randomization trials are also resource-intensive and inefficient. 201 To ensure that enough responders are available for the maintenance phase, these trials often require large, over-powered induction cohorts or additional open-label feeder cohorts. This inefficiency is exacerbated by slow patient recruitment rates, especially when multiple competing trials are being conducted simultaneously. Declining enrolment rates, rising costs, and longer study durations have made traditional RCTs increasingly unsustainable in IBD research. To address these challenges, innovative trial designs and Bayesian approaches have been explored. Bayesian trials, unlike frequentist methods, incorporate prior knowledge to minimize the number of patients receiving placebo and can reduce overall sample sizes.202,203 For example, the EXPLORER study used Bayesian methods to estimate the efficacy of triple-combination therapy in CD, based on data from previous trials. 204
Adaptive trial designs, which allow for flexible adjustments during the trial, are also being integrated into IBD research. These designs, such as multi-arm, multistage trials or seamless trials, enhance trial efficiency and ethical considerations. Moreover, master protocols, including “basket” trials (testing a single treatment across multiple disease types) and “umbrella” trials (testing multiple treatments within a single disease), could streamline trial processes by sharing resources and infrastructure. 203
Despite these innovations, challenges remain, particularly for long-term disease-modification trials. These studies often require extended follow-up, larger sample sizes, and careful management to capture rare but clinically significant outcomes such as stoma formation or cancer development. Cluster randomization, where entire groups (e.g., medical practices) rather than individuals are randomized, could be used in strategy trials for IBD, but such trials require careful consideration of statistical inefficiencies and potential biases.
Head-to-head trials and their challenges
Head-to-head trials in IBD are becoming more common, especially as the number of available biologics grows. 205 For instance, the SEAVUE trial compared adalimumab with ustekinumab in biologic-naïve patients with CD but failed to show a clear superiority of one drug over the other. 206 However, the trial’s limitations, such as the inclusion of patients with relatively moderate disease and the lack of dose optimization, highlight the difficulties in designing fair and conclusive head-to-head studies. The results of other trials, such as the SEQUENCE and VIVID-1 studies, comparing risankizumab, ustekinumab, and mirikizumab, further illustrate the complexity of these trials.207,208 Different designs and patient populations can lead to variable outcomes, making it difficult to draw definitive conclusions about the relative efficacy of these treatments. While head-to-head trials provide critical information about the positioning of new drugs in the treatment landscape, they are often more complex and expensive to conduct. 8
Declining enrollment and recruitment challenges
One of the most pressing issues facing IBD trials today is the decline in patient enrollment. 209 As more trials are conducted globally, the pool of eligible patients becomes increasingly competitive. The average recruitment rate for CD clinical trials dropped from 0.65 to 0.10 patients per site per month between 1998 and 2018. 210 Moreover, trials often require moderate-to-severe clinical activity as an inclusion criteria, that might not be present even in patients with severe endoscopic activity, particularly in CD.211,212 To address this challenge, pharmaceutical companies and contract research organizations have expanded their recruitment efforts to new regions, including Eastern Europe, Asia, and South America.
While this geographic expansion has helped increase trial enrollment, it also introduces new complexities. Different regions have varying healthcare standards, cultural practices, and ethnicities, all of which can influence trial outcomes. Furthermore, disparities in the quality of healthcare between sites in different countries may affect the consistency of trial results. To mitigate these issues, some trials have adopted centralized reading of key outcome measures, such as endoscopic evaluations, to standardize assessments across sites. 213 However, the inclusion of more diverse populations also enhances the generalizability of trial results, making them more applicable to a broader range of patients worldwide.
Despite these efforts, declining enrollment rates continue to pose significant risks to the drug pipeline. 211 Slower recruitment leads to extended trial timelines, higher costs, and, in some cases, the premature termination of studies. These delays increase the overall cost of drug development. Ultimately, the high cost and slow pace of development may discourage innovation and reduce the number of new drugs entering the market. Moreover, the high cost of development is often passed on to healthcare systems and patients in the form of elevated drug prices. In many cases, new and expensive drugs are reserved for use only after cheaper, less effective treatments have failed, delaying access to the most advanced therapies. 214
Inclusivity
Historically, clinical trials for IBD have struggled with generalizability due to rigid inclusion criteria. These criteria often excluded patients based on factors such as age, race, geographic location, or advanced disease, leading to trial populations that were predominantly white and skewed toward patients in tertiary care centers.215,216 This lack of diversity in trial populations is concerning because IBD’s pathophysiology and treatment responses can vary significantly by race and ethnicity, influencing outcomes such as thiopurine-induced leukopenia and herpes zoster, which are more prevalent in non-white populations.215,217
In addition to racial disparities, patients from rural areas and non-tertiary care settings have often been excluded, despite modern trials attempting to be more inclusive by recruiting from a wider range of care settings and geographic regions. This is becoming even more relevant as the prevalence of IBD rises in underrepresented regions like Eastern Europe, South America, and Asia-Pacific. The importance of inclusive trials is further highlighted by the growing recognition of disease heterogeneity in IBD. This encompasses a range of diverse phenotypes, such as chronic pouchitis, upper GI manifestations, proctitis, Inflammatory Bowel Disease Unclassified (IBD-U), and fistulizing CD.218,219
For example, the EMA’s approval of vedolizumab for chronic pouchitis, based on the EARNEST trial, highlighted the importance of trials targeting specific subpopulations with unmet medical needs.
One of the emerging challenges in IBD trials is managing patient populations with varying disease durations and previous treatment failures. 220 Patients who have undergone multiple lines of therapy, particularly those previously exposed to anti-TNF inhibitors, are less likely to respond to subsequent treatments, creating a need for trials that balance the inclusion of both treatment-naïve and treatment-experienced patients. However, the recruitment of bio-naïve patients has become increasingly difficult. 221 As these advanced therapies become more accessible worldwide, patient willingness to participate in placebo-controlled trials diminishes, particularly when existing therapies are effective.
Outcomes
Trial design must also address the issue of disease severity and symptom presentation, which does not always correlate with measurable inflammatory markers. Regulatory bodies, like the FDA and EMA, require that trials use stringent clinical and endoscopic severity thresholds for inclusion, often leading to high rates of screening failure.222,223 For instance, a patient with significant endoscopic disease but minimal symptoms may be excluded, even though such patients may still benefit from intervention. Moreover, this disconnect is further complicated by the reliance on tools like the CDAI and the SES-CD, which, while useful, do not fully capture the complexity of disease activity, particularly in cases involving small bowel or transmural disease.224,225 Despite advancements, trial outcomes must also evolve to better reflect clinical practice and long-term patient needs. 226 While achieving clinical and endoscopic remission remains the gold standard in trials, the definition of “remission” varies between regulatory authorities. For example, the FDA’s criteria for CD focus on achieving both clinical remission (CDAI < 150) and endoscopic remission (SES-CD ⩽ 2), whereas European guidance places more emphasis on mucosal healing and symptom relief. 201 This variation in trial endpoints can lead to differences in how new therapies are evaluated and approved across different regions.
The increasing reliance on PROs in clinical trials is a promising development, but challenges remain.227,228 Regulatory guidance from bodies like the FDA emphasizes the importance of incorporating PROs to assess treatment risks and benefits. However, a fully validated IBD-specific PRO has not yet been developed. The current use of SF and AP as proxies for CD, while responsive to treatment, only provide a limited view of the patient experience.229,230
Incorporating non-invasive methods for disease monitoring, such as intestinal ultrasound and MRI enterography, could also improve the ability to assess disease activity without the need for repeated endoscopies, especially in CD where transmural inflammation is a key concern. 231 The use of imaging techniques, however, is still limited in trials due to operational challenges, such as variability in access and expertise across trial sites. 232
Conclusion
Numerous clinical trials are underway to evaluate the safety and efficacy of novel therapies, including molecules like S1PR modulators, selective JAK inhibitors, anti-TL1A antibodies, and PPARγ agonists. Several drugs show promise in achieving clinical remission and endoscopic response, potentially surpassing existing medications. This evolving therapeutic landscape offers hope for improved disease management, particularly for patients with luminal and perianal CD, as well as those requiring therapies that target fibrosis. However, the development pipeline for IBD therapies faces notable challenges, including issues related to trial speed, costs, and ethical considerations. The use of placebos, while still valuable in some situations, is becoming increasingly controversial, prompting calls for more comparative trials as ethical concerns around placebo-controlled studies continue to grow. Moving forward, adopting novel trial designs and analytic methods may help bridge these gaps, supporting faster and more effective drug development. Ultimately, advancing therapies that are effective, accessible, and quickly initiated is essential for achieving early mucosal healing, enhancing PROs, and promoting durable remission in CD.
Acknowledgments
None.
Footnotes
ORCID iDs: Luisa Bertin  https://orcid.org/0009-0001-9816-4171
https://orcid.org/0009-0001-9816-4171
Martina Crepaldi  https://orcid.org/0009-0008-8388-9699
https://orcid.org/0009-0008-8388-9699
Daria Maniero  https://orcid.org/0000-0003-2468-7292
https://orcid.org/0000-0003-2468-7292
Sonia Facchin  https://orcid.org/0000-0002-6774-590X
https://orcid.org/0000-0002-6774-590X
Marco Scarpa  https://orcid.org/0000-0002-0217-6523
https://orcid.org/0000-0002-0217-6523
Andrea Buda  https://orcid.org/0000-0002-3099-8612
https://orcid.org/0000-0002-3099-8612
Fabiana Zingone  https://orcid.org/0000-0003-1133-1502
https://orcid.org/0000-0003-1133-1502
Edoardo Vincenzo Savarino  https://orcid.org/0000-0002-3187-2894
https://orcid.org/0000-0002-3187-2894
Contributor Information
Luisa Bertin, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Martina Crepaldi, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Miriana Zanconato, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Greta Lorenzon, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Daria Maniero, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Caterina de Barba, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Erica Bonazzi, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Sonia Facchin, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Marco Scarpa, Chirurgia Generale 3 Unit, Azienda Ospedale Università di Padova, Padua, Italy.
Cesare Ruffolo, Chirurgia Generale 3 Unit, Azienda Ospedale Università di Padova, Padua, Italy.
Imerio Angriman, Chirurgia Generale 3 Unit, Azienda Ospedale Università di Padova, Padua, Italy.
Andrea Buda, Gastroenterology Unit, Department of Oncological Gastrointestinal Surgery, Santa Maria del Prato Hospital, Feltre, Italy.
Fabiana Zingone, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Brigida Barberio, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padua, Italy.
Edoardo Vincenzo Savarino, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padova, Via Giustiniani, 2, Padua 35128, Italy.
Declarations
Author declaration: The Associate Editor of Therapeutic Advances in Gastroenterology is an author of this paper; therefore, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Luisa Bertin: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Martina Crepaldi: Conceptualization; Writing – original draft.
Miriana Zanconato: Conceptualization; Writing – original draft.
Greta Lorenzon: Conceptualization; Writing – review & editing.
Daria Maniero: Conceptualization; Writing – review & editing.
Caterina De Barba: Conceptualization; Writing – review & editing.
Erica Bonazzi: Conceptualization; Writing – review & editing.
Sonia Facchin: Conceptualization; Writing – review & editing.
Marco Scarpa: Conceptualization; Writing – review & editing.
Cesare Ruffolo: Conceptualization; Writing – review & editing.
Imerio Angriman: Conceptualization; Writing – review & editing.
Andrea Buda: Conceptualization; Writing – review & editing.
Fabiana Zingone: Conceptualization; Writing – review & editing.
Brigida Barberio: Conceptualization; Writing – review & editing.
Edoardo Vincenzo Savarino: Conceptualization; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Edoardo Vincenzo Savarino has served as speaker for Abbvie, Agave, AGPharma, Alfasigma, Aurora Pharma, CaDiGroup, Celltrion, Dr Falk, EG Stada Group, Fenix Pharma, Fresenius Kabi, Galapagos, Janssen, JB Pharmaceuticals, Innovamedica/Adacyte, Malesci, MayolyBiohealth, Omega Pharma, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Tillots, Unifarco; has served as a consultant for Abbvie, Agave, Alfasigma, Biogen, Bristol-Myers Squibb, Celltrion, DiademaFarmaceutici, Dr Falk, Fenix Pharma, Fresenius Kabi, Janssen, JB Pharmaceuticals, Merck & Co., Nestlè, Reckitt Benckiser, Regeneron, Sanofi, SILA, Sofar, Synformulas GmbH, Takeda, Unifarco; he received research support from Pfizer, Reckitt Benckiser, SILA, Sofar, Unifarco, Zeta Farmaceutici. Fabiana Zingone has served as a speaker for EG Stada Group, Fresenius Kabi, Janssen, Pfizer, Takeda, Unifarco, Malesci, and Kedrion and has served as a consultant for Galapagos. Brigida Barberio has served as a speaker for Abbvie, Agave, Alfasigma, AGpharma, Janssen, Lilly, MSD, Pfizer, Sofar, Takeda, and Unifarco. Sonia Facchin has served as a consultant for SILA, Unifarco, and Zeta Farmaceutici and has served as a speaker for Unifarco and SILA. The other authors declare no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Dolinger M, Torres J, Vermeire S. Crohn’s disease. Lancet 2024; 403: 1177–1191. [DOI] [PubMed] [Google Scholar]
- 2. Barberio B, Zingone F, Savarino EV. Inflammatory bowel disease and sleep disturbance: as usual, quality matters. Dig Dis Sci 2021; 66: 3–4. [DOI] [PubMed] [Google Scholar]
- 3. Barberio B, Zamani M, Black CJ, et al. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021; 6: 359–370. [DOI] [PubMed] [Google Scholar]
- 4. Barberio B, Massimi D, Cazzagon N, et al. Prevalence of primary sclerosing cholangitis in patients with inflammatory bowel disease: a systematic review and meta-analysis. Gastroenterology 2021; 161: 1865–1877. [DOI] [PubMed] [Google Scholar]
- 5. Revés J, Mascarenhas A, Temido MJ, et al. Early intervention with biologic therapy in Crohn’s disease: how early is early? J Crohns Colitis 2023; 17: 1752–1760. [DOI] [PubMed] [Google Scholar]
- 6. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160: 1570–1583. [DOI] [PubMed] [Google Scholar]
- 7. Law CCY, Tkachuk B, Lieto S, et al. Early biologic treatment decreases risk of surgery in Crohn’s disease but not in ulcerative colitis: systematic review and meta-analysis. Inflamm Bowel Dis 2024; 30: 1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barberio B, Gracie DJ, Black CJ, et al. Efficacy of biological therapies and small molecules in induction and maintenance of remission in luminal Crohn’s disease: systematic review and network meta-analysis. Gut 2023; 72: 264–274. [DOI] [PubMed] [Google Scholar]
- 9. Gordon H, Minozzi S, Kopylov U, et al. ECCO Guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2024; 15: 1531–1555. [DOI] [PubMed] [Google Scholar]
- 10. Barberio B, Black CJ, Savarino EV, et al. Ciclosporin or infliximab as rescue therapy in acute glucorticosteroid-refractory ulcerative colitis: systematic review and network meta-analysis. J Crohns Colitis 2021; 15: 733–741. [DOI] [PubMed] [Google Scholar]
- 11. Zingone F, Barberio B, Compostella F, et al. Good efficacy and safety of vedolizumab in Crohn’s disease and ulcerative colitis in a real-world scenario. Therap Adv Gastroenterol 2020; 13: 1756284820936536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raine T, Danese S. Breaking through the therapeutic ceiling: what will it take? Gastroenterology 2022; 162: 1507–1511. [DOI] [PubMed] [Google Scholar]
- 13. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 2019; 16: 185–196. [DOI] [PubMed] [Google Scholar]
- 14. Vuyyuru SK, Shackelton LM, Hanzel J, et al. Targeting IL-23 for IBD: rationale and progress to date. Drugs 2023; 83: 873–891. [DOI] [PubMed] [Google Scholar]
- 15. D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet 2022; 399: 2015–2030. [DOI] [PubMed] [Google Scholar]
- 16. Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet 2022; 399: 2031–2046. [DOI] [PubMed] [Google Scholar]
- 17. Alsoud D, Sabino J, Franchimont D, et al. Real-world effectiveness and safety of risankizumab in patients with moderate to severe multirefractory Crohn’s disease: a Belgian multicentric cohort study. Inflamm Bowel Dis. Epub ahead of print January 2024. DOI: 10.1093/ibd/izad315. [DOI] [PubMed] [Google Scholar]
- 18. Zinger A, Choi D, Choi N, et al. Risankizumab effectiveness and safety in Crohn’s disease: real-world data from a large tertiary center. Clin Gastroenterol Hepatol 2024; 22: 1336–1338.e2. [DOI] [PubMed] [Google Scholar]
- 19. Fumery M, Defrance A, Roblin X, et al. Effectiveness and safety of risankizumab induction therapy for 100 patients with Crohn’s disease: a GETAID multicentre cohort study. Aliment Pharmacol Ther 2023; 57: 426–434. [DOI] [PubMed] [Google Scholar]
- 20. Danese S, Beaton A, Duncan EA, et al. Long-term safety of brazikumab in the open-label period of a randomized phase 2a study of patients with Crohn’s disease. BMC Gastroenterol 2023; 23: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sandborn WJ, D’Haens GR, Reinisch W, et al. Guselkumab for the treatment of Crohn’s disease: induction results from the phase 2 GALAXI-1 study. Gastroenterology 2022; 162: 1650–1664.e8. [DOI] [PubMed] [Google Scholar]
- 22. Danese S, Panaccione R, Feagan BG, et al. Efficacy and safety of 48 weeks of guselkumab for patients with Crohn’s disease: maintenance results from the phase 2, randomised, double-blind GALAXI-1 trial. Lancet Gastroenterol Hepatol 2024; 9: 133–146. [DOI] [PubMed] [Google Scholar]
- 23. Afzali A, Danese S, Panaccione R, et al. S851 Efficacy and safety of guselkumab for Crohn’s disease through 3 years: GALAXI-1 long-term extension. Am Coll Gastroenterol 2023; 118: S630. [Google Scholar]
- 24. Panaccione R, Danese S, Feagan BG, et al. Efficacy and safety of guselkumab therapy in patients with moderately to severely active Crohn’s disease: results of the GALAXI 2 & 3 phase 3 studies. Gastroenterology 2024; 166: 1057b–1057b2. [Google Scholar]
- 25. Jairath V, Sands BE, Bossuyt P, et al. OP35 Efficacy of mirikizumab in comparison to ustekinumab in patients with moderate to severe Crohn’s disease: results from the phase 3 VIVID 1 study. J Crohns Colitis 2024; 18: i62–i64. [Google Scholar]
- 26. Chua L, Otani Y, Friedrich S, et al. P1044 Mirikizumab pharmacokinetics and exposure-response in a phase 2 study in patients with moderately to severely active Crohn’s disease. J Crohns Colitis 2024; 18: i1882. [Google Scholar]
- 27. Torres J, Bonovas S, Doherty G, et al. ECCO Guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020; 14: 4–22. [DOI] [PubMed] [Google Scholar]
- 28. Macaluso FS, Papi C, Orlando A, et al. Use of biologics for the management of Crohn’s disease: IG-IBD clinical guidelines based on the GRADE methodology. Dig Liver Dis 2023; 55: 442–453. [DOI] [PubMed] [Google Scholar]
- 29. Barberio B, Zingone F, D’Incà R, et al. Infliximab originator, infliximab biosimilar, and adalimumab are more effective in Crohn’s disease than ulcerative colitis: a real-life cohort study. Clin Transl Gastroenterol 2020; 11: e00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barberio B, Cingolani L, Canova C, et al. A propensity score-weighted comparison between adalimumab originator and its biosimilars, ABP501 and SB5, in inflammatory bowel disease: a multicenter Italian study. Therap Adv Gastroenterol 2021; 14: 17562848211031420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol 2022; 13: 1037983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petti L, Rizzo G, Rubbino F, et al. Unveiling role of sphingosine-1-phosphate receptor 2 as a brake of epithelial stem cell proliferation and a tumor suppressor in colorectal cancer. J Exp Clin Cancer Res 2020; 39: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peyrin-Biroulet L, Christopher R, Behan D, et al. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev 2017; 16: 495–503. [DOI] [PubMed] [Google Scholar]
- 34. Verstockt B, Vetrano S, Salas A, et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2022; 19: 351–366. [DOI] [PubMed] [Google Scholar]
- 35. Degagné E, Saba JD. S1pping fire: Sphingosine-1-phosphate signaling as an emerging target in inflammatory bowel disease and colitis-associated cancer. Clin Exp Gastroenterol 2014; 7: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D’Haens G, Danese S, Davies M, et al. A phase II, multicentre, randomised, double-blind, placebo-controlled study to evaluate safety, tolerability, and efficacy of amiselimod in patients with moderate to severe active Crohn’s disease. J Crohns Colitis 2022; 16: 746–756. [DOI] [PubMed] [Google Scholar]
- 37. Shirley M. Etrasimod: first approval. Drugs. Epub ahead of print February 2024. DOI: 10.1007/s40265-024-01997-7. [DOI] [PubMed] [Google Scholar]
- 38. D’Haens G, Dubinsky MC, Peyrin-Biroulet L, et al. P632 Etrasimod induction therapy in moderately to severely active Crohn’s disease: results from a phase 2, randomised, double-blind substudy. J Crohns Colitis 2023; 17: i764–i765. [Google Scholar]
- 39. Kim J-W, Kim S-Y. The era of Janus kinase inhibitors for inflammatory bowel disease treatment. Int J Mol Sci 2021; 22: 11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao M-Y, Zhang W, Rao G-W. Targeting Janus kinase (JAK) for fighting diseases: the research of JAK inhibitor drugs. Curr Med Chem 2022; 29: 5010–5040. [DOI] [PubMed] [Google Scholar]
- 41. Herrera-deGuise C, Serra-Ruiz X, Lastiri E, et al. JAK inhibitors: a new dawn for oral therapies in inflammatory bowel diseases. Front Med (Lausanne) 2023; 10: 1089099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu X, Li J, Fu M, et al. The JAK/STAT signaling pathway: from bench to clinic. Sig Transduct Target Ther 2021; 6: 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salas A, Hernandez-Rocha C, Duijvestein M, et al. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020; 17: 323–337. [DOI] [PubMed] [Google Scholar]
- 44. Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 1485–1493.e2. [DOI] [PubMed] [Google Scholar]
- 45. Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017; 66: 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017; 389: 266–275. [DOI] [PubMed] [Google Scholar]
- 47. D’Haens GR, Lee S, Taylor SA, et al. Filgotinib for the treatment of small bowel Crohn’s disease: the DIVERGENCE 1 trial. Gastroenterology 2023; 165: 289–292.e3. [DOI] [PubMed] [Google Scholar]
- 48. Reinisch W, Colombel J-F, D’Haens GR, et al. Efficacy and safety of filgotinib for the treatment of perianal fistulizing Crohn’s disease [DIVERGENCE 2]: a phase 2, randomized, placebo-controlled trial. J Crohns Colitis 2024; 18: 864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loftus EV, Panés J, Lacerda AP, et al. Upadacitinib induction and maintenance therapy for Crohn’s disease. N Engl J Med 2023; 388: 1966–1980. [DOI] [PubMed] [Google Scholar]
- 50. Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 2013; 12: 147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Migone TS, Zhang J, Luo X, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 2002; 16: 479–492. [DOI] [PubMed] [Google Scholar]
- 52. Zhan C, Patskovsky Y, Yan Q, et al. Decoy strategies: the structure of TL1A:DcR3 complex. Structure 2011; 19: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Solitano V, Jairath V, Ungaro F, et al. TL1A inhibition for inflammatory bowel disease treatment: from inflammation to fibrosis. Med 2024; 5: 386–400. [DOI] [PubMed] [Google Scholar]
- 54. Furfaro F, Alfarone L, Gilardi D, et al. TL1A: a new potential target in the treatment of inflammatory bowel disease. Curr Drug Targets 2021; 22: 760–769. [DOI] [PubMed] [Google Scholar]
- 55. Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 2008; 40: 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clarke AW, Poulton L, Shim D, et al. An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease. MAbs 2018; 10: 664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kokkotis G, Bamias G. TL1A as a therapeutic target in inflammatory bowel disease. Expert Rev Clin Immunol 2022; 18: 551–555. [DOI] [PubMed] [Google Scholar]
- 58. Michelsen KS, Thomas LS, Taylor KD, et al. IBD-associated TL1A gene (TNFSF15) haplotypes determine increased expression of TL1A protein. PLoS One 2009; 4: e4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Danese S, Klopocka M, Scherl EJ, et al. Anti-TL1A antibody PF-06480605 safety and efficacy for ulcerative colitis: a phase 2a single-arm study. Clin Gastroenterol Hepatol 2021; 19: 2324–2332.e6. [DOI] [PubMed] [Google Scholar]
- 60. Sands B, Peyrin-Biroulet L, Danese S, et al. OP40 PRA023 demonstrated efficacy and favorable safety as induction therapy for moderately to severely active UC: phase 2 ARTEMIS-UC study results. J Crohns Colitis 2023; 17: i56–i59. [Google Scholar]
- 61. Feagan BG, Sands B, Siegel CA, et al. DOP87 the anti-TL1A antibody PRA023 demonstrated proof-of-concept in Crohn’s disease: phase 2a APOLLO-CD study results. J Crohns Colitis 2023; 17: i162–i164. [Google Scholar]
- 62. Raphael G, Damera G, Angeles T, et al. P1061 TEV-48574, an anti-TL1A antibody in development for use in IBD, is safe and well tolerated following 16 weeks of subcutaneous treatment in adults with severe uncontrolled T2-low/non T2 asthma. J Crohns Colitis 2024; 18: i1908. [Google Scholar]
- 63. Reinisch W, Stoyanov S, Raphael G, et al. P998 Phase 2 basket design study evaluating the efficacy and safety of an anti-TL1A antibody (TEV-48574) in moderate to severe ulcerative colitis or Crohn’s disease (RELIEVE UCCD). J Crohns Colitis 2024; 18: i1811. [Google Scholar]
- 64. Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997; 91: 521–530. [DOI] [PubMed] [Google Scholar]
- 65. Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol 2001; 78: 57–110. [DOI] [PubMed] [Google Scholar]
- 66. Hundhausen C, Misztela D, Berkhout TA, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 2003; 102: 1186–1195. [DOI] [PubMed] [Google Scholar]
- 67. Nishimura M, Umehara H, Nakayama T, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol 2002; 168: 6173–6180. [DOI] [PubMed] [Google Scholar]
- 68. Goda S, Imai T, Yoshie O, et al. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol 2000; 164: 4313–4320. [DOI] [PubMed] [Google Scholar]
- 69. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012; 12: 180–190. [DOI] [PubMed] [Google Scholar]
- 70. Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 2016; 16: 295–309. [DOI] [PubMed] [Google Scholar]
- 72. Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 2015; 15: 283–294. [DOI] [PubMed] [Google Scholar]
- 73. Allegretti J, Canavan J, Mitsialis V, et al. Low dose IL-2 for the treatment of moderate to severe ulcerative colitis. Gastroenterology 2021; 160: S9–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bono JD, Champiat S, Danlos F-X, et al. 715 ICT01 plus low dose SC IL-2 produces a robust anti-tumor immune activation in advanced cancer patients (EVICTION-2 Study). J Immunother Cancer 2023; 11(1). [Google Scholar]
- 75. Aggeletopoulou I, Mouzaki A, Thomopoulos K, et al. miRNA molecules—late breaking treatment for inflammatory bowel diseases? Int J Mol Sci 2023; 24: 2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tazi J, Begon-Pescia C, Campos N, et al. Specific and selective induction of miR-124 in immune cells by the quinoline ABX464: a transformative therapy for inflammatory diseases. Drug Discov Today 2021; 26: 1030. [DOI] [PubMed] [Google Scholar]
- 77. Singh NP, Singh UP, Singh B, et al. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One 2011; 6: e23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011; 141: 237–248, 248.e1. [DOI] [PubMed] [Google Scholar]
- 79. Zhao Y, Ma T, Chen W, et al. MicroRNA-124 promotes intestinal inflammation by targeting aryl hydrocarbon receptor in Crohn’s disease. J Crohns Colitis 2016; 10: 703–712. [DOI] [PubMed] [Google Scholar]
- 80. Vermeire S, Sands BE, Tilg H, et al. ABX464 (obefazimod) for moderate-to-severe, active ulcerative colitis: a phase 2b, double-blind, randomised, placebo-controlled induction trial and 48 week, open-label extension. Lancet Gastroenterol Hepatol 2022; 7: 1024–1035. [DOI] [PubMed] [Google Scholar]
- 81. Dulai P, Sands BE, Danese S, et al. P985 Efficacy and safety of de-escalation from 50 mg to 25 mg of oral, once-daily, obefazimod for the third and fifth year of open-label maintenance treatment in patients with moderately to severely active ulcerative colitis (UC): an interim analysis. J Crohns Colitis 2024; 18: i1787. [Google Scholar]
- 82. Vermeire S, Solitano V, Peyrin-Biroulet L, et al. Obefazimod: a first-in-class drug for the treatment of ulcerative colitis. J Crohns Colitis 2023; 17: 1689–1697. [DOI] [PubMed] [Google Scholar]
- 83. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 84. Zundler S, Becker E, Schulze LL, et al. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut 2019; 68: 1688–1700. [DOI] [PubMed] [Google Scholar]
- 85. Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol 2019; 20: 970–979. [DOI] [PubMed] [Google Scholar]
- 86. Nelson SM, Nguyen TM, McDonald JW, et al. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2018; 2018: CD006097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sandborn WJ, Panés J, Danese S, et al. Etrolizumab as induction and maintenance therapy in patients with moderately to severely active Crohn’s disease (BERGAMOT): a randomised, placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2023; 8: 43–55. [DOI] [PubMed] [Google Scholar]
- 88. Sandborn WJ, Lee SD, Tarabar D, et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut 2018; 67: 1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vermeire S, Danese S, Sandborn WJ, et al. Efficacy and safety of the anti-mucosal addressin cell adhesion molecule-1 antibody ontamalimab in patients with moderate-to-severe ulcerative colitis or Crohn’s disease. J Crohns Colitis 2024; 18: 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ray A, Cui D, Lee D, et al. P306 MORF-057, an oral selective α4β7 integrin inhibitor for Inflammatory Bowel Disease, leads to specific target engagement in a single and multiple ascending dose study in healthy subjects. J Crohns Colitis 2021; 15: S333–S335. [Google Scholar]
- 91. Wendland M, Czeloth N, Mach N, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A 2007; 104: 6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Keshav S, Vaňásek T, Niv Y, et al. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS One 2013; 8: e60094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Marks D. AZD7798: a new CCR9 depleting antibody to treat Crohn’s disease, https://eposters.ddw.org/ddw/2023/ddw-2023/379907/daniel.marks.azd7798.a.new.ccr9.depleting.antibody.to.treat.crohn.s.disease.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D2%2Aspeaker%3D939349%2Alabel%3D26122 (2024).
- 94. Savarino E, Bertani L, Ceccarelli L, et al. Antimicrobial treatment with the fixed-dose antibiotic combination RHB-104 for Mycobacterium avium subspecies paratuberculosis in Crohn’s disease: pharmacological and clinical implications. Expert Opin Biol Ther 2019; 19: 79–88. [DOI] [PubMed] [Google Scholar]
- 95. Zarei-Kordshouli F, Geramizadeh B, Khodakaram-Tafti A. Prevalence of Mycobacterium avium subspecies paratuberculosis IS 900 DNA in biopsy tissues from patients with Crohn’s disease: histopathological and molecular comparison with Johne’s disease in Fars province of Iran. BMC Infect Dis 2019; 19: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Khan IA, Pilli S, Surendranath A, et al. Prevalence and association of Mycobacterium avium subspecies paratuberculosis with disease course in patients with ulcero-constrictive ileocolonic disease. PLoS One 2016; 11: e0152063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shaffer JL, Hughes S, Linaker BD, et al. Controlled trial of rifampicin and ethambutol in Crohn’s disease. Gut 1984; 25: 203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Borody TJ, Leis S, Warren EF, et al. Treatment of severe Crohn’s disease using antimycobacterial triple therapy—approaching a cure? Dig Liver Dis 2002; 34: 29–38. [DOI] [PubMed] [Google Scholar]
- 99. Leiper K, Morris AI, Rhodes JM. Open label trial of oral clarithromycin in active Crohn’s disease. Aliment Pharmacol Ther 2000; 14: 801–806. [DOI] [PubMed] [Google Scholar]
- 100. Agrawal G, Clancy A, Huynh R, et al. Profound remission in Crohn’s disease requiring no further treatment for 3–23 years: a case series. Gut Pathog 2020; 12: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Agrawal G, Hamblin H, Clancy A, et al. Anti-mycobacterial antibiotic therapy induces remission in active paediatric Crohn’s disease. Microorganisms 2020; 8: 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gui GP, Thomas PR, Tizard ML, et al. Two-year-outcomes analysis of Crohn’s disease treated with rifabutin and macrolide antibiotics. J Antimicrob Chemother 1997; 39: 393–400. [DOI] [PubMed] [Google Scholar]
- 103. Afdhal NH, Long A, Lennon J, et al. Controlled trial of antimycobacterial therapy in Crohn’s disease. Clofazimine versus placebo. Dig Dis Sci 1991; 36: 449–453. [DOI] [PubMed] [Google Scholar]
- 104. Swift GL, Srivastava ED, Stone R, et al. Controlled trial of anti-tuberculous chemotherapy for two years in Crohn’s disease. Gut 1994; 35: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Selby W, Pavli P, Crotty B, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology 2007; 132: 2313–2319. [DOI] [PubMed] [Google Scholar]
- 106. Agrawal G, Borody T, Turner R, et al. Combining infliximab, anti-MAP and hyperbaric oxygen therapy for resistant fistulizing Crohn’s disease. Future Sci OA 2015; 1: FSO77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Waldner MJ, Neurath MF. Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin Immunol 2014; 26: 75–79. [DOI] [PubMed] [Google Scholar]
- 108. Akiyama M, Hayashi Y, Suzuki K, et al. Successful treatment of IgG4-related disease with tocilizumab monotherapy. Autoimmun Rev 2023; 22: 103296. [DOI] [PubMed] [Google Scholar]
- 109. Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 2004; 126: 989–996; discussion 947. [DOI] [PubMed] [Google Scholar]
- 110. Monemi S, Berber E, Sarsour K, et al. Incidence of gastrointestinal perforations in patients with rheumatoid arthritis treated with tocilizumab from clinical trial, postmarketing, and real-world data sources. Rheumatol Ther 2016; 3: 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shaw S, Bourne T, Meier C, et al. Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs 2014; 6: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Danese S, Vermeire S, Hellstern P, et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut 2019; 68: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wagner F, Schreiber S, Bagger Y, et al. Safety, tolerability, and pharmacokinetics of single- and multiple-ascending doses of olamkicept: Results from randomized, placebo-controlled, first-in-human phase I trials. Clin Transl Sci 2024; 17: e13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schreiber S, Aden K, Bernardes JP, et al. Therapeutic interleukin-6 trans-signaling inhibition by olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology 2021; 160: 2354–2366.e11. [DOI] [PubMed] [Google Scholar]
- 115. Zhang S, Chen B, Wang B, et al. Effect of induction therapy with olamkicept vs placebo on clinical response in patients with active ulcerative colitis: a randomized clinical trial. JAMA 2023; 329: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen B, Zhang S, Wang B, et al. DOP01 Efficacy and safety of the IL-6 trans-signalling inhibitor olamkicept: a phase 2 randomized, placebo-controlled trial in moderately to severely active Ulcerative Colitis. J Crohns and Colitis 2021; 15(Supplement_1): S041–S042. [Google Scholar]
- 117. Irving AT, Mimuro H, Kufer TA, et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe 2014; 15: 623–635. [DOI] [PubMed] [Google Scholar]
- 118. Chin AI, Dempsey PW, Bruhn K, et al. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 2002; 416: 190–194. [DOI] [PubMed] [Google Scholar]
- 119. Stronati L, Negroni A, Merola P, et al. Mucosal NOD2 expression and NF-kappaB activation in pediatric Crohn’s disease. Inflamm Bowel Dis 2008; 14: 295–302. [DOI] [PubMed] [Google Scholar]
- 120. Negroni A, Stronati L, Pierdomenico M, et al. Activation of NOD2-mediated intestinal pathway in a pediatric population with Crohn’s disease. Inflamm Bowel Dis 2009; 15: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 121. Törüner M, Ünal NG. Epigenetics of inflammatory bowel diseases. Turk J Gastroenterol 2023; 34: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Liang B, Wang Y, Xu J, et al. Unlocking the potential of targeting histone-modifying enzymes for treating IBD and CRC. Clin Epigenetics 2023; 15: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tsaprouni LG, Ito K, Powell JJ, et al. Differential patterns of histone acetylation in inflammatory bowel diseases. J Inflamm (Lond) 2011; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Facchin S, Bertin L, Bonazzi E, et al. Short-chain fatty acids and human health: from metabolic pathways to current therapeutic implications. Life (Basel) 2024; 14: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Choi SW, Braun T, Henig I, et al. Vorinostat plus tacrolimus/methotrexate to prevent GVHD after myeloablative conditioning, unrelated donor HCT. Blood 2017; 130: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Patra S, Praharaj PP, Klionsky DJ, et al. Vorinostat in autophagic cell death: a critical insight into autophagy-mediated, -associated and -dependent cell death for cancer prevention. Drug Discov Today 2022; 27: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bettenworth D, Baker ME, Fletcher JG, et al. A global consensus on the definitions, diagnosis and management of fibrostenosing small bowel Crohn’s disease in clinical practice. Nat Rev Gastroenterol Hepatol 2024; 21: 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shen B, Kochhar G, Navaneethan U, et al. Practical guidelines on endoscopic treatment for Crohn’s disease strictures: a consensus statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol Hepatol 2020; 5: 393–405. [DOI] [PubMed] [Google Scholar]
- 129. East JE, Brooker JC, Rutter MD, et al. A pilot study of intrastricture steroid versus placebo injection after balloon dilatation of Crohn’s strictures. Clin Gastroenterol Hepatol 2007; 5: 1065–1069. [DOI] [PubMed] [Google Scholar]
- 130. Di Nardo G, Oliva S, Passariello M, et al. Intralesional steroid injection after endoscopic balloon dilation in pediatric Crohn’s disease with stricture: a prospective, randomized, double-blind, controlled trial. Gastrointest Endosc 2010; 72: 1201–1208. [DOI] [PubMed] [Google Scholar]
- 131. Bettenworth D, Mücke MM, Lopez R, et al. Efficacy of endoscopic dilation of gastroduodenal Crohn’s disease strictures: a systematic review and meta-analysis of individual patient data. Clin Gastroenterol Hepatol 2019; 17: 2514–2522.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Thienpont C, D’Hoore A, Vermeire S, et al. Long-term outcome of endoscopic dilatation in patients with Crohn’s disease is not affected by disease activity or medical therapy. Gut 2010; 59: 320–324. [DOI] [PubMed] [Google Scholar]
- 133. Asairinachan A, An V, Daniel ES, et al. Endoscopic balloon dilatation of Crohn’s strictures: a safe method to defer surgery in selective cases. ANZ J Surg 2017; 87: E240–E244. [DOI] [PubMed] [Google Scholar]
- 134. Greener T, Shapiro R, Klang E, et al. Clinical outcomes of surgery versus endoscopic balloon dilation for stricturing Crohn’s disease. Dis Colon Rectum 2015; 58: 1151–1157. [DOI] [PubMed] [Google Scholar]
- 135. Atreja A, Aggarwal A, Dwivedi S, et al. Safety and efficacy of endoscopic dilation for primary and anastomotic Crohn’s disease strictures. J Crohns and Colitis 2014; 8: 392–400. [DOI] [PubMed] [Google Scholar]
- 136. Lian L, Stocchi L, Remzi FH, et al. Comparison of endoscopic dilation vs surgery for anastomotic stricture in patients with Crohn’s disease following ileocolonic resection. Clin Gastroenterol Hepatol 2017; 15: 1226–1231. [DOI] [PubMed] [Google Scholar]
- 137. Lan N, Stocchi L, Ashburn JH, et al. Outcomes of endoscopic balloon dilation vs surgical resection for primary ileocolic strictures in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2018; 16: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 138. Bettenworth D, Gustavsson A, Atreja A, et al. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn’s disease. Inflamm Bowel Dis 2017; 23: 133–142. [DOI] [PubMed] [Google Scholar]
- 139. Teich N, Wallstabe I, Schiefke I. Topic infliximab injection for refractory rectal stenosis in Crohn’s disease: long-term follow-up in two patients. Int J Colorectal Dis 2017; 32: 1289–1294. [DOI] [PubMed] [Google Scholar]
- 140. Hendel J, Karstensen JG, Vilmann P. Serial intralesional injections of infliximab in small bowel Crohn’s strictures are feasible and might lower inflammation. United European Gastroenterol J 2014; 2: 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Biancone L, Cretella M, Tosti C, et al. Local injection of infliximab in the postoperative recurrence of Crohn’s disease. Gastrointest Endosc 2006; 63: 486–492. [DOI] [PubMed] [Google Scholar]
- 142. Swaminath A, Lichtiger S. Dilation of colonic strictures by intralesional injection of infliximab in patients with Crohn’s colitis. Inflamm Bowel Dis 2008; 14: 213–216. [DOI] [PubMed] [Google Scholar]
- 143. Kamal S, Lo SW, McCall S, et al. Unveiling the potential of JAK inhibitors in inflammatory bowel disease. Biologics 2024; 4: 177–186. [Google Scholar]
- 144. Jiang F, Liu M, Wang H, et al. Wu Mei Wan attenuates CAC by regulating gut microbiota and the NF-kB/IL6-STAT3 signaling pathway. Biomed Pharmacother 2020; 125: 109982. [DOI] [PubMed] [Google Scholar]
- 145. Yan B, Li X, Zhou L, et al. Inhibition of IRAK 1/4 alleviates colitis by inhibiting TLR 4/NF-κB pathway and protecting the intestinal barrier. Bosn J Basic Med Sci 2022; 22: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lee W, Chung HK, Kim J, et al. LMT503, a novel therapy for inflammatory bowel disease through macrophage polarizations by metabolic reprogramming of activated macrophage. Inflamm Bowel Dis 2023; 29: S4–S5. [Google Scholar]
- 147. Klein OR, Bonfim C, Abraham A, et al. Transplant for non-malignant disorders: an International Society for Cell & Gene Therapy Stem Cell Engineering Committee report on the role of alternative donors, stem cell sources and graft engineering. Cytotherapy 2023; 25: 463–471. [DOI] [PubMed] [Google Scholar]
- 148. Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA 2015; 314: 2524–2534. [DOI] [PubMed] [Google Scholar]
- 149. Lindsay JO, Hind D, Swaby L, et al. Safety and efficacy of autologous haematopoietic stem-cell transplantation with low-dose cyclophosphamide mobilisation and reduced intensity conditioning versus standard of care in refractory Crohn’s disease (ASTIClite): an open-label, multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol 2024; 9: 333–345. [DOI] [PubMed] [Google Scholar]
- 150. Brierley CK, Castilla-Llorente C, Labopin M, et al. Autologous haematopoietic stem cell transplantation for Crohn’s disease: a retrospective survey of long-term outcomes from the European Society for Blood and Marrow Transplantation. J Crohns Colitis 2018; 12: 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Burt RK, Craig R, Yun L, et al. A pilot feasibility study of non-myeloablative allogeneic hematopoietic stem cell transplantation for refractory Crohn disease. Bone Marrow Transplant 2020; 55: 2343–2346. [DOI] [PubMed] [Google Scholar]
- 152. Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol 2020; 21: e168–e178. [DOI] [PubMed] [Google Scholar]
- 153. Zundler S, Vitali F, Kharboutli S, et al. Case Report: IBD-like colitis following CAR T cell therapy for diffuse large B cell lymphoma. Front Oncol 2023; 13: 149450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Morris EC, Neelapu SS, Giavridis T, et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 2022; 22: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fang X, Neyrinck AP, Matthay MA, et al. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 2010; 285: 26211–26222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Sengupta V, Sengupta S, Lazo A, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 2020; 29: 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Foussat A, Cottrez F, Brun V, et al. A comparative study between T regulatory type 1 and CD4+CD25+ T cells in the control of inflammation. J Immunol 2003; 171: 5018–5026. [DOI] [PubMed] [Google Scholar]
- 158. Canavan JB, Scottà C, Vossenkämper A, et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut 2016; 65: 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Irving PM, Seah D, Centritto A, et al. P999 First in human treatment of Crohn’s disease with autologous ex-vivo expanded polyclonal, gut-targeted regulatory T cells: initial results of the TRIBUTE feasibility study. J Crohns Colitis 2024; 18: i1812. [Google Scholar]
- 160. Christofides A, Konstantinidou E, Jani C, et al. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021; 114: 154338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wang Z, Wang M, Xu X, et al. PPARs/macrophages: a bridge between the inflammatory response and lipid metabolism in autoimmune diseases. Biochem Biophys Res Commun 2023; 684: 149128. [DOI] [PubMed] [Google Scholar]
- 162. Berger J, Wagner JA. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol Therap 2002; 4: 163–174. [DOI] [PubMed] [Google Scholar]
- 163. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med 2002; 53: 409–435. [DOI] [PubMed] [Google Scholar]
- 164. He Y, B’nai Taub A, Yu L, et al. PPARγ acetylation orchestrates adipose plasticity and metabolic rhythms. Adv Sci 2023; 10: 2204190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Guo N, Woeller CF, Feldon SE, et al. Peroxisome proliferator-activated receptor gamma ligands inhibit transforming growth factor-beta-induced, hyaluronan-dependent, T cell adhesion to orbital fibroblasts. J Biol Chem 2011; 286: 18856–18867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lin Q, Fang L-P, Zhou W-W, et al. Rosiglitazone inhibits migration, proliferation, and phenotypic differentiation in cultured human lung fibroblasts. Exp Lung Res 2010; 36: 120–128. [DOI] [PubMed] [Google Scholar]
- 167. Liu Y, Dai B, Xu C, et al. Rosiglitazone inhibits transforming growth factor-β1 mediated fibrogenesis in ADPKD cyst-lining epithelial cells. PLoS One 2011; 6: e28915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jeon K-I, Kulkarni A, Woeller CF, et al. Inhibitory effects of PPARγ ligands on TGF-β1-induced corneal myofibroblast transformation. Am J Pathol 2014; 184: 1429–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wei W-Y, Ma Z-G, Xu S-C, et al. Pioglitazone protected against cardiac hypertrophy via inhibiting AKT/GSK3β and MAPK signaling pathways. PPAR Res 2016; 2016: 9174190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wang W, Liu F, Chen N. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonists attenuate the profibrotic response induced by TGF-beta1 in renal interstitial fibroblasts. Mediators Inflamm 2007; 2007: 62641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zhou B, Buckley ST, Patel V, et al. Troglitazone attenuates TGF-β1-induced EMT in alveolar epithelial cells via a PPARγ-independent mechanism. PLoS One 2012; 7: e38827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Zhang G-Y, Yi C-G, Li X, et al. Troglitazone suppresses transforming growth factor-beta1-induced collagen type I expression in keloid fibroblasts. Br J Dermatol 2009; 160: 762–770. [DOI] [PubMed] [Google Scholar]
- 173. Fan F, Li Y, Duan X, et al. Rosiglitazone attenuates activation of human Tenon’s fibroblasts induced by transforming growth factor-β1. Graefes Arch Clin Exp Ophthalmol 2012; 250: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 174. Shimizu K, Shiratori K, Kobayashi M, et al. Troglitazone inhibits the progression of chronic pancreatitis and the profibrogenic activity of pancreatic stellate cells via a PPARgamma-independent mechanism. Pancreas 2004; 29: 67–74. [DOI] [PubMed] [Google Scholar]
- 175. Schwab M, Reynders V, Loitsch S, et al. PPARgamma is involved in mesalazine-mediated induction of apoptosis and inhibition of cell growth in colon cancer cells. Carcinogenesis 2008; 29: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 176. Katkar GD, Sayed IM, Anandachar MS, et al. Artificial intelligence-rationalized balanced PPARα/γ dual agonism resets dysregulated macrophage processes in inflammatory bowel disease. Commun Biol 2022; 5: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Medina C, Santos-Martinez MJ, Santana A, et al. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol 2011; 224: 461–472. [DOI] [PubMed] [Google Scholar]
- 178. Santacroce G, Lenti MV, Di Sabatino A. Therapeutic targeting of intestinal fibrosis in Crohn’s disease. Cells 2022; 11: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ihara S, Hirata Y, Koike K. TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol 2017; 52(7): 777–787. [DOI] [PubMed] [Google Scholar]
- 180. Barcelo J, Samain R, Sanz-Moreno V. Preclinical to clinical utility of ROCK inhibitors in cancer. Trends Cancer 2023; 9: 250–263. [DOI] [PubMed] [Google Scholar]
- 181. Zanin-Zhorov A, Chen W, Moretti J, et al. Selectivity matters: selective ROCK2 inhibitor ameliorates established liver fibrosis via targeting inflammation, fibrosis, and metabolism. Commun Biol 2023; 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Knipe RS, Probst CK, Lagares D, et al. The Rho kinase isoforms ROCK1 and ROCK2 each contribute to the development of experimental pulmonary fibrosis. Am J Respir Cell Mol Biol 2018; 58: 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yu B, Sladojevic N, Blair JE, et al. Targeting Rho-associated coiled-coil forming protein kinase (ROCK) in cardiovascular fibrosis and stiffening. Expert Opin Therap Targets 2020; 24: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Adamina M, Minozzi S, Warusavitarne J, et al. ECCO Guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis 2024; 18: 1556–1582. [DOI] [PubMed] [Google Scholar]
- 185. Panés J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol 2017; 14: 652–664. [DOI] [PubMed] [Google Scholar]
- 186. Han S, Sun HM, Hwang K-C, et al. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit Rev Eukaryot Gene Expr 2015; 25: 145–152. [DOI] [PubMed] [Google Scholar]
- 187. Laureti S, Gionchetti P, Cappelli A, et al. Refractory complex Crohn’s perianal fistulas: a role for autologous microfragmented adipose tissue injection. Inflamm Bowel Dis 2020; 26: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Nazari H, Alborzi F, Heirani-Tabasi A, et al. Evaluating the safety and efficacy of mesenchymal stem cell-derived exosomes for treatment of refractory perianal fistula in IBD patients: clinical trial phase I. Gastroenterol Rep (Oxf) 2022; 10: goac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wei J, Zhang Y, Chen C, et al. Efficacy and safety of allogeneic umbilical cord-derived mesenchymal stem cells for the treatment of complex perianal fistula in Crohn’s disease: a pilot study. Stem Cell Res Ther 2023; 14: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lightner AL, Sengupta V, Qian S, et al. Bone marrow mesenchymal stem cell-derived extracellular vesicle infusion for the treatment of respiratory failure from COVID-19: a randomized, placebo-controlled dosing clinical trial. Chest 2023; 164: 1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Tang Y, Zhou Y, Li H-J. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther 2021; 12: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kaspi H, Semo J, Abramov N, et al. MSC-NTF (NurOwn®) exosomes: a novel therapeutic modality in the mouse LPS-induced ARDS model. Stem Cell Res Ther 2021; 12: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. De Jong OG, Van Balkom BWM, Schiffelers RM, et al. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol 2014; 5: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Krishnan A, Muthusamy S, Fernandez FB, et al. Mesenchymal stem cell-derived extracellular vesicles in the management of COVID19-associated lung injury: a review on publications, clinical trials and patent landscape. Tissue Eng Regen Med 2022; 19: 659–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Honap S, Jairath V, Danese S, et al. Navigating the complexities of drug development for inflammatory bowel disease. Nat Rev Drug Discov 2024; 23: 546–562. [DOI] [PubMed] [Google Scholar]
- 196. Honap S, Zou G, Danese S, et al. Personalized (N-of-1) clinical trials for inflammatory bowel disease: opportunities and challenges. Clin Gastroenterol Hepatol. 2024. Epub ahead of print September 2024. DOI: 10.1016/j.cgh.2024.08.028. [DOI] [PubMed] [Google Scholar]
- 197. Lichtenberg P, Heresco-Levy U, Nitzan U. The ethics of the placebo in clinical practice. J Med Ethics 2004; 30: 551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Millum J, Grady C. The ethics of placebo-controlled trials: methodological justifications. Contemp Clin Trials 2013; 36: 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Din S, Segal J, Blackwell J, et al. Harms with placebo in trials of biological therapies and small molecules as induction therapy in inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2024; 9: 1020–1029. [DOI] [PubMed] [Google Scholar]
- 200. Gros B, Blackwell J, Segal J, et al. Harms with placebo in trials of biological therapies and small molecules as maintenance therapy in inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2024; 9: 1030–1040. [DOI] [PubMed] [Google Scholar]
- 201. Ma C, Jairath V, Feagan BG, et al. Interpreting modern randomized controlled trials of medical therapy in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2024; 21: 792–808. [DOI] [PubMed] [Google Scholar]
- 202. van Zyl CJJ. Frequentist and Bayesian inference: a conceptual primer. New Ideas in Psychology 2018; 51: 44–49. [Google Scholar]
- 203. Ma C, Solitano V, Danese S, et al. The future of clinical trials in inflammatory bowel disease. Clin Gastroenterol Hepatol. Epub ahead of print July 2024. DOI: 10.1016/j.cgh.2024.06.036. [DOI] [PubMed] [Google Scholar]
- 204. Colombel J-F, Ungaro RC, Sands BE, et al. Vedolizumab, adalimumab, and methotrexate combination therapy in Crohn’s disease (EXPLORER). Clin Gastroenterol Hepatol 2024; 22: 1487–1496.e12. [DOI] [PubMed] [Google Scholar]
- 205. Pouillon L, Travis S, Bossuyt P, et al. Head-to-head trials in inflammatory bowel disease: past, present and future. Nat Rev Gastroenterol Hepatol 2020; 17: 365–376. [DOI] [PubMed] [Google Scholar]
- 206. Sands BE, Irving PM, Hoops T, et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 2022; 399: 2200–2211. [DOI] [PubMed] [Google Scholar]
- 207. Peyrin-Biroulet L, Chapman JC, Colombel J-F, et al. Risankizumab versus ustekinumab for moderate-to-severe Crohn’s disease. N Engl J Med 2024; 391: 213–223. [DOI] [PubMed] [Google Scholar]
- 208. Aslam M, Ali MH, Irfan H. Mirikizumab: a promising breakthrough in Crohn’s disease treatment. Health Sci Rep 2024; 7: e2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Uzzan M, Bouhnik Y, Abreu M, et al. Declining enrolment and other challenges in IBD clinical trials: causes and potential solutions. J Crohns Colitis 2023; 17: 1066–1078. [DOI] [PubMed] [Google Scholar]
- 210. Solitano V, Prins H, Archer M, et al. Toward patient centricity: why do patients with inflammatory bowel disease participate in pharmaceutical clinical trials? A mixed-methods exploration of study participants. Crohns Colitis 360 2024; 6: otae019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Uzzan M, Georgiev G, Peyrin-Biroulet L, et al. Screen failures and causes in inflammatory bowel disease randomized controlled trials: a study of 16 913 screened patients. Inflamm Bowel Dis. Epub ahead of print September 2024. DOI: 10.1093/ibd/izae233. [DOI] [PubMed] [Google Scholar]
- 212. Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 1994; 35: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Eichler H-G, Sweeney F. The evolution of clinical trials: can we address the challenges of the future? Clin Trials 2018; 15: 27–32. [DOI] [PubMed] [Google Scholar]
- 214. Rankala R, Mustonen A, Voutilainen M, et al. Costs of medications used to treat inflammatory bowel disease. Scand J Gastroenterol 2024; 59: 34–38. [DOI] [PubMed] [Google Scholar]
- 215. Cohen NA, Silfen A, Rubin DT. Inclusion of under-represented racial and ethnic minorities in randomized clinical trials for inflammatory bowel disease. Gastroenterology 2022; 162: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Strigáč A, Caban M, Małecka-Wojciesko E, et al. Safety and effectiveness of thiopurines and small molecules in elderly patients with inflammatory bowel diseases. J Clin Med 2024; 13: 4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Venkateswaran N, Sultan K. Racial and ethnic disparities in clinical presentation, management, and outcomes of patients with inflammatory bowel disease: a narrative review. Transl Gastroenterol Hepatol 2024; 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Travis S, Silverberg MS, Danese S, et al. Vedolizumab for the treatment of chronic pouchitis. N Engl J Med 2023; 388: 1191–1200. [DOI] [PubMed] [Google Scholar]
- 219. Gorospe J, Windsor J, Hracs L, et al. Trends in inflammatory bowel disease incidence and prevalence across epidemiologic stages: a global systematic review with meta-analysis. Inflamm Bowel Dis 2024; 30: S00. [Google Scholar]
- 220. Bertin L, Crepaldi M, Zanconato M, et al. Refractory Crohn’s disease: perspectives, unmet needs and innovations. Clin Exp Gastroenterol 2024; 17: 261–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Sedano R, Guizzetti L, McDonald C, et al. Clinical, endoscopic, and radiological effectiveness of ustekinumab in bio-naïve versus bio-experienced patients with Crohn’s disease: real-world experience from a large canadian center. Inflamm Bowel Dis 2023; 29: 866–874. [DOI] [PubMed] [Google Scholar]
- 222. Food and Drug Administration. Crohn’s disease: developing drugs for treatment, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/crohns-disease-developing-drugs-treatment (2022, accessed 22 September 2024).
- 223. Therapeutic Goods Administration. Guideline on the development of new medicinal products for the treatment of Crohn’s disease | Therapeutic Goods Administration (TGA), https://www.tga.gov.au/resources/resource/international-scientific-guidelines/guideline-development-new-medicinal-products-treatment-crohns-disease (2022, accessed 17 October 2024).
- 224. Fierens L, Carney N, Novacek G, et al. A core outcome set for inflammatory bowel diseases: development and recommendations for implementation in clinical practice through an international multi-stakeholder consensus process. J Crohns Colitis 2024; 18: 1583–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Khanna R, Ma C, Jairath V, et al. Endoscopic assessment of inflammatory bowel disease activity in clinical trials. Clin Gastroenterol Hepatol 2022; 20: 727–736.e2. [DOI] [PubMed] [Google Scholar]
- 226. Le Berre C, Ricciuto A, Peyrin-Biroulet L, et al. Evolving short- and long-term goals of management of inflammatory bowel diseases: getting it right, making it last. Gastroenterology 2022; 162: 1424–1438. [DOI] [PubMed] [Google Scholar]
- 227. Higgins PDR, Harding G, Leidy NK, et al. Development and validation of the Crohn’s disease patient-reported outcomes signs and symptoms (CD-PRO/SS) diary. J Patient Rep Outcomes 2018; 2: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Horrigan JM, Louis E, Spinelli A, et al. The real-world global use of patient-reported outcomes for the care of patients with inflammatory bowel disease. Crohns Colitis 360 2023; 5: otad006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Lewis JD, Rutgeerts P, Feagan BG, et al. Correlation of stool frequency and abdominal pain measures with simple endoscopic score for Crohn’s disease. Inflamm Bowel Dis 2020; 26: 304–313. [DOI] [PubMed] [Google Scholar]
- 230. Dovizio M, Hartz S, Buzzoni C, et al. Real-world treatment patterns and healthcare resource use for ulcerative colitis and Crohn’s disease in italy. Adv Ther 2024; 41: 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Danese S, Vermeire S, D’Haens G, et al. Treat to target versus standard of care for patients with Crohn’s disease treated with ustekinumab (STARDUST): an open-label, multicentre, randomised phase 3b trial. Lancet Gastroenterol Hepatol 2022; 7: 294–306. [DOI] [PubMed] [Google Scholar]
- 232. Novak KL, Nylund K, Maaser C, et al. Expert consensus on optimal acquisition and development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: a reliability and inter-rater variability study on intestinal ultrasonography in Crohn’s disease. J Crohns Colitis 2021; 15: 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]