Abstract
1. cis-Benzene glycol dehydrogenase was purified to a homogeneous state from a species of Pseudomonas grown with benzene as the major carbon source. 2. The enzyme was specific for the cis-isomer of its substrate and required NAD+ as hydrogen acceptor. 3. Partial inactivation of the enzyme, which was observed during purification, could be reversed by the addition of Fe2+ and GSH. 4. A molecular weight of 440000 was calculated from data obtained by sedimentation-velocity and diffusion analysis in the ultracentrifuge. Sodium dodecyl sulphate polyacrylamide-gel electrophoresis indicated a subunit of molecular weight 110000. 5. p-Chloromercuribenzoic acid and 1,10-phenanthroline were shown to inhibit the enzyme.
Full text
PDF

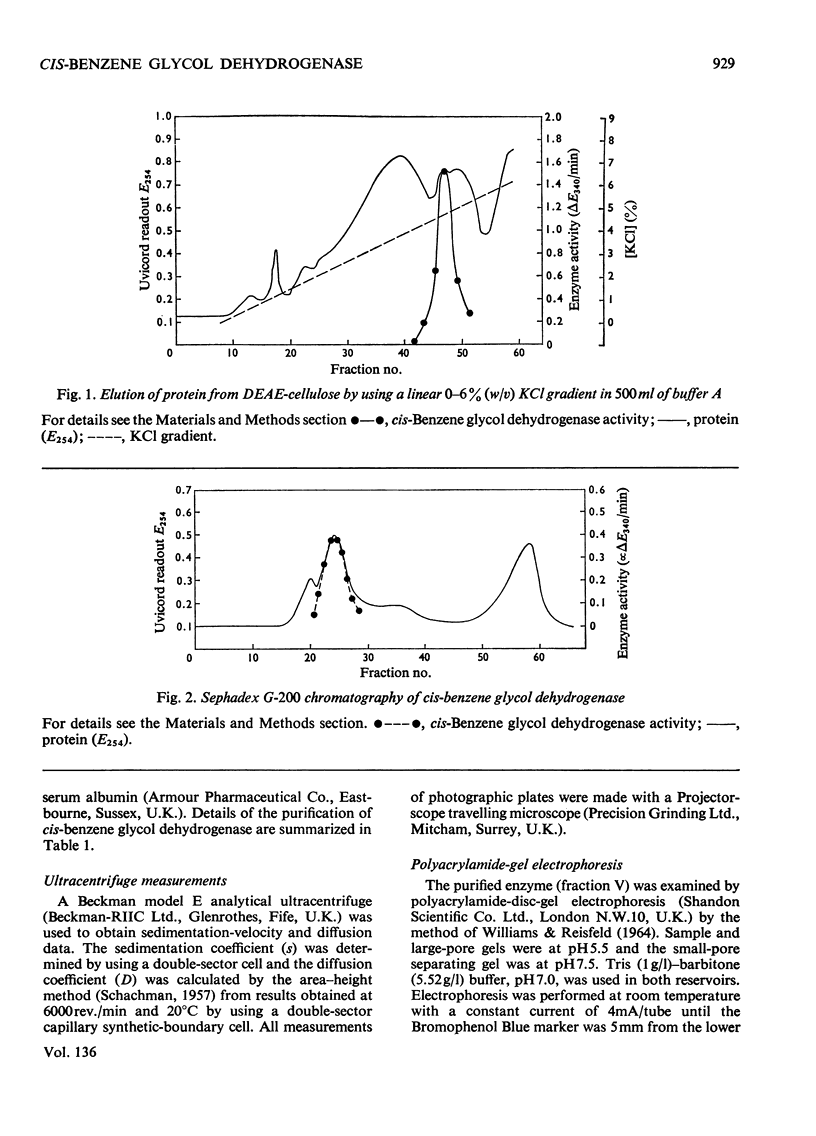
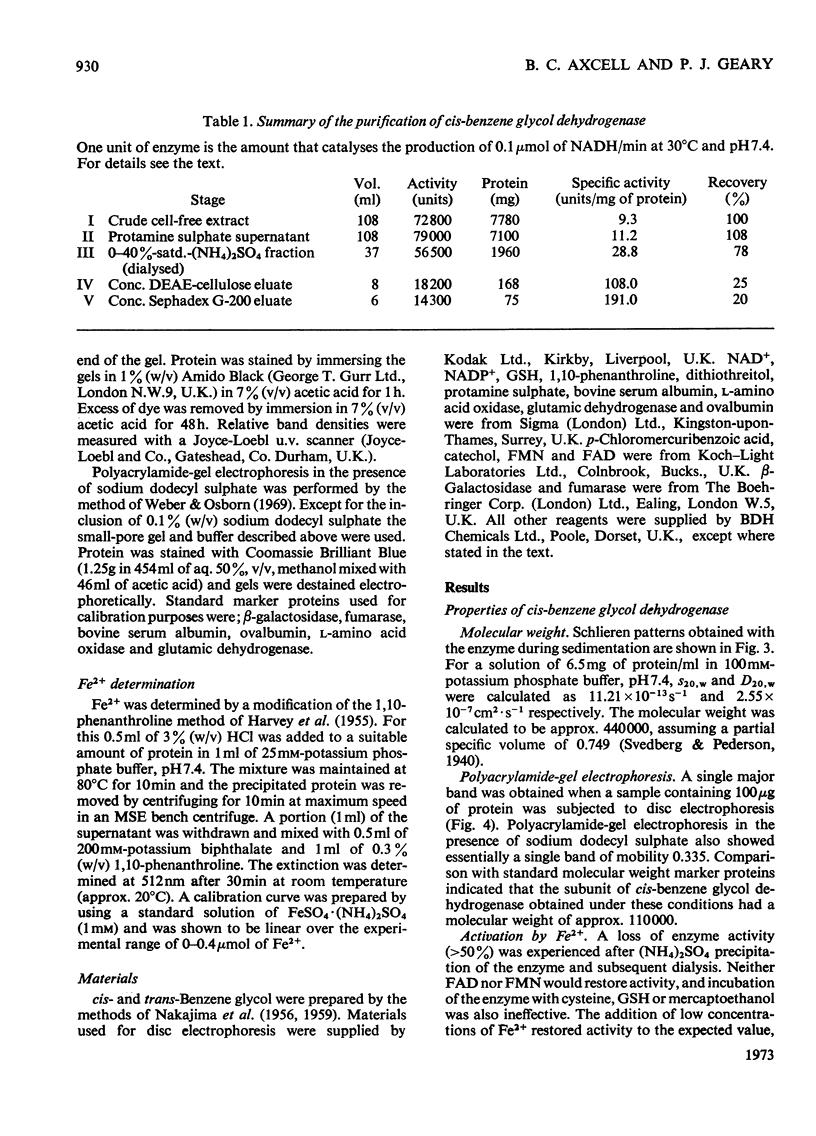
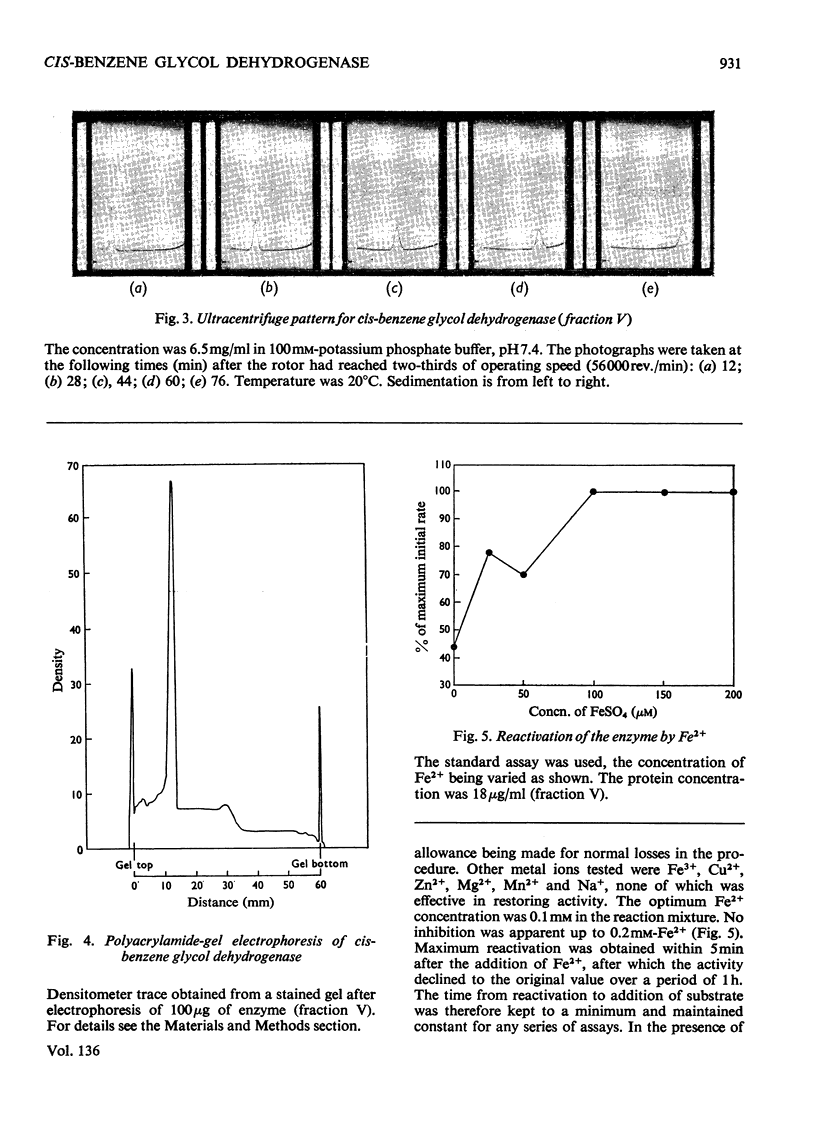
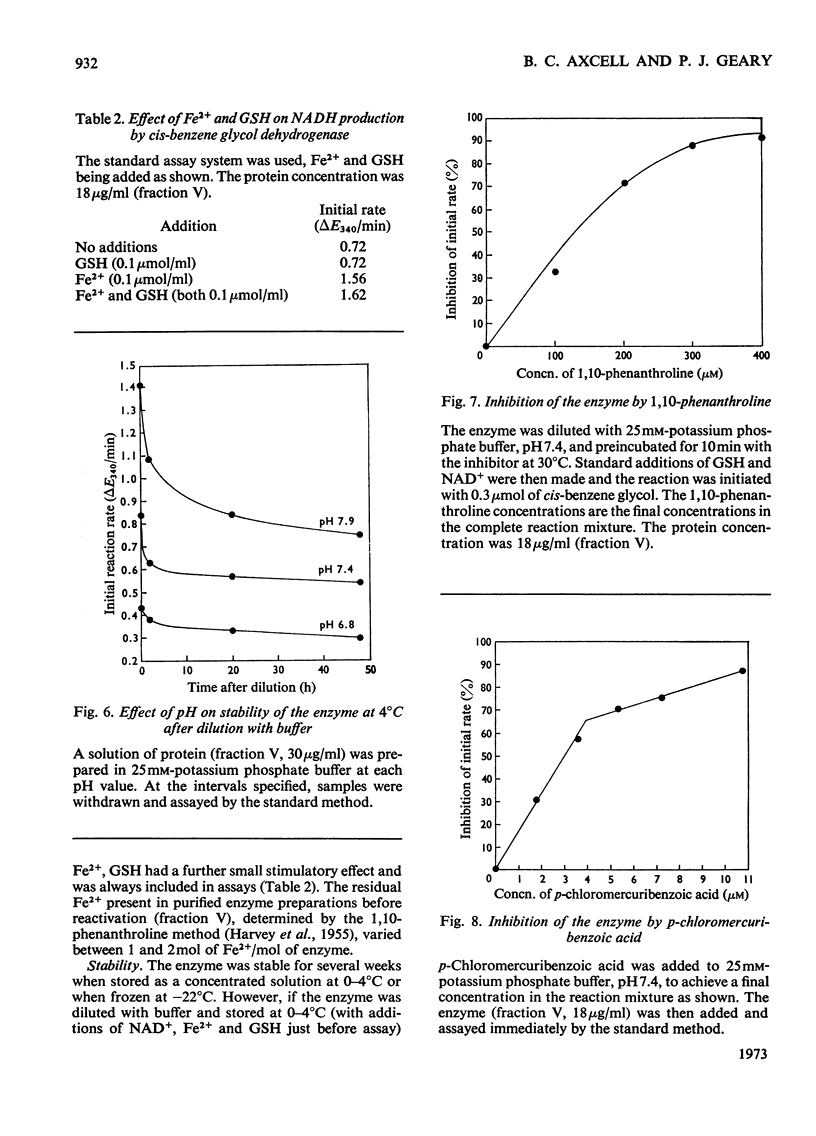


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gibson D. T., Koch J. R., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968 Jul;7(7):2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of aromatic compounds in bacteria. Purification and properties of the catechol-forming enzyme, 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid (NAD + ) oxidoreductase (decarboxylating). J Biol Chem. 1972 Aug 25;247(16):4960–4965. [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



