Abstract
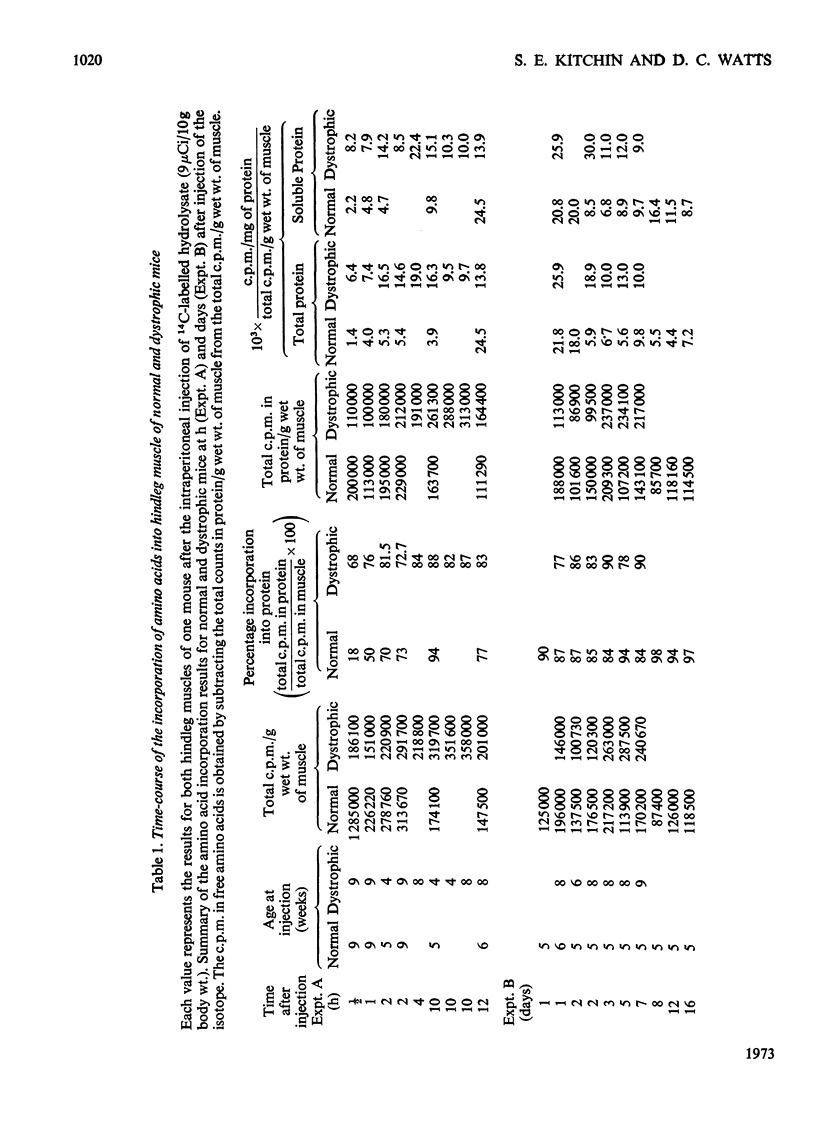
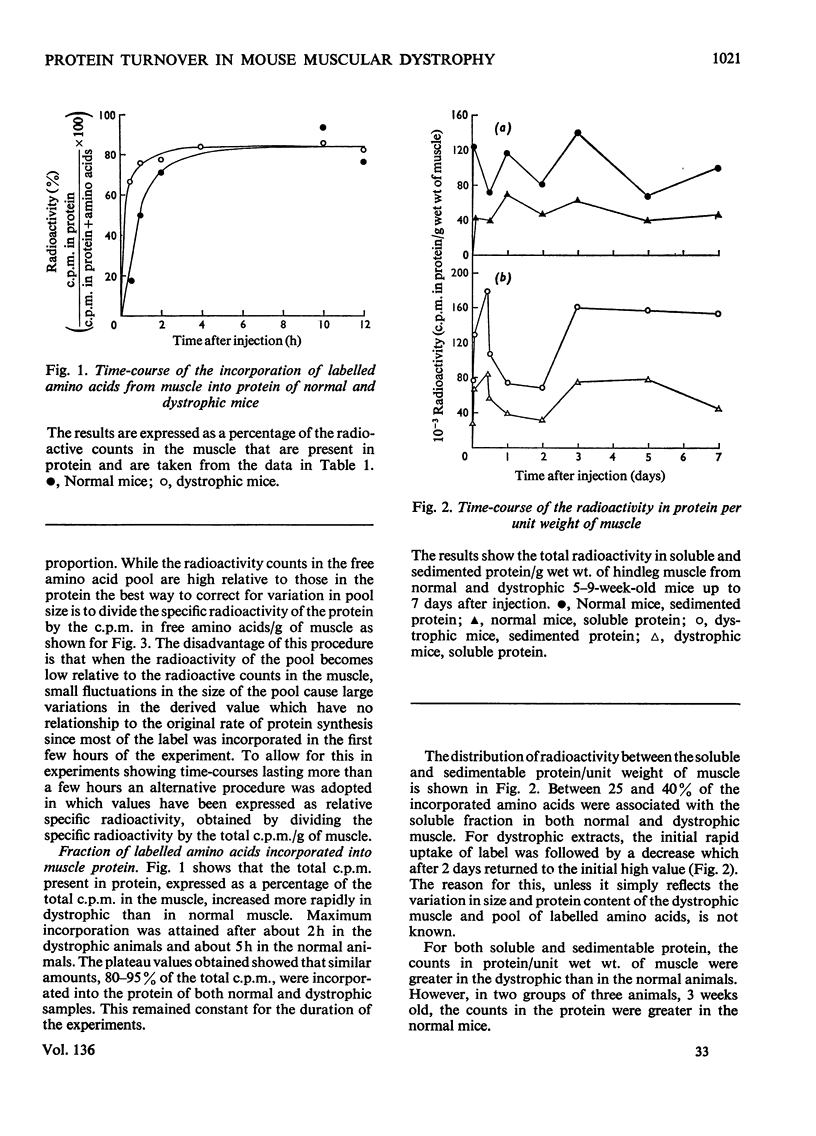
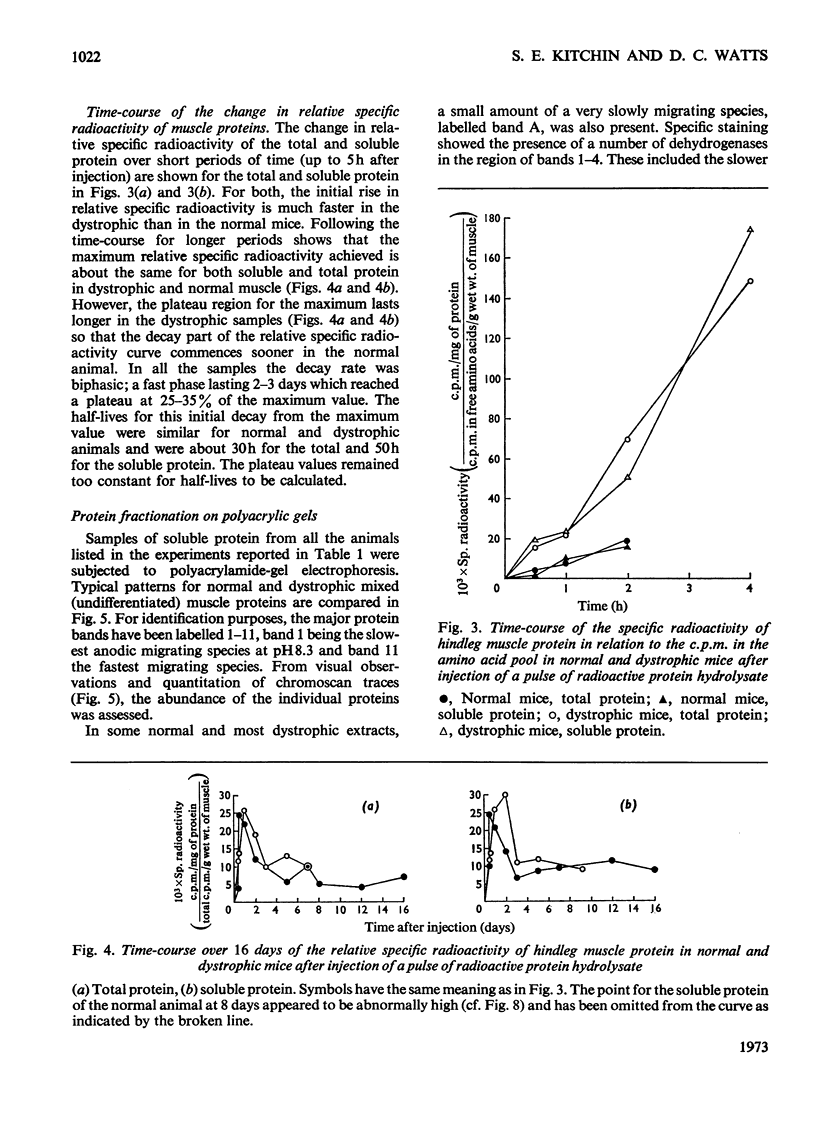
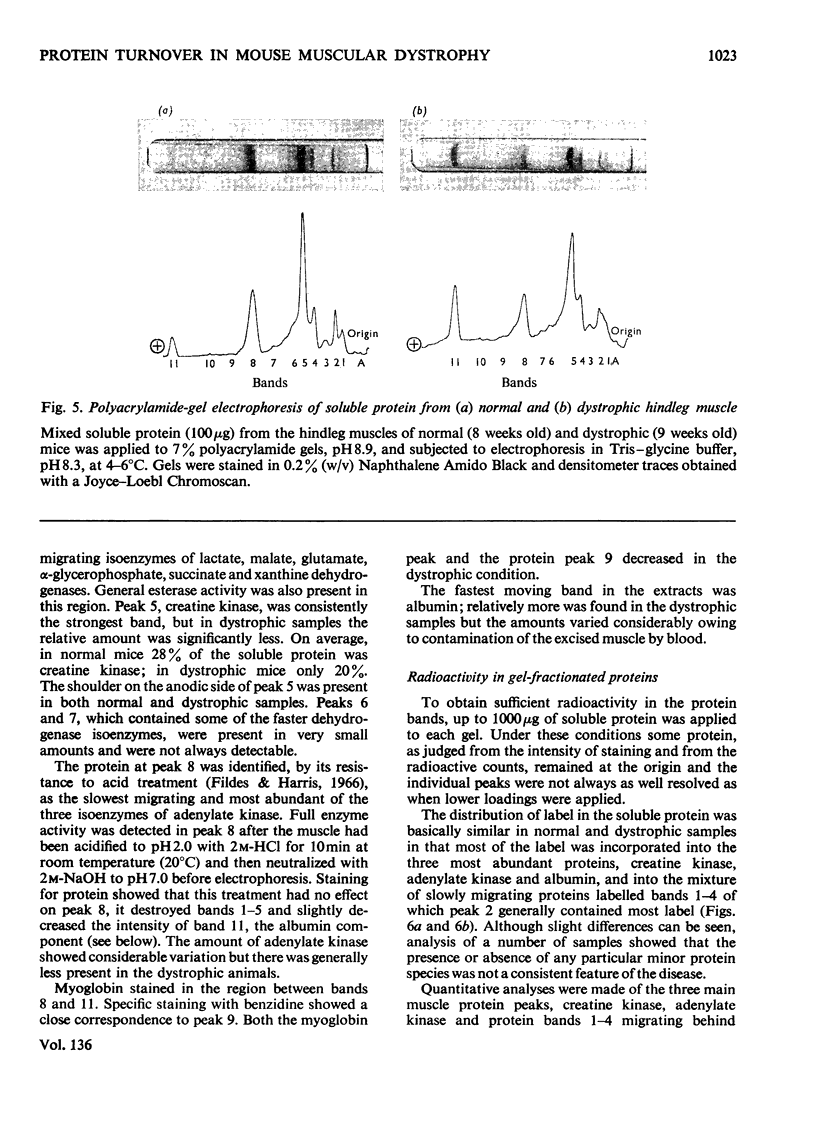
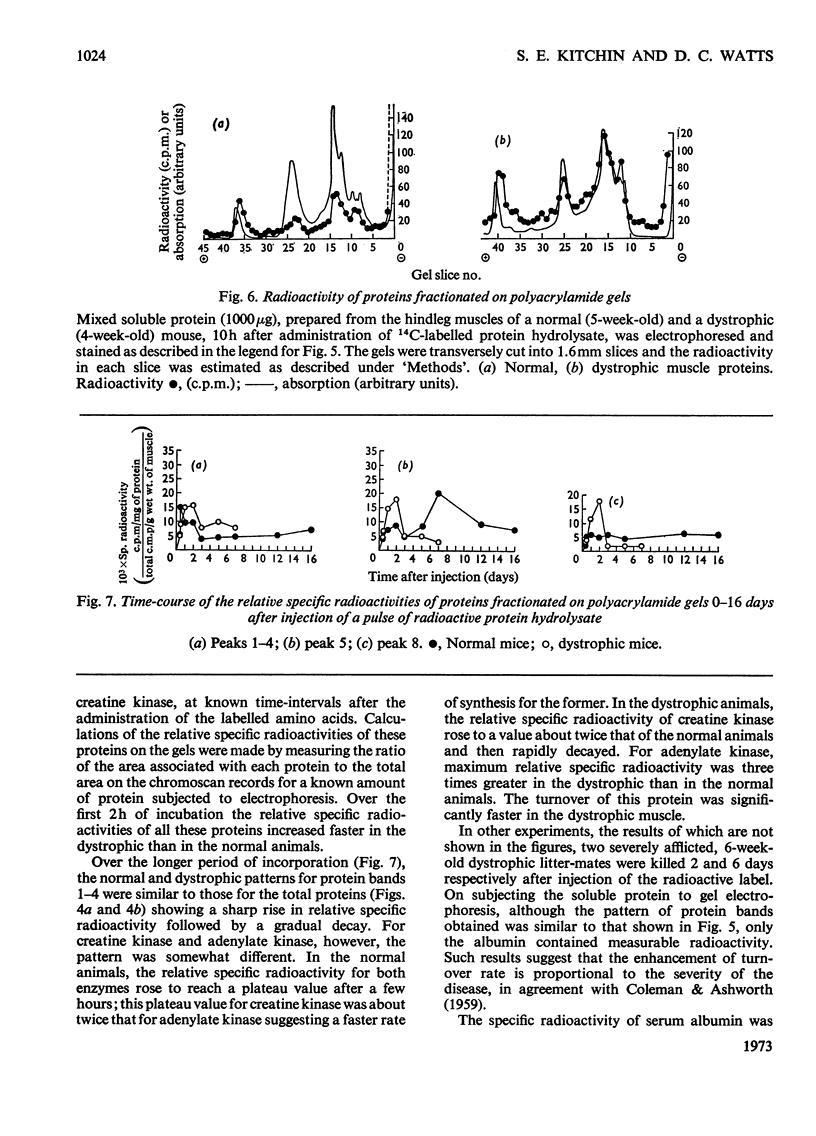
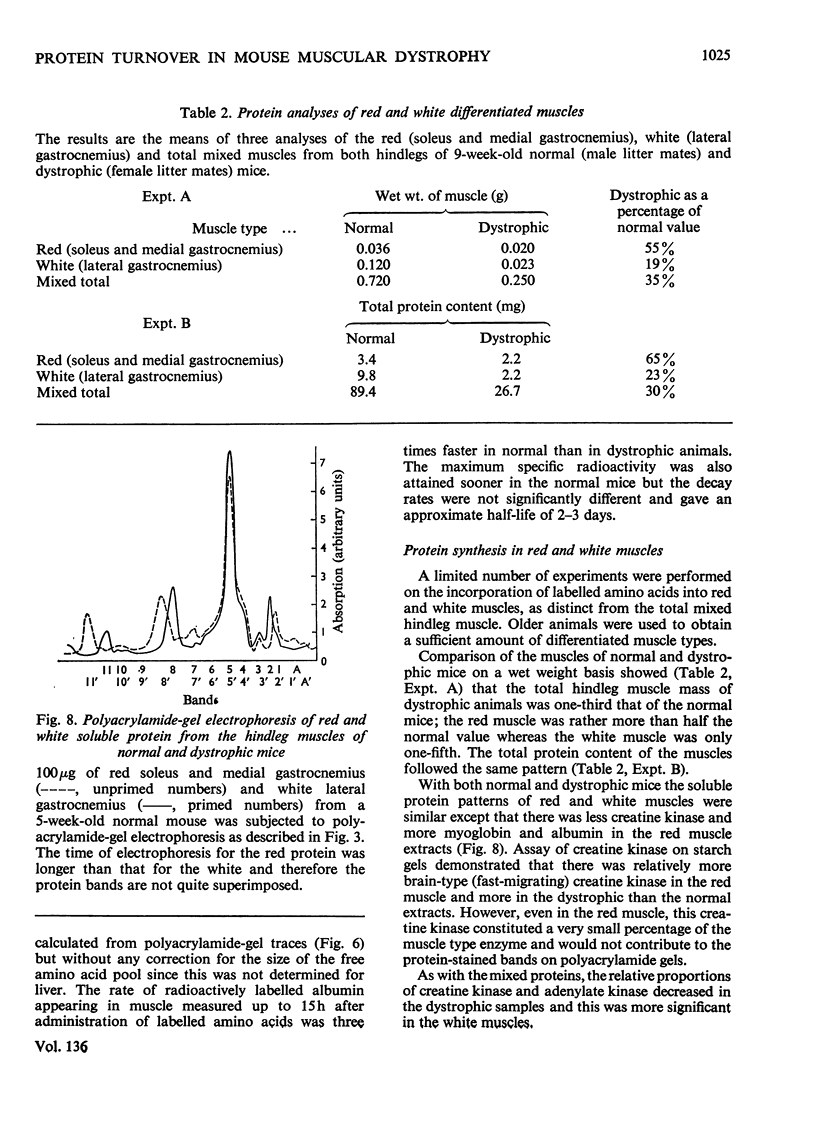
1. The incorporation of amino acids into hindleg muscle proteins of normal and dystrophic mice was measured ½h to 16 days after administration of the radioactive pulse. 2. Dystrophic animals showed a faster initial rate of incorporation into total and soluble proteins in the first few hours after injection, but the extent of incorporation relative to the size of the amino acid pool was similar in both. There was little difference between the overall degradation rates although this started later in the dystrophic proteins. An initial fast phase of degradation reached a plateau after 3 days whereupon the residual label in the protein remained constant up to 16 days after injection. 3. Analyses of individual radioactive proteins fractionated by polyacrylamide-gel electrophoresis showed that the distribution of label was similar in all the soluble proteins from normal and dystrophic muscle. Time-course experiments revealed that in dystrophic mice the two major soluble proteins of the muscle, creatine kinase and adenylate kinase, initially incorporated 2–3 times more label relative to the initial size of the precursor pool. This label was then lost equally rapidly and the final plateau value was much less than that in normal mice. This initial peak of activity was not observed in normal mice. 4. A group of dehydrogenases showed similar initial turnover patterns in both dystrophic and normal mice but the final plateau value was much higher in the former. 5. The results provide support for the hypothesis that there is no obvious defect in the protein synthetic machinery of dystrophic muscle. However, certain proteins do show anomalous turnover patterns relative to those in normal animals. A single structural gene mutation giving rise to one particularly unstable and readily degradable muscle protein is excluded as the cause of the dystrophy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basch R. S. An improved method for counting tritium and carbon-14 in acrylamide gels. Anal Biochem. 1968 Oct 10;26(1):184–188. doi: 10.1016/0003-2697(68)90044-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Fildes R. A., Harris H. Genetically determined variation of adenylate kinase in man. Nature. 1966 Jan 15;209(5020):261–263. doi: 10.1038/209261a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Protein synthesis in tonic and phasic skeletal muscles. Nature. 1967 Dec 23;216(5121):1219–1220. doi: 10.1038/2161219a0. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Holmes D., Pennington R. J. Studies on proteolytic activity in commercial myoglobin preparations. Biochem J. 1971 Dec;125(3):865–868. doi: 10.1042/bj1250865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooton B. T., Watts D. C. Adenosine 5 -triphosphate--creatine phosphotransferase from dystrophic mouse skeletal muscle. A genetic lesion associated with the catalytic-site thiol group. Biochem J. 1966 Sep;100(3):637–646. doi: 10.1042/bj1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooton B. T., Watts D. C. Levels of protein and non-protein sulphydryl groups in the skeletal muscle of normal and dystrophic Bar Harbor mice. Clin Chim Acta. 1967 Apr;16(1):173–176. doi: 10.1016/0009-8981(67)90286-0. [DOI] [PubMed] [Google Scholar]
- KRUH J., DREYFUS J. C., SCHAPIRA G., GEY G. O., Jr Abnormalities of muscle protein metabolism in mice with muscular dystrophy. J Clin Invest. 1960 Jul;39:1180–1184. doi: 10.1172/JCI104132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn R. R. A proteolytic system involving myofibrils and a soluble factor from normal and atrophying muscle. Lab Invest. 1969 Feb;20(2):202–206. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NICHOL C. J. SULFHYDRYL AND DISULFIDE CONCENTRATIONS IN DYSTROPHIC MOUSE MUSCLE. Can J Biochem. 1964 Nov;42:1643–1645. doi: 10.1139/o64-176. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- SIMON E. J., GROSS C. S., LESSELL I. M. Turnover of muscle and liver proteins in mice with hereditary muscular dystrophy. Arch Biochem Biophys. 1962 Jan;96:41–46. doi: 10.1016/0003-9861(62)90447-2. [DOI] [PubMed] [Google Scholar]
- Schirmer R. H., Thuma E. Sensitivity of adenylate kinase isozymes from normal and dystrophic human muscle to sulfhydryl reagents. Biochim Biophys Acta. 1972 Apr 7;268(1):92–97. doi: 10.1016/0005-2744(72)90201-x. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Methods for starch-gel electrophoresis of sarcoplasmic proteins. An investigation of the relative mobilities of the glycolytic enzymes from the muscles of a variety of species. Biochem J. 1968 Mar;107(2):139–150. doi: 10.1042/bj1070139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava U. Biochemical changes in progressive muscular dystrophy. IX. Synthesis of native myosin, actin, and tropomyosin in the skeletal muscle of mouse as a function of muscular dystrophy. Can J Biochem. 1972 Apr;50(4):409–415. doi: 10.1139/o72-055. [DOI] [PubMed] [Google Scholar]
- Srivastava U. Biochemical changes in progressive muscular dystrophy. VII. Studies on the biosynthesis of protein and RNA in various cellular fractions of the muscle of normal and dystrophic mice. Can J Biochem. 1968 Jan;46(1):35–41. doi: 10.1139/o68-006. [DOI] [PubMed] [Google Scholar]
- Watts D. C., Reid J. D. Comparison of the protein-synthesizing machinery in the skeletal muscle of normal and dystrophic Bar Harbor mice. Biochem J. 1969 Nov;115(3):377–382. doi: 10.1042/bj1150377. [DOI] [PMC free article] [PubMed] [Google Scholar]



