Abstract
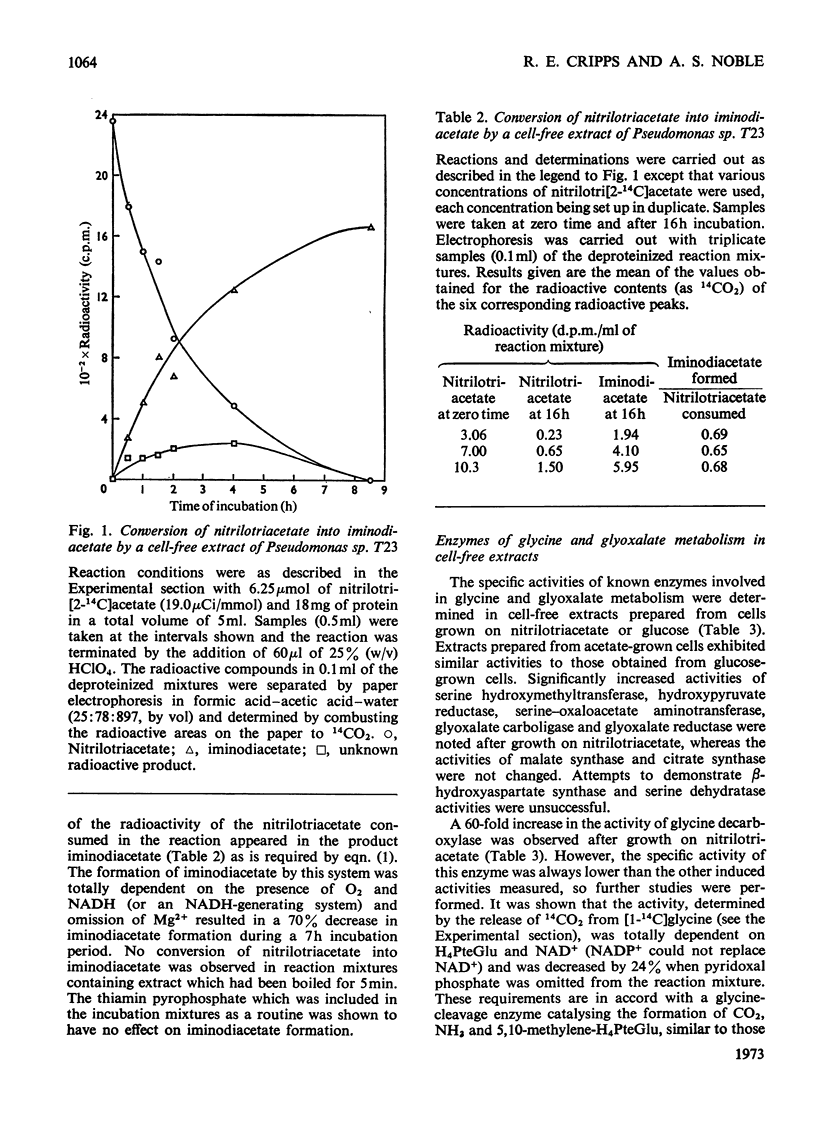
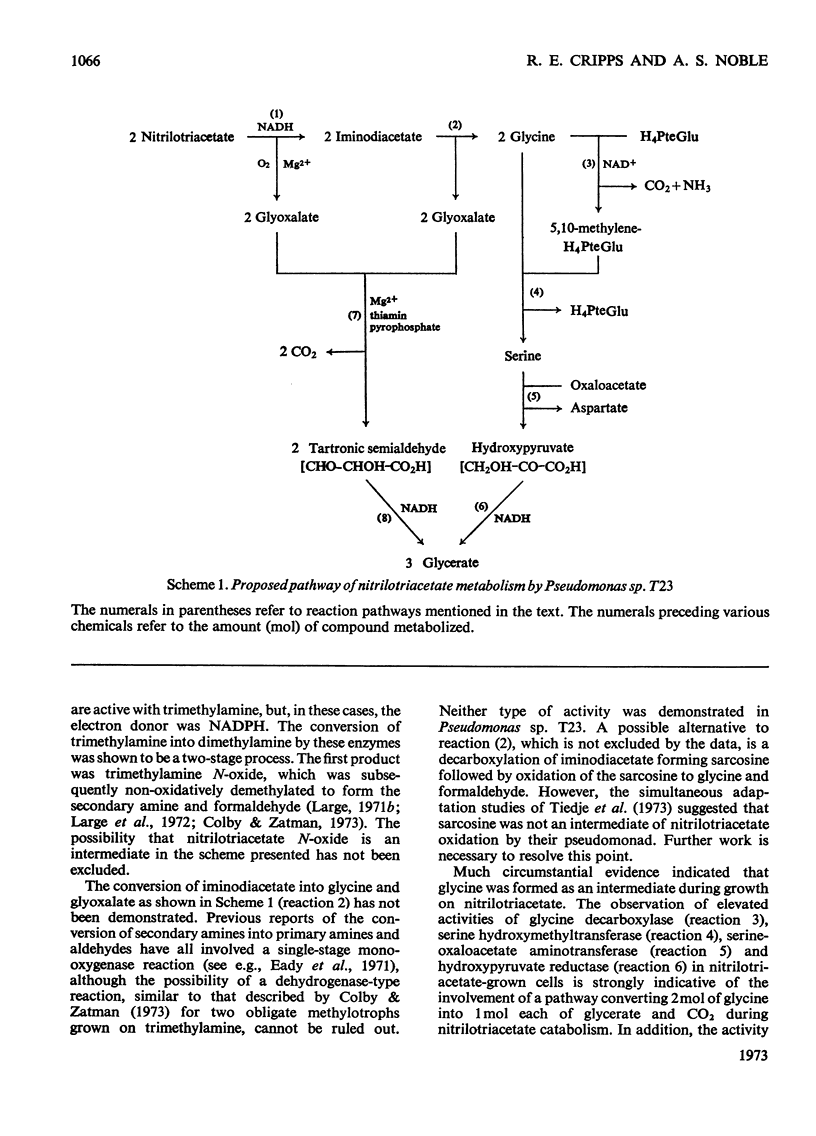
1. An organism that grows on nitrilotriacetate as sole source of carbon and energy was isolated in pure culture and was identified as a pseudomonad. 2. Cell-free extracts of the nitrilotriacetate-grown pseudomonad contain an enzyme that catalyses the NADH-and O2-dependent oxidation of nitrilotriacetate to iminodiacetate and glyoxalate. This enzyme is absent from extracts of glucose-grown cells. 3. Compared with growth on glucose, growth on nitrilotriacetate results in increased activities of enzymes of glycine and serine metabolism, namely serine hydroxymethyltransferase, glycine decarboxylase, serine–oxaloacetate aminotransferase and hydroxypyruvate reductase. 4. Cell-free extracts of the nitrilotriacetate-grown organism contain the enzyme glyoxalate carboligase and, when supplemented with NADH, Mg2+ and thiamin pyrophosphate, can catalyse the anaerobic conversion of glyoxalate into glycerate. 5. These results are incorporated in a scheme which shows the oxidative metabolism of nitrilotriacetate by the successive removal of C2 units to form 2mol of glyoxalate and 1mol of glycine per mol of nitrilotriacetate degraded. The glyoxalate and glycine are then both metabolized to glycerate by separate pathways, via tartronic semialdehyde and serine respectively. The role of this scheme in the growth of the organism on nitrilotriacetate is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAVALLINI D. The coupled oxidation of pyruvate with glutathione and cysteine. Biochem J. 1951 Jun;49(1):1–5. doi: 10.1042/bj0490001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Trimethylamine metabolism in obligate and facultative methylotrophs. Biochem J. 1973 Jan;132(1):101–112. doi: 10.1042/bj1320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps R. E. The microbial metabolism of thiophen-2-carboxylate. Biochem J. 1973 Jun;134(2):353–366. doi: 10.1042/bj1340353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANCIS J., HUTZLER J., LEVITZ M. THIN-LAYER CHROMATOGRAPHY AND SPECTROPHOTOMETRY OF ALPHA-KETOACID HYDRAZONES. Biochim Biophys Acta. 1963 Oct 8;78:85–90. doi: 10.1016/0006-3002(63)91612-3. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Jarman T. R., Large P. J. Microbial oxidation of amines. Partial purification of a mixed-function secondary-amine oxidase system from Pseudomonas aminovorans that contains an enzymically active cytochrome-P-420-type haemoprotein. Biochem J. 1971 Nov;125(2):449–459. doi: 10.1042/bj1250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht D. D., Joseph H. A. Bacterial degradation of nitrilotriacetic acid (NTA). Can J Microbiol. 1971 Dec;17(12):1553–1556. doi: 10.1139/m71-247. [DOI] [PubMed] [Google Scholar]
- Forsberg C., Lindqvist G. On biological degradation of nitrilotriacetate (NTA). Life Sci. 1967 Sep 15;6(18):1961–1962. doi: 10.1016/0024-3205(67)90255-x. [DOI] [PubMed] [Google Scholar]
- GIBBS R. G., MORRIS J. G. ASSAY AND PROPERTIES OF BETA-HYDROXYASPARTATE ALDOLASE FROM MICROCOCCUS DENITRIFICANS. Biochim Biophys Acta. 1964 Jun 1;85:501–503. doi: 10.1016/0926-6569(64)90318-9. [DOI] [PubMed] [Google Scholar]
- Hammond A. L. Phosphate replacements: problems with the washday miracle. Science. 1971 Apr 23;172(3981):361–363. doi: 10.1126/science.172.3981.361. [DOI] [PubMed] [Google Scholar]
- Harder W., Quayle J. R. Aspects of glycine and serine biosynthesis during growth of Pseudomonas AM1 on C compounds. Biochem J. 1971 Mar;121(5):763–769. doi: 10.1042/bj1210763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall J., Quayle J. R. Pathways leading to and from serine during growth of Pseudomonas AM1 on C1 compounds or succinate. Biochem J. 1970 Apr;117(3):563–572. doi: 10.1042/bj1170563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., GOTTO A. M. The metabolism of C2 compounds in micro-organisms. 6. Synthesis of cell constituents from glycollate by Pseudomonas sp. Biochem J. 1961 Jan;78:69–82. doi: 10.1042/bj0780069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., SADLER J. R. The metabolism of C2-compounds in micro-organisms. VIII. A dicarboxylic acid cycle as a route for the oxidation of glycollate by Escherichia coli. Biochem J. 1961 Dec;81:503–513. doi: 10.1042/bj0810503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Reactions of glycine synthesis and glycine cleavage catalyzed by extracts of Arthrobacter globiformis grown on glycine. Arch Biochem Biophys. 1969 Jul;132(2):359–369. doi: 10.1016/0003-9861(69)90377-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J. Non-oxidative demethylation of trimethylamine N-oxide by Pseudomonas aminovorans. FEBS Lett. 1971 Nov 1;18(2):297–300. doi: 10.1016/0014-5793(71)80470-2. [DOI] [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J. The oxidative cleavage of alkyl-nitrogen bonds in micro-organisms. Xenobiotica. 1971 Jul-Oct;1(4):457–467. doi: 10.3109/00498257109041511. [DOI] [PubMed] [Google Scholar]
- McGilvray D., Morris J. G. Utilization of L-threonine by a species of Arthrobacter. A novel catabolic role for "aminoacetone synthase". Biochem J. 1969 May;112(5):657–671. doi: 10.1042/bj1120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I., POTOCHNY M. L. NUTRITIONAL AND REGULATORY ASPECTS OF SERINE METABOLISM IN ESCHERICHIA COLI. J Bacteriol. 1964 Sep;88:611–619. doi: 10.1128/jb.88.3.611-619.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I. THE PATHWAY AND CONTROL OF SERINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Biol Chem. 1963 Dec;238:3934–3944. [PubMed] [Google Scholar]
- SAGERS R. D., GUNSALUS I. C. Intermediatry metabolism of Diplococcus glycinophilus. I. Glycine cleavage and one-carbon interconversions. J Bacteriol. 1961 Apr;81:541–549. doi: 10.1128/jb.81.4.541-549.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Duthie J. R. The biodegradability and treatability of NTA. J Water Pollut Control Fed. 1968 Feb;40(2):306–319. [PubMed] [Google Scholar]
- Tiedje J. M., Mason B. B., Warren C. B., Malec E. J. Metabolism of nitrilotriacetate by cells of Pseudomonas species. Appl Microbiol. 1973 May;25(5):811–818. doi: 10.1128/am.25.5.811-818.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A. The biosynthetic pathway of serine in salmonella typhimurium. Biochim Biophys Acta. 1962 Jul 30;62:193–195. doi: 10.1016/0006-3002(62)90515-2. [DOI] [PubMed] [Google Scholar]
- Warren C. B., Malec E. J. Biodegradation of nitrilotriacetic acid and related imino aand amino acids in river water. Science. 1972 Apr 21;176(4032):277–279. doi: 10.1126/science.176.4032.277. [DOI] [PubMed] [Google Scholar]


