Abstract
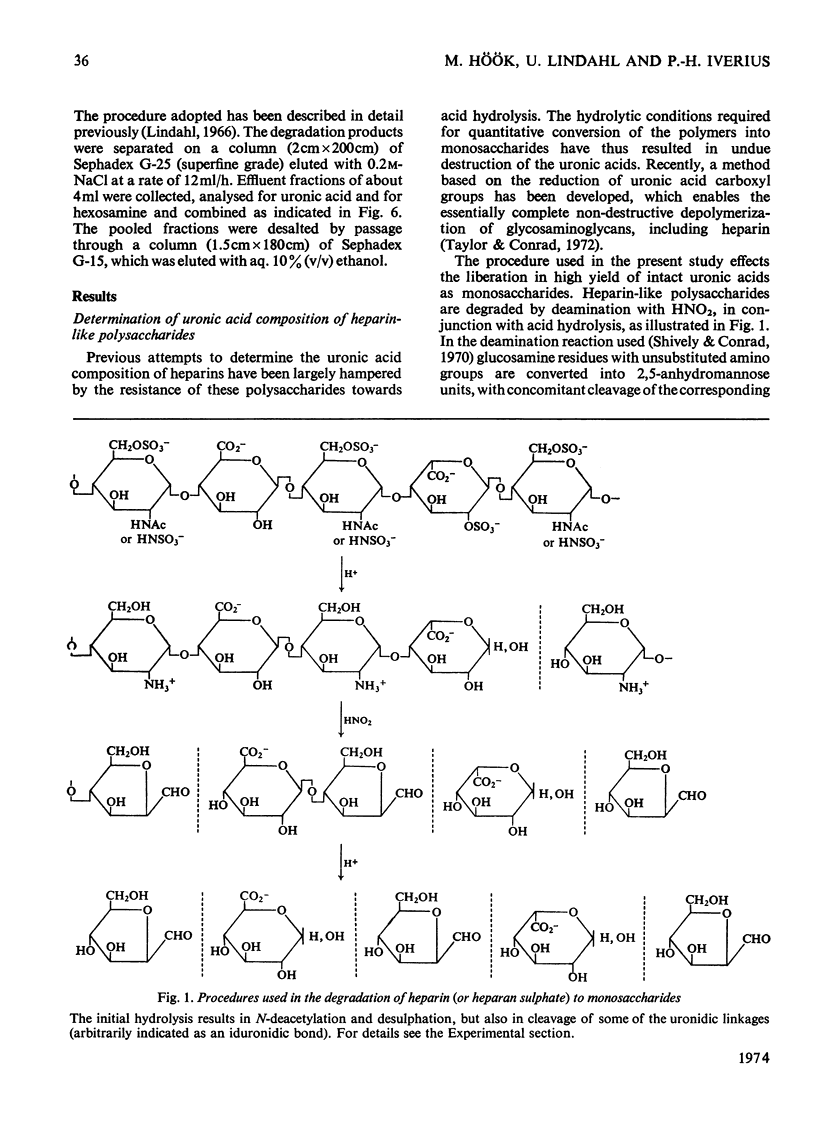
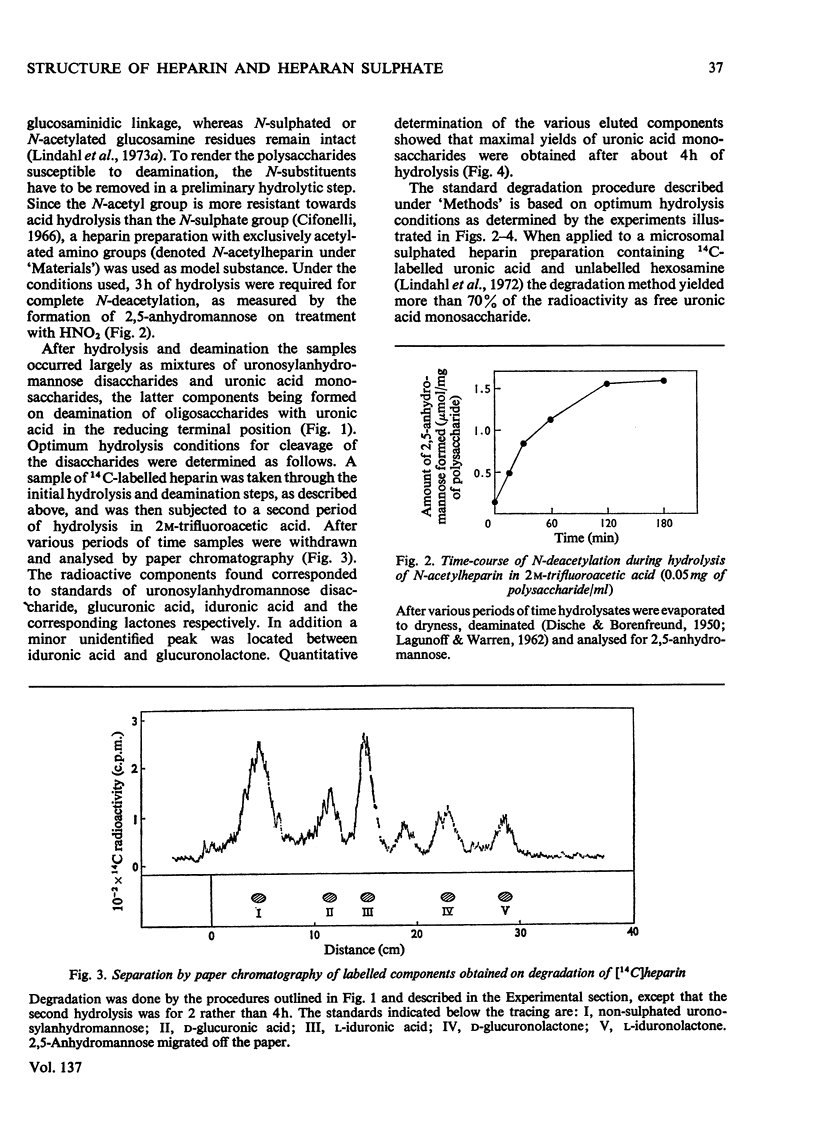
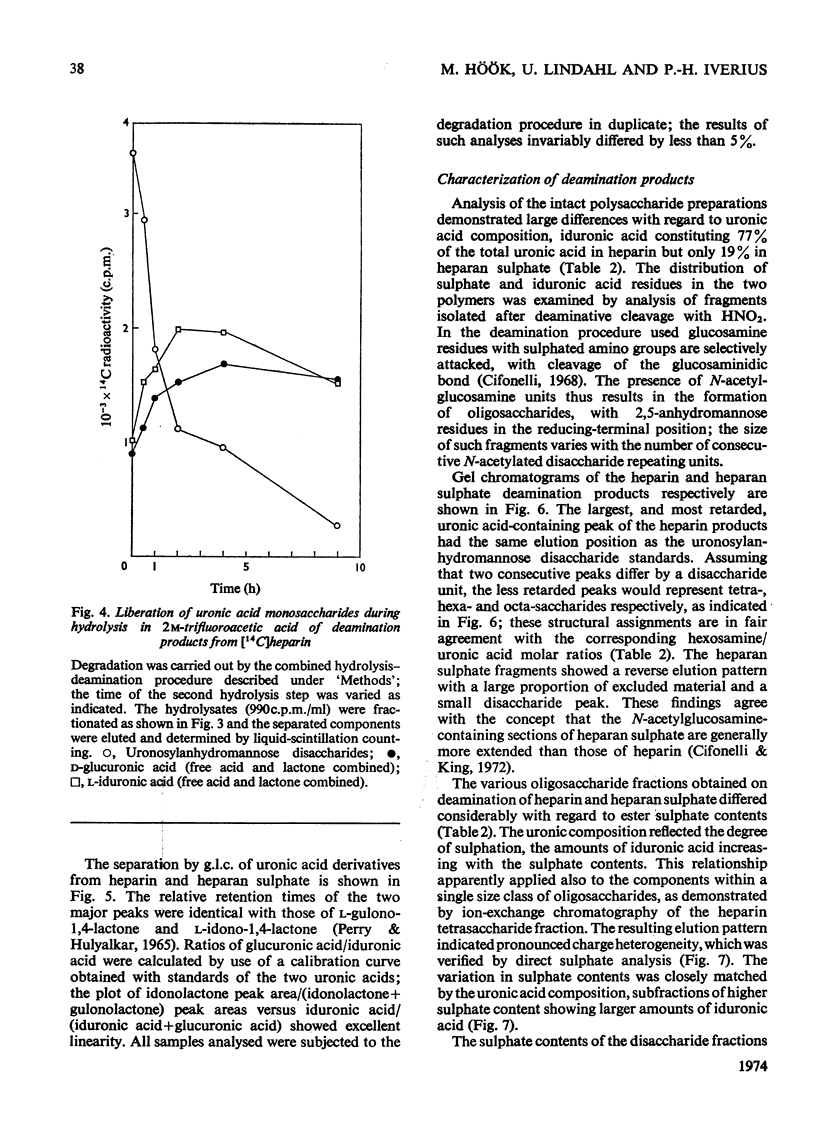
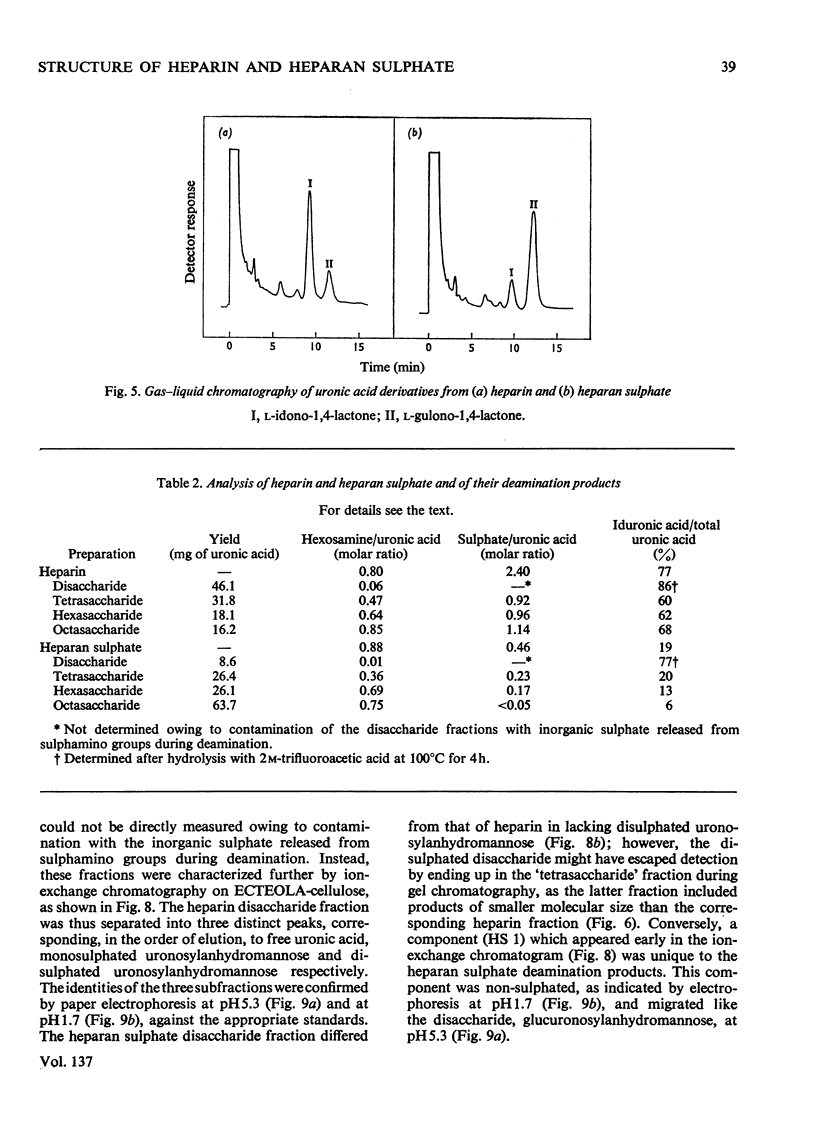
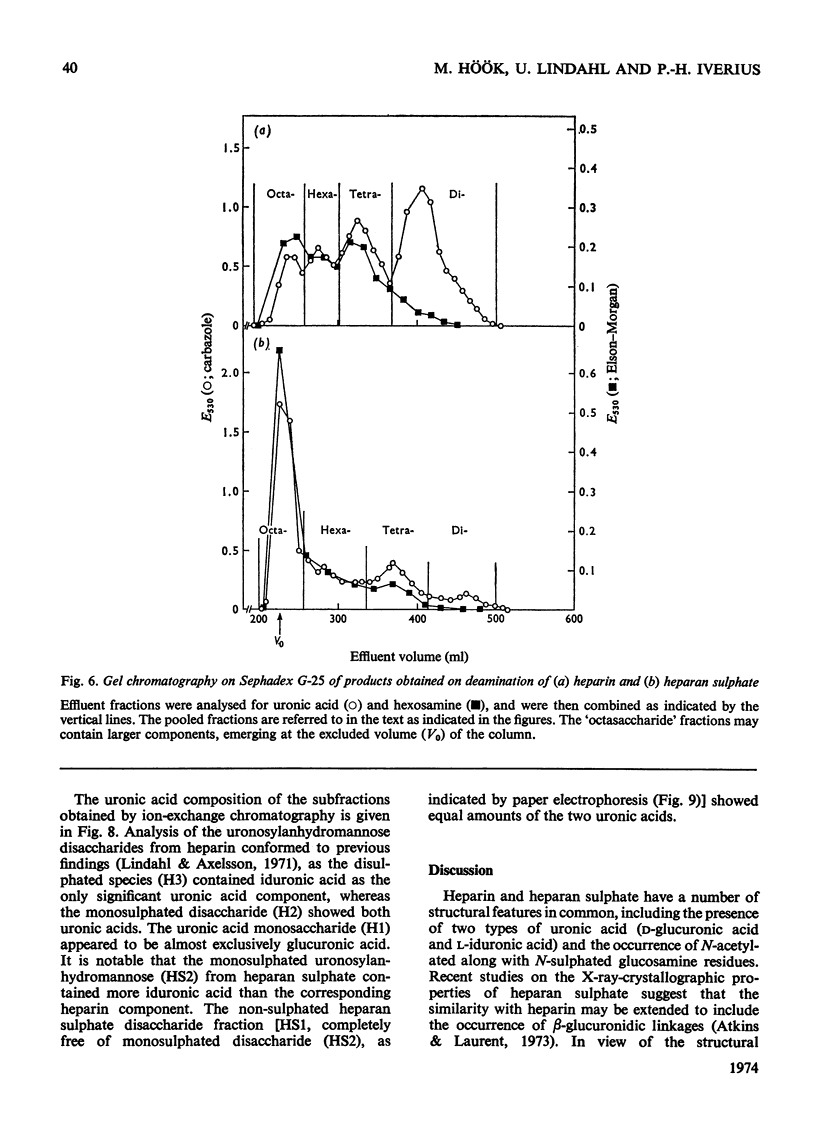
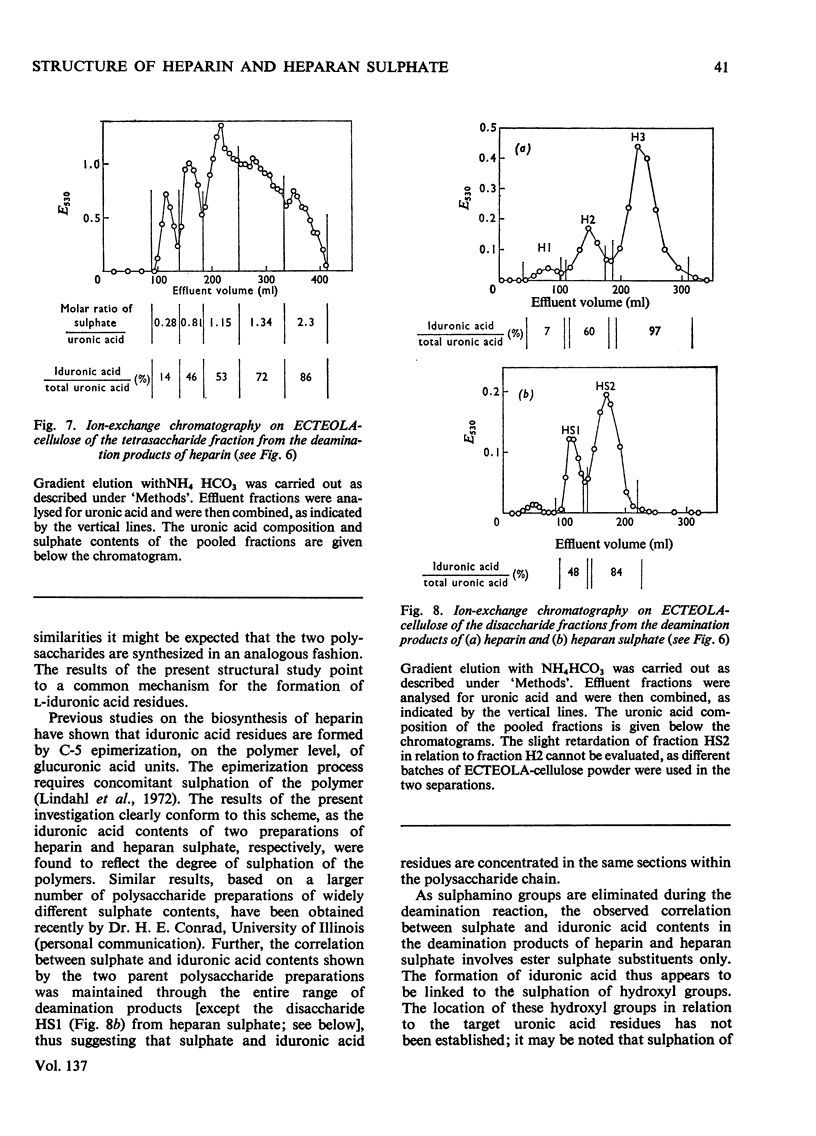
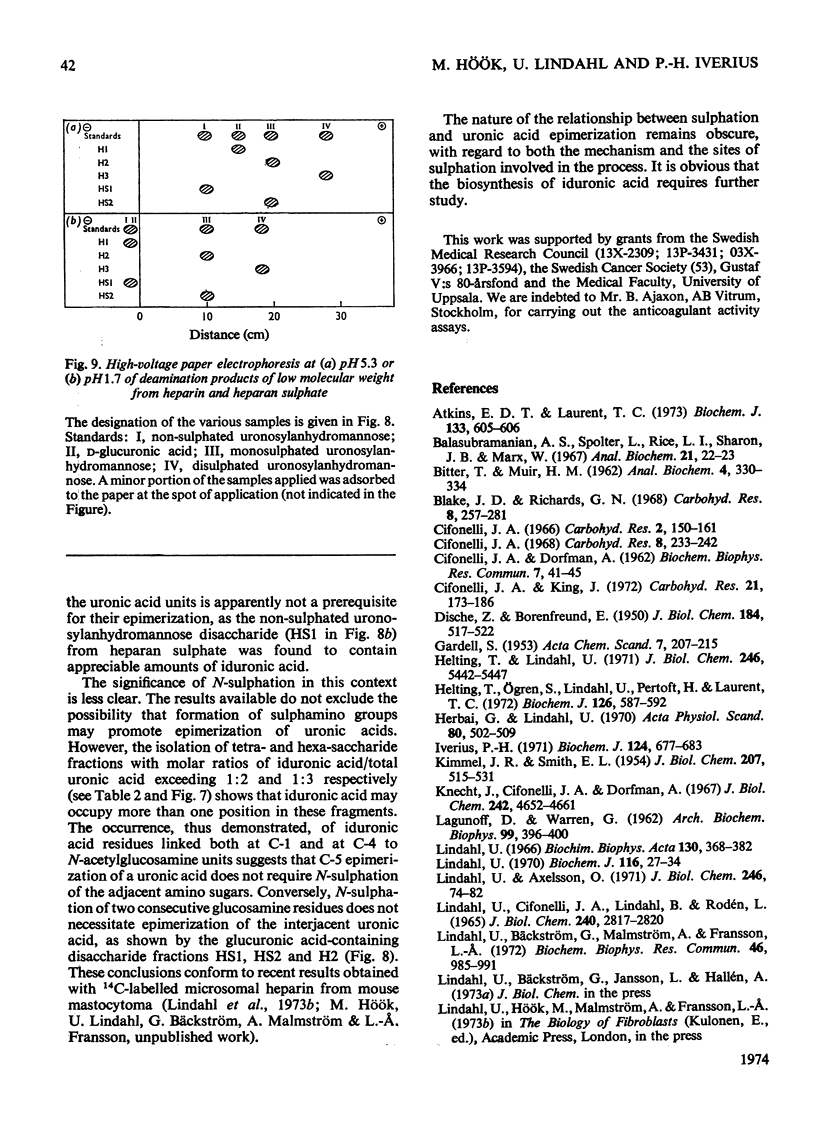
1. A method was developed for determination of the uronic acid composition of heparin-like glycosaminoglycans. Polymers or oligosaccharides are degraded to monosaccharides by a combination of acid hydrolysis and deamination with HNO2. The resulting uronic acid monosaccharides (accounting for about 70% of the uronic acid contents of the starting materials) are isolated and converted into the corresponding aldono-1,4-lactones, which are separated by g.l.c. The calculated ratios of glucuronic acid/iduronic acid are reproducible within 5%. 2. Samples of heparin from pig intestinal mucosa (molar ratio of sulphate/disaccharide unit, 2.40) and heparan sulphate from human aorta (sulphate/disaccharide ratio, 0.46) were subjected to uronic acid analysis. l-Iduronic acid constituted 77% and 19% respectively of the total uronic acid contents. 3. The correlation between the contents of sulphate and iduronic acid indicated by this finding also applied to the fractionated deamination products of the two polymers. The sulphated fragments varied in size from disaccharide to octasaccharide (or larger) and showed sulphate/disaccharide molar ratios in the range of 0.05–2.0. The proportion of iduronic acid increased with increasing ester sulphate contents of the oligosaccharides. 4. Previous studies on the biosynthesis of heparin in a cell-free system have shown that l-iduronic acid residues are formed by C-5 epimerization of d-glucuronic acid units at the polymer level; the process requires concomitant sulphation of the polymer. The results obtained in the present structural study conform to these findings, and suggest further that similar mechanisms may operate in the biosynthesis of heparan sulphate. The epimerization reaction appears to be linked to the sulphation of hydroxyl groups but does not seem to require sulphation of the target uronic acid residues. The significance of sulphamino groups in relation to the formation of iduronic acid is unknown.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins E. D., Laurent T. C. X-ray-diffraction patterns from chondroitin 4-sulphate, dermatan sulphate and heparan sulphate. Biochem J. 1973 Jul;133(3):605–606. doi: 10.1042/bj1330605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Balasubramanian A. S., Spolter L., Rice L. I., Sharon J. B., Marx W. Preparation of 3'-phosphoadenylyl sulfate in substrate quantities using mastocytoma enzymes. Anal Biochem. 1967 Oct;21(1):22–33. doi: 10.1016/0003-2697(67)90078-4. [DOI] [PubMed] [Google Scholar]
- CIFONELLI J. A., DORFMAN A. The uronic acid of heparin. Biochem Biophys Res Commun. 1962 Feb 20;7:41–45. doi: 10.1016/0006-291x(62)90141-9. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem. 1950 Jun;184(2):517–522. [PubMed] [Google Scholar]
- Helting T., Lindahl U. Occurrence and biosynthesis of beta-glucuronidic linkages in heparin. J Biol Chem. 1971 Sep 10;246(17):5442–5447. [PubMed] [Google Scholar]
- Helting T., Ogren S., Lindahl U., Pertoft H., Laurent T. Glycosaminoglycan synthesis in mouse mastocytoma. Biochem J. 1972 Feb;126(3):587–592. doi: 10.1042/bj1260587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbai G., Lindahl U. Regional differences in the incorporation rates of 3H-acetate and 35S-sulfate into chondroitin sulfate of mouse costal cartilage in vitro. Acta Physiol Scand. 1970 Dec;80(4):502–509. doi: 10.1111/j.1748-1716.1970.tb04817.x. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Knecht J., Cifonelli J. A., Dorfman A. Structural studies on heparitin sulfate of normal and Hurler tissues. J Biol Chem. 1967 Oct 25;242(20):4652–4661. [PubMed] [Google Scholar]
- LAGUNOFF D., WARREN G. Determination of 2-deoxy-2-sulfoaminohexose content of mucopolysaccharides. Arch Biochem Biophys. 1962 Dec;99:396–400. doi: 10.1016/0003-9861(62)90285-0. [DOI] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lindahl U. Attempted isolation of a heparin proteoglycan from bovine liver capsule. Biochem J. 1970 Jan;116(1):27–34. doi: 10.1042/bj1160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Axelsson O. Identification of iduronic acid as the major sulfated uronic acid of heparin. J Biol Chem. 1971 Jan 10;246(1):74–82. [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Malmström A., Fransson L. A. Biosynthesis of L-iduronic acid in heparin: epimerization of D-glucuronic acid on the polymer level. Biochem Biophys Res Commun. 1972 Jan 31;46(2):985–991. doi: 10.1016/s0006-291x(72)80238-9. [DOI] [PubMed] [Google Scholar]
- Lindahl U. Further characterization of the heparin-protein linkage region. Biochim Biophys Acta. 1966 Dec 28;130(2):368–382. doi: 10.1016/0304-4165(66)90233-9. [DOI] [PubMed] [Google Scholar]
- Lohmander S. Ion exchange chromatography of glucosamine and galactosamine in microgram amounts with quantitative determination and specific radioactivity assay. Biochim Biophys Acta. 1972 May 16;264(3):411–417. doi: 10.1016/0304-4165(72)90003-7. [DOI] [PubMed] [Google Scholar]
- PERRY M. B., HULYALKAR R. K. THE ANALYSIS OF HEXURONIC ACIDS IN BIOLOGICAL MATERIALS BY GAS-LIQUID PARTITION CHROMATOGRAPHY. Can J Biochem. 1965 May;43:573–584. doi: 10.1139/o65-067. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Stoichiometry of the nitrous acid deaminative cleavage of model amino sugar glycosides and glycosaminoglycuronans. Biochemistry. 1970 Jan 6;9(1):33–43. doi: 10.1021/bi00803a005. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal Biochem. 1968 Dec;26(3):439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- Wessler E. Electrophoresis of acidic glycosaminoglycans in hydrochloric acid: a micro method for sulfate determination. Anal Biochem. 1971 May;41(1):67–69. doi: 10.1016/0003-2697(71)90192-8. [DOI] [PubMed] [Google Scholar]


