Abstract
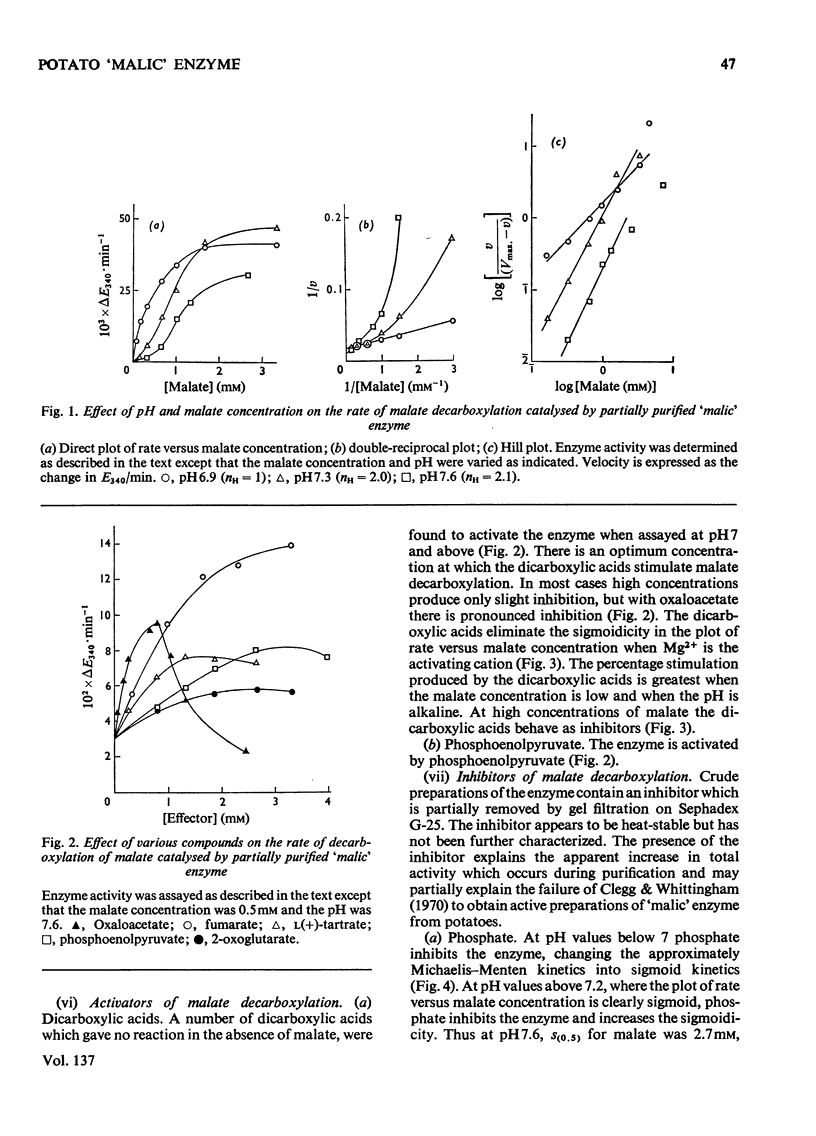
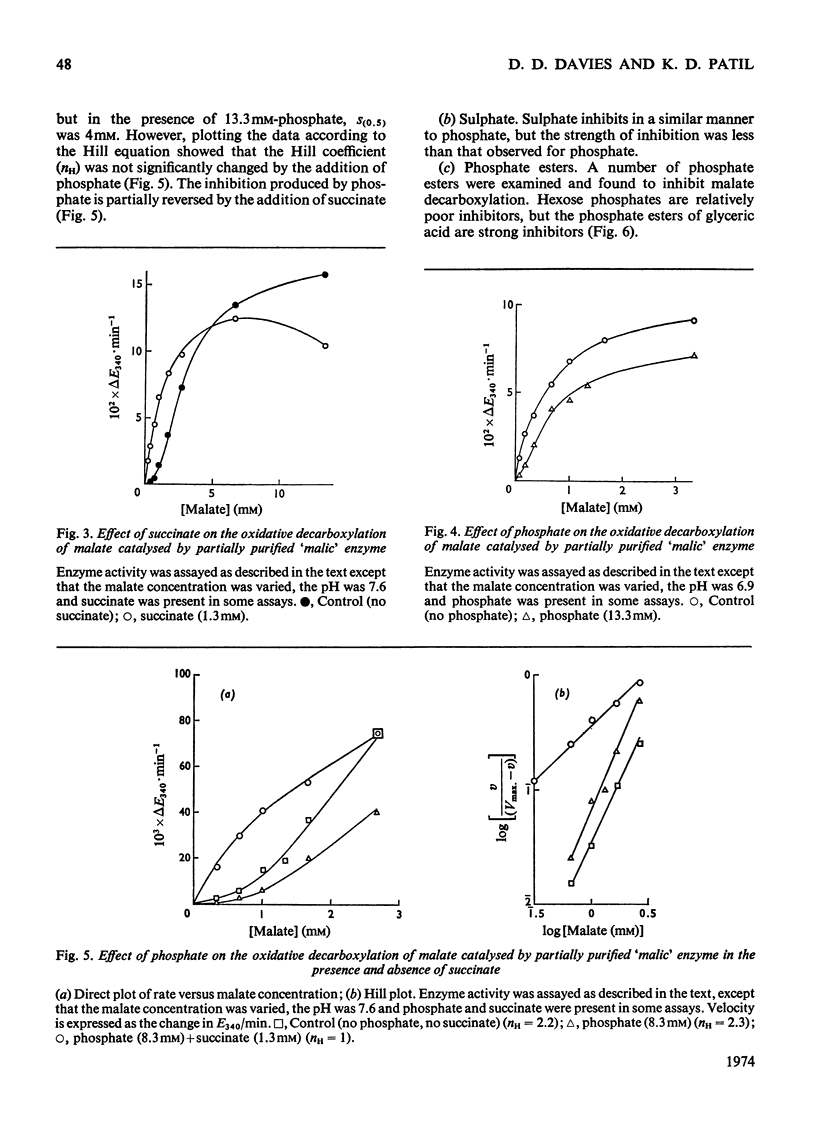
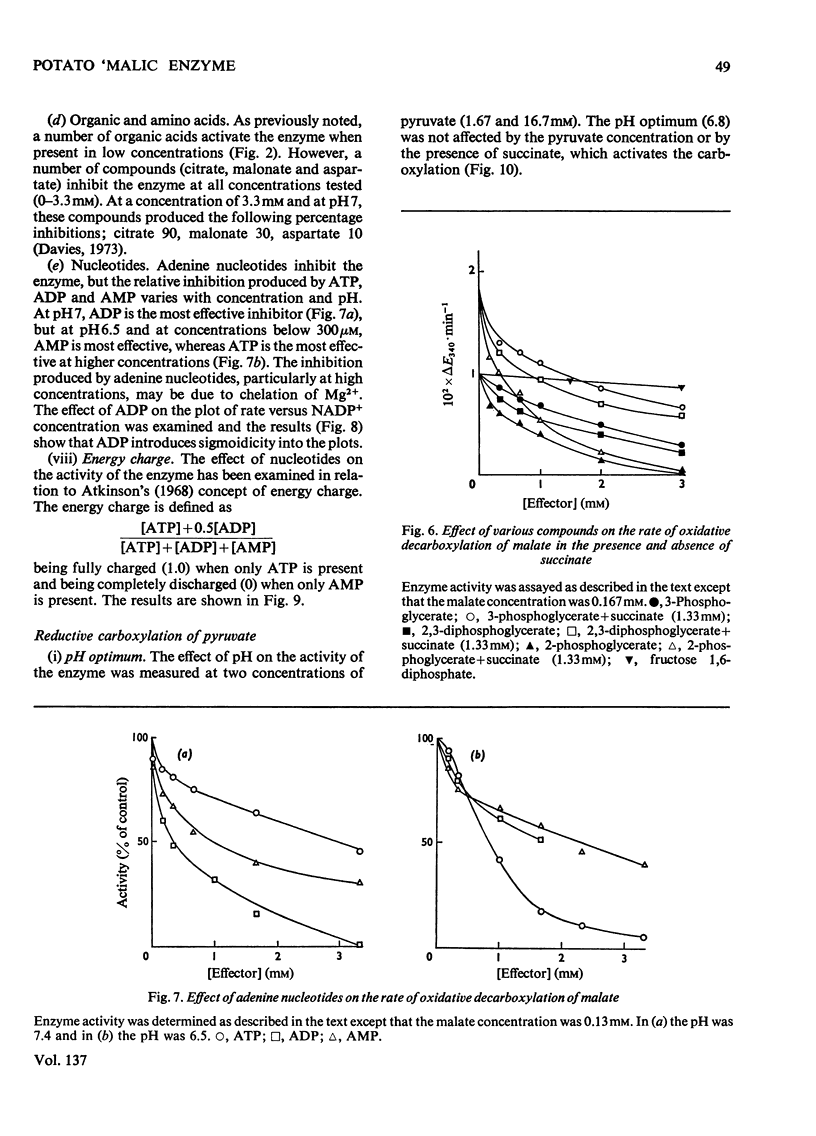
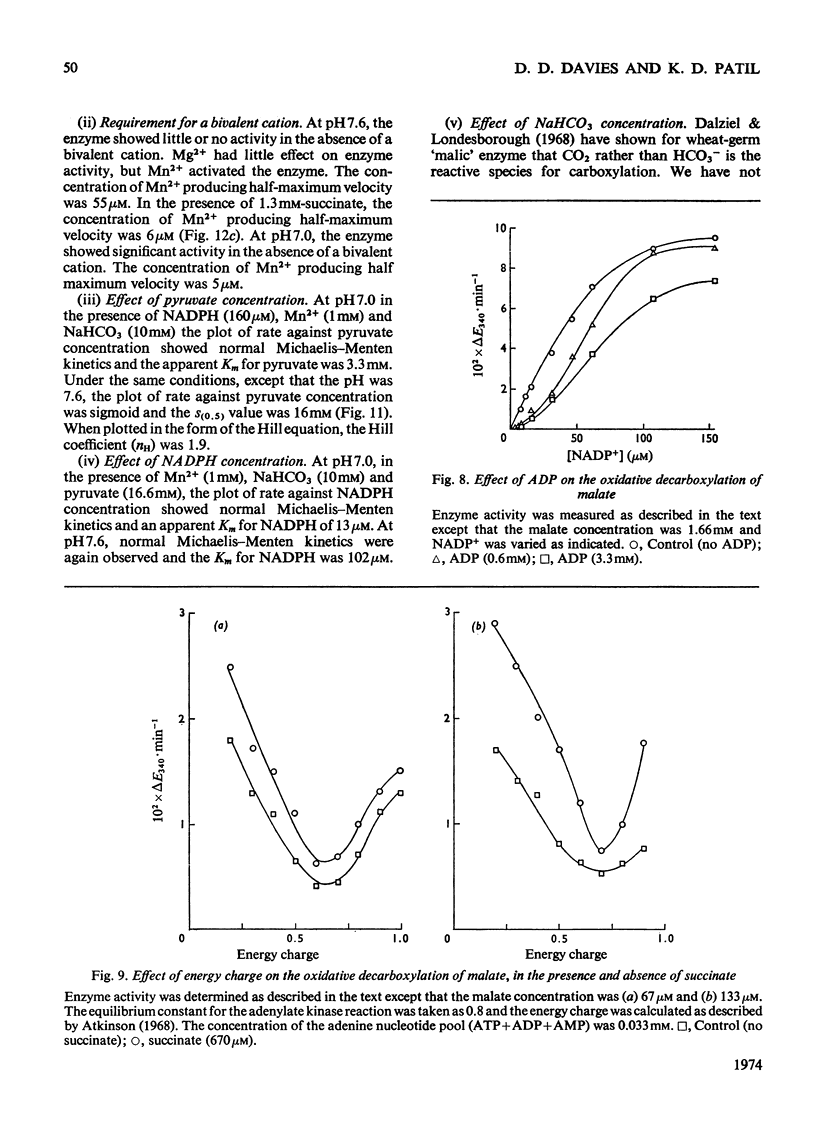
A purification of `malic' enzyme from potato is described. The purified enzyme is specific for NADP and requires a bivalent cation for activity. At pH values below 7 the plot of rate versus malate concentration approximates to normal Michaelis–Menten kinetics. At pH values above 7 the plot of rate versus malate concentration is sigmoid. A number of dicarboxylic acids activate the enzyme and remove the sigmoidicity. The enzyme is inhibited by phosphate, triose phosphates and AMP. In general, effectors of the oxidative decarboxylation of malate behave in the same manner in the reductive carboxylation of pyruvate. The response of the enzyme to energy charge is reported and the physiological significance of the response to metabolites is discussed in relation to the proposed role of the enzyme in the control of pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Bomsel J. L., Pradet A. Study of adenosine 5'-mono-,di- and triphosphates in plant tissues. IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: theoretical and experimental study. Biochim Biophys Acta. 1968 Aug 20;162(2):230–242. doi: 10.1016/0005-2728(68)90105-9. [DOI] [PubMed] [Google Scholar]
- Brandon P. C., van Boekel-Mol T. N. Properties of purified malic enzyme in relation to crassulacean acid metabolism. Eur J Biochem. 1973 May;35(1):62–69. doi: 10.1111/j.1432-1033.1973.tb02810.x. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel K., Londesborough J. C. The mechanisms of reductive carboxylation reactions. Carbon dioxide or bicarbonate as substrate of nicotinamide-adenine dinucleotide phosphate-linked isocitrate dehydrogenase and malic enzyme. Biochem J. 1968 Nov;110(2):223–230. doi: 10.1042/bj1100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D. Control of and by pH. Symp Soc Exp Biol. 1973;27:513–529. [PubMed] [Google Scholar]
- Davies D. D., Davies S. Purification and properties of L(+)-lactate dehydrogenase from potato tubers. Biochem J. 1972 Oct;129(4):831–839. doi: 10.1042/bj1290831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley D. R. Purification and properties of apple fruit malic enzyme. Plant Physiol. 1966 Feb;41(2):214–220. doi: 10.1104/pp.41.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HARARY I., KOREY S. R., OCHOA S. Biosynthesis of dicarboxylic acids by carbon dioxide fixation. VII. Equilibrium of malic enzyme reaction. J Biol Chem. 1953 Aug;203(2):595–604. [PubMed] [Google Scholar]
- Haglund H. Isoelectric focusing in pH gradients--a technique for fractionation and characterization of ampholytes. Methods Biochem Anal. 1971;19:1–104. doi: 10.1002/9780470110386.ch1. [DOI] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Pacold I., Anderson L. E. Energy charge control of the Calvin cycle enzyme 3-phosphoglyceric acid kinase. Biochem Biophys Res Commun. 1973 Mar 5;51(1):139–143. doi: 10.1016/0006-291x(73)90519-6. [DOI] [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J. Additional factors influencing enzyme responses to the adenylate energy charge. J Biol Chem. 1973 Jan 25;248(2):461–466. [PubMed] [Google Scholar]
- WALKER D. A. Physiological studies on acid metabolism. 7. Malic enzyme from Kalanchoe crenata: effects of carbon dioxide concentration. Biochem J. 1960 Feb;74:216–223. doi: 10.1042/bj0740216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman G. S. Influence of ammonium and nitrate nutrition on the pyridine and adenine nucleotides of soybean and sunflower. Plant Physiol. 1972 Feb;49(2):142–145. doi: 10.1104/pp.49.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. F., Davies D. D. Regulation of phosphoenolpyruvate carboxylase of Zea mays by metabolites. Biochem J. 1973 Mar;131(3):451–458. doi: 10.1042/bj1310451. [DOI] [PMC free article] [PubMed] [Google Scholar]


