Abstract
Medicinal plants, also known as herbs, have been discovered and utilized in traditional medical practice since prehistoric times. Medicinal plants have been proven rich in thousands of natural products that hold great potential for the development of new drugs. Previously, we reviewed the types of Chinese traditional medicines that a Tang Dynasty monk Jianzhen (Japanese: Ganjin) brought to Japan from China in 742. This article aims to review the origin of Kampo (Japanese traditional medicine), and to present the overview of neurodegenerative diseases and retinitis pigmentosa as well as medicinal plants in some depth. Through the study of medical history of the origin of Kampo, we found that herbs medicines contain many neuroprotective ingredients. It provides us a new perspective on extracting neuroprotective components from herbs medicines to treat neurodegenerative diseases. Retinitis pigmentosa (one of the ophthalmic neurodegenerative diseases) is an incurable blinding disease and has become a popular research direction in global ophthalmology. To date, treatments for retinitis pigmentosa are very limited worldwide. Therefore, we intend to integrate the knowledge and skills from different disciplines, such as medical science, pharmaceutical science and plant science, to take a new therapeutic approach to treat neurodegenerative diseases. In the future, we will use specific active ingredients extracted from medicinal plants to treat retinitis pigmentosa. By exploring the potent bioactive ingredients present in medicinal plants, a valuable opportunity will be offered to uncover novel approaches for the development of drugs which target for retinitis pigmentosa.
Keywords: retinitis pigmentosa, ophthalmology, botany, pharmacology, medical history, compound, drug discovery, degenerative diseases
1. Introduction
Plants have long served as the primary source of medicinal compounds since the advent of humanity. Plants harbor a vast array of compounds, and these various compounds have tremendous potential in the development of future medicines. Here, we explore the realm of herbal medicine and plant science in relation to retinal neurodegenerative diseases. There has been limited research focused on extracting active ingredients from plants specifically for the treatment of retinitis pigmentosa. Therefore, a multidisciplinary approach is necessary to uncover effective solutions for these conditions. Collaborations across various disciplines such as plant science (1, 2), pharmaceutical science (3, 4), and medical science (5–10) can yield synergistic outcomes and contribute to the development of more sophisticated treatments for retinitis pigmentosa.
2. Overview of the neurodegenerative diseases
Neurodegenerative diseases (NDDs) are characterized by the gradual loss of neurons and/or their myelin sheath, leading to functional deterioration over time. Within the field of neurology, prominent neurodegenerative diseases include Alzheimer disease (AD), Parkinson disease (PD), amyotrophic lateral sclerosis (ALS), and more. Similarly, in the realm of ophthalmology, neurodegenerative diseases encompass retinitis pigmentosa, age-related macular degeneration, glaucoma, and other related conditions.
Neurodegenerative diseases are driven by various shared pathogenic mechanisms, including: (1) abnormal protein dynamics characterized by protein misfolding and aggregation; (2) oxidative stress resulting from the formation of reactive oxygen species and free radicals; (3) dysfunction of neurotrophic factors; (4) mitochondrial dysfunction; (5) neuroimmune inflammation; (6) failure of neuronal Golgi apparatus; (7) disruption of cell/axonal transport; and (8) altered cell signaling. The convergence of these diverse pathogenic factors ultimately leads to multifaceted neuronal cell death (11–14). Effective therapeutic strategies aim to tackle these pathogenic mechanisms to halt or delay disease progression.
Developing therapeutic strategies for neurodegenerative diseases has profoundly drawn much attention from the public and medical field. On December 23, 2021, President Biden signed the Accelerated Access to Critical Treatments (ACT) for amyotrophic lateral sclerosis (ALS) Act (ACT for ALS) into law as Public Law 117–79. This legislation mandates the Department of Health and Human Services (HHS) to collaborate with the Food and Drug Administration (FDA) and the National Institutes of Health (NIH) in establishing public-private partnerships focused on rare neurodegenerative diseases. These partnerships will be facilitated through collaborative agreements or contracts, with the goal of enhancing our understanding of these diseases and expediting the development of treatments for ALS and other related conditions (whitehouse.org). The FDA has devised a comprehensive five-year plan to promote innovation, has streamlined processes, and accelerated the development of drugs specifically tailored for treating rare neurodegenerative diseases, including ALS (fda.org). Consequently, scientists around the world are actively engaged in research, development, and advancement of drugs aimed at addressing the treatment needs of individuals with rare neurodegenerative diseases, steering us toward a brighter future in this field.
3. Overview of the origin of Kampo (Japanese traditional medicine)
Monk Jianzhen, also known as Ganjin in Japanese, is renowned in Japan for his teachings on Buddhist precepts (Vinaya). Additionally, he played a crucial role in introducing Chinese herbal medicines to Japan. He not only cultivated medicinal plants within the country but also instructed his disciples on the identification and prescription of medicinal materials. Unfortunately, he lost his eyesight upon arriving in Nara, Japan (15). This event can be seen as the planting of a seed in the land, which subsequently led to numerous developments, including the field of pharmacognosy, the formulation of various Kampo medicines, and the treatment of countless people.
Jianzhen had five unsuccessful attempts to travel to Japan which finally led to achieving success on his sixth try. He lost his eyesight, when he arrived in Japan. During that era, these was no advanced medical technology for diagnosing the cause of blindness. Numerous sight-threatening conditions, such as cataract, glaucoma, age-related macular degeneration (AMD), diabetic retinopathy, as well as ophthalmological neurodegenerative disorders like retinitis pigmentosa (RP), remained undiagnosed and untreated. In contrast, contemporary medical advancements enable the diagnosis of these eye diseases and provide treatments to improve vision. Among the prominent areas of research in ophthalmology, retinitis pigmentosa stands out. Regrettably, most patients with this disease have no choice but to endure progressive vision loss.
4. Overview of the retinitis pigmentosa
Inherited retinal diseases (IRDs) are a group of inherited eye disorders that alter the structure and function of the retina, leading to vision loss and sometimes blindness. Eye is highly compartmentalized and shows the immune privilege during retinal degeneration (16, 17) which becomes an ideal tissue to evaluate genetic and pharmacological therapies. There are many types of IRDs, such as Retinitis pigmentosa (RP), Rod dystrophy or rod-cone dystrophy, Usher syndrome (USH), Bietti crystalline dystrophy (BCD), Alport syndrome, Leber congenital amaurosis (LCA) or early onset retinal dystrophy (EORD), Cone dystrophy, Cone-rod dystrophy (CORD), Macula dystrophy, Stargardt’s disease, Best disease, X-linked retinoschisis (XLRS). Most of IRDs affect the photoreceptor cells, which reduces or prevents the retina’s response to light and causes vision loss. Photoreceptors are light-sensing neurons that capture and convert light photons to electrical signals at the specialized primary cilia called outer segments (18). Two classes of photoreceptors, rods and cones, are found in the outer nuclear layer (ONL) of the retina that is located at the back of an eye. Rods are more sensitive in the condition of dim light, whereas cones are more sensitive in daylight and responsible for color vision (19, 20). In addition, cones are concentrated in the central area (i.e., macula) of a human retina (21). Cones localize in the center of the retina at the fovea. There are approximately 6 million cones and more than 100 million rods in a human retina (22). The most common IRD is retinitis pigmentosa (RP). RP is characterized by a progressive loss of rods followed by the concomitant loss of cones (23). The disease syndrome is commonly exemplified by night blindness or nyctalopia at early stages, accompanied by the deterioration of abnormal rod-driven electroretinogram. As RP progresses to later stages, gradual ocular fundus changes become noticeable, primarily encompassing a triad of optic disk pallor, attenuation of retinal blood vessels, and the dispersion of bone spicule-like pigments (24). Extensive loss of photoreceptors eventually leads to tunnel vision or blindness. RP inheritance can be autosomal dominant, autosomal recessive, or X-linked recessive (23). The advancement of genetic testing has identified the genetic causes of IRDs for approximately two thirds of associated patients (25, 26), including cases of RP. RP-associated genes include rod-function genes such as RHO, ABCA4, CNGA1, and CNGB1, and rod-specific transcription factor genes NRL and NR2E3 (27–29) (retnet.org).
RP is currently uncurable for the majority of patients. Gene therapy, particularly gene augmentation/replacement, yields a new hope for these patients. LUXTURNA (voretigene neparvovec-rzyl) is a prescription gene therapy product used for the treatment of patients with recessive mutations in RPE65 gene (30). Many clinical trials are testing AAV-mediated gene therapies for various forms of RP (31, 32). Unfortunately, gene therapy does not cover all forms and onsets of RPs. Some of the advantages of herbal medicines and extracted compounds in treatment for IRDs include: (1) Genetic testing cannot detect all RP-associated genes or compound heterozygosity. Hence, gene therapy may not be applicable to patients with unknown genetic causes. These patients would opt for other forms of medication to delay the disease progression. Pharmacological interventions are gaining great popularity for early-stage RP (33–35). Many preclinical tests involve the therapeutic applications of antioxidants and other natural products to RP-related animal and/cell models (details are discussed in the following section). (2) Gene therapy usually targets early-onset IRDs. There is no clinical trial of genetic interventions for mid- and late-onset RP. In addition, the clinical diagnosis of mid- and late-onset neurodegenerative diseases often suffers from the low specificity of individual assessment methods and biomarkers (36). Hence, antioxidants provide the conservative treatment to patients with mid- and late-onset RP. (3) Different forms of RP demonstrate variable onsets and patterns of disease progression (37, 38). The accuracy of forecasts for the disease progression is unavailable. The herbal medicines would prolong the window of opportunity for symptom monitoring and further interventions. (4) The herbal medicines would serve as a general therapeutic strategy, regardless of the specific mutations, aging conditions, and spatial patterns. (5) The herbal medicines would become a valuable second-line therapy to enhance the efficacy of gene therapy, chemical intervention (39–42) and cell transplantation (43).
5. Overview of the medicinal plant
5.1. The history of medicinal plant use
The history of medicinal plants dates back thousands of years, with various archeological discoveries attesting to their early use. For instance, a cave in South Africa revealed 77,000-year-old beds constructed from the anti-mosquito plant Cryptocarya woodii (44). Dental calculus from Neanderthals dating back 49,000 years (during the Paleolithic Age) contained residues of Asteraceae plants such as Matricaria chamomilla (chamomile) and Achillea millefolium (common yarrow) (45), that have functions of alleviating toothache. In Xiaoshan, Hangzhou, Zhejiang, an 8,000-year-old tea tree seed was found at the Kuahuqiao site (46), alongside pottery cauldron cooking utensils containing plant residues associated with herbal medicine. Archeologists also discovered a range of medicinal plants, including Ziziphus jujube, Gorgon fruit, and water chestnut, in the ruins of the 5,000-year-old Liangzhu ancient city (47).
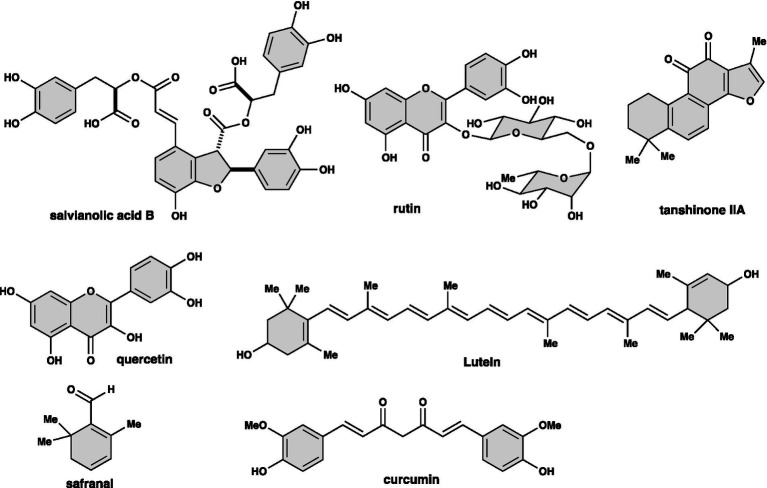
Traditional Chinese medicine (TCM) holds a prominent position with its long-standing history of thousands of years, serving as a global representative of traditional medicine. In the quest for disease treatment and health maintenance, more and more people have turned to natural medicines and green plants (Figure 1). Traditional medicine practices are often passed down through generations orally. Jianzhen’s prescription, for example, has been verbally transmitted for over 1,000 years and has now been compiled into a book by Lei Yutian, a 52nd generation inheritor, making it available to the public (15).
Figure 1.
Structures of the main component of some Chinese herbal medicines.
China, specifically, boasts numerous traditional Chinese medicine formulas that demonstrate potent therapeutic effects, although their standardization still requires international recognition. Such traditional medicinal practices can be found worldwide and harbor significant therapeutic potential. However, many of these herbal medicines are at risk of disappearing due to insufficient documentation. Establishing relevant regulations and policies for their protection would elevate the standards of traditional herbal medicines to international levels, ultimately benefiting a greater number of patients.
In TCM, various parts of natural plants, such as roots, stems, and leaves, are utilized as medicinal materials (Figure 1). Each Chinese medicine recipe contains hundreds of known and unknown chemical compounds. Presently, China recognizes over 10,000 types of traditional Chinese medicines, serving as a vast reservoir of organic compounds waiting to be explored. From these organic precursor compounds, diverse drugs for numerous diseases can be developed in the future.
Japan, with its distinct approach, approves Kampo medicines based on governmental assessments of their safety, effectiveness, quality, and manufacturing control. These medicines are prescribed alongside Western medicines under national health insurance and are also available as over-the-counter drugs in pharmacies. The materials used in Kampo medicines are derived from medicinal plants (Figure 2). The plant names were checked with http://mpns.kew.org. It has been discovered through recent research that the origin of the structured and systematic prescriptions in Japanese Kampo medicine can be attributed to Jianzhen, who arrived in Japan during the Nara period in the eighth century. Some herbal medicines brought by Jianzhen are preserved in the Shoso-in of Todaiji Temple, and certain prescriptions are included in the oldest medical book, “Ishin-ho,” compiled by Tanba Yasuyori in 984, albeit with limited details available.
Figure 2.
Medicinal plants for Kampo Medicines in Medical Botanical Garden (Okayama University, Japan). (A) Scutellaria baicalensis Georgi, (B) Ocimum basilicum L., (C) Typha orientalis C.Presl, Nymphaea tetragona georgi, (D) Catharanthus roseus (L.) G.Don, (E) Ephedra intermedia Schrenk & C.A.Mey., (F) Bupleurum chinense DC., (G) Stemona sessilifolia (Miq.) Miq., (H) Lagerstroemia indica L., (I) Agrimonia pilosa Ledeb., (J) Senna obtusifolia (L.) H.S.Irwin & Barneby, (K) Ficus erecta Thunb., (L) Fagopyrum cymosum (Trevir.) Meisn., (M) Isodon japonicus (Burm.f.) H.Hara, (N) Ziziphus jujuba Mill. (O) Chamaecrista nomame (Makino) H.Ohashi.
5.2. Current market value of medicinal plant compounds
Medicinal plant compounds have a long history, and many early medicines were derived from natural metabolites found in plants. Prominent examples of plant-derived medications include aspirin, quinine, and digoxin. Plants contain a vast array of compounds, including secondary metabolites, which can be broadly categorized into three groups: phenolics, terpenoids, and alkaloids (48). Numerous natural compounds from these plants which now serve as active ingredients in many modern pharmaceuticals. The global pharmaceutical market, valued at around US$1.1 trillion annually, relies on the drugs derived from natural products. Among these sources, plants contribute 25%, microorganisms contribute 13%, and animals contribute 3% (49). The utilization of natural products offers several advantages compared to other sources. These compounds exhibit chemical novelties that can serve as starting points for developing potential drug candidates targeting complex diseases. Moreover, naturally derived ingredients possess chemical diversity, intricate bi- and tri-dimensional structures, and can be efficiently absorbed and metabolized within the body (50). Medicinal plant compounds encompass a variety of active ingredients and secondary metabolites that demonstrate favorable properties, including anti-inflammatory, anti-bacterial, antiviral, anti-cancer, antioxidant, and anti-apoptotic effects. In this article, we provide a review of select medicinal plant components employed in the treatment of retinitis pigmentosa, and we anticipate future research directions and advancements in the application of medicinal plants within this field. The significant role of medicinal plants as invaluable resources for the development of new drugs within the global pharmaceutical industry cannot be overstated (Table 1).
Table 1.
Representative herbal medicines for the treatment of Retinitis Pigmentosa.
| No. | Chinese herbal medicines | Extraction source | Pharmacological effects in the treatment of retinitis pigmentosa | Animal model of retinitis pigmentosa |
|---|---|---|---|---|
| 1 | Lycium barbarum polysaccharide (LBP) | Goji berry fruit (Lycium barbarum L.) | Antioxidant effect | rd1 |
| 2 | Salvianolic acid B | The root and rhizome of Danshen (Salvia miltiorrhiza Bunge) | Antioxidant effect | rd10 |
| 3 | Tanshinone IIA | The root of Danshen (Salvia miltiorrhiza Bunge) | Antioxidant effect | rd10 |
| 4 | Rutin | Citrus fruits, buckwheat, asparagus, and apples, etc. | Antioxidant effect | rd10 |
| 5 | Quercetin | Peppers, onions, berries, broccoli and red apples, etc. | Antioxidant effect | rd10 |
| 6 | Lutein | Spinach, kale, and broccoli, etc. | Antioxidant effect | rd10 |
| 7 | Safranal | Stigmas of saffron (Crocus sativus L.) | Antioxidant effect, antiapoptotic effect | P23H |
| 8 | Curcumin | Turmeric ginger (Zingiber officinale Roscoe) | Antioxidant effect | P23H |
6. Phytogenic compounds currently in development to treat retinitis pigmentosa
Herbal medicines currently being developed for the treatment of retinitis pigmentosa include Lycium barbarum polysaccharide, salvianolic acid B, tanshinone IIA, rutin, quercetin, lutein, Safranal, curcumin, etc. (Figure 1), which mainly protect retinal nerve cell damage through the antioxidant properties of herbal medicine. Numerous clinical and epidemiological studies have demonstrated the beneficial effects of these plant-derived compounds on ocular diseases (51). More importantly, these compounds have been tested in animal models of retinitis pigmentosa (details are listed below).
Major animal models of retinitis pigmentosa are described as follows. (1) Mice homozygous for the retinal degeneration 1 (rd1) mutation have an early-onset severe rod degeneration mainly due to a nonsense mutation in exon 7 of the Pde6b gene (52, 53) which causes undetectable PDE6B protein. At postnatal day (P) 10, the rod outer segment shows signs of disruption, the apoptosis of photoreceptor cells increases with a rapid loss of rods by P14 (age of eye opening). Rod degeneration happens in prior to cone degeneration in all regions of the retina. By P21, less than 2% of rods can be found in rd1/rd1 mice but more than half of cones are still present (54). Expression of rod-enriched genes is downregulated in rd1/rd1 mice (55, 56). (2) The retinal degeneration 10 (rd10) mouse carries a missense mutation (R560C) in exon 13 of the Pde6b gene (57), causing a reduced PDE6B protein expression. Photoreceptor loss occurs from the central retina at P16 and spreads to the peripheral retina around P20 in homozygous mutants, for which the peak of apoptosis happens between P18 and P25. ONL is fully degenerated by P60 (58). (3) Autosomal dominant RhoP23H is the most frequent RP mutation (59). Rod outer segments appear shorter in RhoP23H/+ mouse retinas at P35, and about 50% ONL neurons are lost in mutants at P63 (60). The pathogenic mechanism indicates that a misfolded monomer of P23H opsin induces aggregation of mutant rhodopsin protein with WT counterpart and prevents the formation of rod outer segment.
Lycium barbarum polysaccharide (LBP). LBPs are a group of water-soluble glycoconjugates and can be extracted from wolfberry or goji berry (Lycium barbarum L.) (61, 62). Goji berry is a common herb of traditional Chinese medicine (63). Regular treatment of LBPs benefited the neuronal survival in studies of animal models (64–67). In particular of retinopathies, LBPs could preserve neurons (ex: rod bipolar cells and amacrine cells) of inner nuclear layer in the model of retinal ischaemia (68), and protect retinal ganglion cells from CoCl2-induced apoptosis (69). Notably, LBPs delayed the ensuing degeneration of retinal ganglion cells and cone photoreceptors in rd1 mice (70), which matched results of a placebo-controlled intervention trial with 12-month LBP oral administration (71). Since the therapeutic window for rods appeared longer in rd10 mice, LBP treatment reduced photoreceptor apoptosis via inhibition of through inhibition of NF-κB and HIF-1α pathways and improved scotopic and photopic electroretinogram responses. In addition, LBP treatment could inhibit the activation of microglia in rd10 retinas (72). However, polysaccharides have limited solubility in conventional solvent system, leading to difficulties in effective extraction and co-delivery with other compounds (73).
Salvianolic acid B (Sal B). Sal B is a 1-benzofuran derived from the root and rhizome of the plant species Danshen (Salvia miltiorrhiza Bunge), which has been commonly used in traditional Chinese medicine for various therapeutic purposes (74, 75). Sal B is well known for its anti-apoptotic and anti-inflammatory properties in treating neurodegenerative diseases via associated pathways of PI3K/Akt (76, 77), AMPK (78, 79), SIRT1 (78, 80) etc. Furthermore, studies have explored the therapeutic effects of Sal B in RPE and lens diseases, indicating its anti-oxidative stress roles via examples of NRF2 signaling (81), TNF-α signaling (82). On the other hand, studies have shown the neuroprotective effects of salvianolic acid A (Sal A), that has similar chemical properties with Sal B, in a photoreceptor degenerative model (83). A marked limitation of applying salvianolic acids in clinical assessments is their low stability in buffers with plasma pH (84).
Tanshinone IIA (Tan IIA). Tan IIA is another compound found in the root of Danshen, and belongs to a group of diterpenes called tanshinones. Tan IIA and its derivative sodium tanshinone IIA sulfonate carry o-naphthoquinone chromophore and provide anti-oxidation protection to retinal (85) and RPE cells (86) in stress-related models. A notable challenge of applying Tan IIA in clinical assessments is the poor oral bioavailability and water solubility (87), even though sodium tanshinone IIA sulfonate has improved water-soluble property (88). Sal B and Tan IIA have not been largely applied to IRD models and patients. Promisingly, the extracts from salvia miltiorrhiza bunge (containing Sal B and Tan IIA) could improve retinal morphology and function in rd10 mice via the inhibition of oxidative stress by regulating the NRF2/HO-1 pathways (89).
Rutin. Rutin is also known as rutoside, a flavonoid that can be extracted in several plants, including tea leaves, citrus fruits, buckwheat, asparagus, and apples (90). Rutin inhibited cataractogenesis by maintaining the activity of antioxidant proteins (91, 92), and also delayed the photoreceptor degeneration in streptozotocin-induced diabetic retinas by directly regulating anti-apoptotic and antioxidant pathways (93, 94). In addition, ginkgo biloba extracts, procyanidin B2 and rutin, promoted RPE cell survival against t-BHP-induced apoptosis, suggesting their therapeutic potentials in treating age-related macular degeneration (AMD) (95). However, rutin has not been largely practiced in IRD-related trials.
Quercetin. Quercetin is a flavonoid found in fruits and vegetables, such as peppers, onions, berries, broccoli and red apples (96, 97), and its extraction is relatively easy (98, 99). The compound has two pharmacodynamic groups: a catechol group in the B ring and a 3-position OH group. Quercetin upregulated antioxidant peroxiredoxins through activation of the pro-survival signaling such as NRF2 and HO-1 signaling in models of AMD (100–102) and diabetic retinopathy (103, 104), and promoted the photoreceptor survival in NaIO3-treated mice (105). Moreover, quercetin downregulated photo-oxidative stress in light-damage photoreceptors by inhibition of the heterodimer binding of c-Jun and c-Fos proteins involved in the AP-1 pathway (106). More importantly, quercetin promoted the cone survival and functions in rd10 mice during the period of persistent rod degeneration by reducing the expression of oxidative stress markers (107). Since its effective antioxidant and anti-inflammatory properties have been well observed in treating ocular diseases (108–110), quercetin can be a good candidate for second-line therapy to RP treatment.
Lutein. Lutein is a dietary carotenoid, found in various plants, particularly in green leafy vegetables such as spinach, kale, and broccoli. Its extraction from plants is not complicated (111–113). The neuroprotective effects of lutein in treating ocular diseases have been well documented (114–116), including its profound therapeutics in AMD treatment (117–119). In addition, 1-week treatment of lutein rescued rods and cones in rd10 mice and reduced the reactive gliosis of Müller cells and inflammatory response (120). A 24-week lutein supplementation significantly preserved the visual field in placebo-controlled clinical trial on RP patients (121), however, inconsistent findings were obtained in other studies (122, 123).
Safranal. Safranal is a component extracted from stigmas of saffron (Crocus sativus L.) (124). Saffron extracts including safranal improved anti-inflammation and retinal functions in glaucoma models (125) and POAG trials (126, 127), and preserved photoreceptor and RPE cell survival in AMD models (128–130) and trials (131–133). In addition, the dietary supplementation of safranal prolonged photoreceptor survival, ameliorated the loss of retinal function, and improved the vascular network in RhoP23H/P23H rats (134). The neuroprotection to rod photoreceptor by safranal can be further exemplified in light-damage models (135, 136). Therefore, safranal or saffron extracts may have the promising therapeutic potential in RP treatment. However, the application of saffron extracts exhibits dose-dependent adverse effects (137). Hence, the clinical and experimental studies with safranal or saffron extracts usually adopted dosages of milligrams, showing minimum adverse effects.
Curcumin. Curcumin is the active compound extracted from turmeric ginger (Zingiber officinale Roscoe) (138, 139). Curcumin is a powerful antioxidant and anti-inflammatory agent that has been used in traditional Chinese medicine and widely used in clinical applications (140). In particular, curcumin treatment upregulated the expression of rod- and cone-specific genes and translocated rhodopsin to rod outer segment in RhoP23H/P23H rats. Curcumin treatment also reduced the endoplasmic reticulum stress in retinas (141). A similar rescue result by curcumin was reported in P23H swine model (142). Neuroprotective effects of curcumin on the photoreceptor survival can be seen in N-methyl-N-nitrosourea (MNU)-treated rats (143). However, clinical studies have shown adverse effects at high doses (>12 g/daily) of curcumin (144).
7. Treatment of retinal neurodegenerative diseases requires multidisciplinary collaboration
Genes, proteins, and lipids in the photoreceptor cells of retinitis pigmentosa animal models were highly oxidized, and oxidative damage was present in retinitis pigmentosa regardless of genotype (145). Retinitis pigmentosa is a disease caused by multiple genetic factors, and the path of disease expansion and rate of degeneration vary from person to person. Treatments that target different genes mutations are expensive and not efficient. The ideal solution would be to develop a treatment that can treat retinitis pigmentosa caused by all the different genetic defects. At the same time, treatments of early stage of degeneration of retinitis pigmentosa caused by genetic defects in rod photoreceptor cells will be required. Oxidative damage is related to the pathophysiology of retinitis pigmentosa such as death of rods and cones, and retinal inflammation may become a common therapeutic target for retinitis pigmentosa. A limitation of current research is that plants for the use in retinitis pigmentosa have not yet been fully developed, and more plants with effective active ingredients are worth developing and applying in this field. Other botanicals, including Tibetan medicines (Saussurea medusa Maxim, known as “snow lotus”) (146–148) and health products (various types of tea and mulberry leaves) also have antioxidant and neuroprotective effects. These herbal medicines can be developed for the treatment of retinal degenerative diseases (149, 150). In the future, we look forward to jointly developing new drugs for retinitis pigmentosa caused by oxidative damage through multidisciplinary collaboration with botanical scientists, pharmaceutical scientists, medical scientists, and ophthalmologists.
8. Future perspectives
In China, doctors currently prescribe several traditional Chinese medicine (TCM) to patients. The main principle of TCM is to restore the balance of yin and yang as well as the harmony of body and mind. Using the “look, smell, ask, and feel” method, doctors collect comprehensive information about the patient’s symptoms and signs. Based on this diagnosis, doctors select appropriate medicines and determine their dosages. A TCM prescription typically consists of multiple drugs, ranging from several to dozens. In TCM, it is believed that every medicine has a 70% therapeutic effect and a 30% potential for side effects. Furthermore, a famous ancient book from the Former Han Dynasty called “Huangdi Neijing” categorizes Chinese medicine into four groups based on toxicity: highly toxic, moderately toxic, mildly toxic, and non-toxic. Medicines can not only cure diseases but also cause them or have lethal effects. As a result, ensuring the safety of TCM prescriptions is paramount. The Pharmacopeia and literature have clear warnings regarding the cautious use or avoidance of toxic medicines.
While some patients travel to Western countries seeking gene therapy, others, due to genetic incompatibility or economic factors, opt for more affordable TCM treatments upon returning to China. Encouragingly, many have experienced positive therapeutic effects. Western medicine primarily targets specific or multiple disease-related factors, while TCM aims to rebalance the overall yin and yang in the body for therapeutic outcomes. Herbal medicine is often considered an alternative or complementary treatment option (151).
Research-based repositories of natural products are available, for example, the National Cancer Institute Natural Products Repository of NIH offers thousands of plant samples and resulting extracts, including Traditional Chinese Medicinal Plant Extracts Library, for drug screening studies. The eight above-mentioned compounds and associated herbs can be found in this repository, although a large majority of herbal extracts have not been tested in the treatment for inherited retinal diseases such as retinitis pigmentosa. In 2004, the FDA formulated the Botanical Drug Guidance, which is applicable to the clinical trials and inspection registration of new botanical drugs. Then the “Botanical Drug Development” guidance was announced in 2016, to address development considerations for late-stage trials and provide recommendations designed to facilitate botanical drug development (fda.org) (152). However, only some botanical New Drug Applications (NDAs) have been approved in the United States so far: Veregen in 2006, Fulyzaq in 2012 (152), Zoryve in 2023 (zoryve.com) and Filsuvez in 2023 (filsuvez.com). Scientists used this guidance to guide the research and development of botanical medicines (153). On the other hand, the European Medicines Agency (EMA) of the European Union proposed a draft “Guideline on quality of herbal medicinal products / traditional herbal medicinal products” in 2005 for the quality control of botanical medicines, then officially announced it in 2006, and a revised version (revision3) was released in 2022 (ema.europa.eu). Although Chinese pharmacy and Western pharmacy are separate disciplines in China, some scholars argue that the two can complement each other and have synergistic effects (154–156), thus surpassing the efficacy of either approach alone. In addition, we found in clinical practice that when doctors are seeing patients in outpatient clinics, genetic testing department and clinical trial staff are participating at the same time. In the future, the treatment of patients with retinitis pigmentosa can be combined with personalized medicine (157, 158) or comprehensive medical care. For example, the genotype of patients with retinitis pigmentosa can be detected first, then genetic testing can more accurately classify/ diagnose inherited retinal diseases and relevant drug treatment can be formulated based on the genotype.
TCM was introduced to Japan and, after over a thousand years of adaptation, has evolved into Kampo medicine, tailored to the Japanese constitution. In the future, TCM may be adjusted to suit the constitution of people from different regions and become a form of medicine applicable to individuals worldwide. Given the diverse medicinal plants found across the globe due to variations in soil, climate, and region, it is crucial to fully explore and develop medicinal herbs derived from these plants. Additionally, developing new drugs from the organic compounds present in these herbal extracts, combining them with gene therapy, cell therapy, and other innovative approaches, holds great value in overcoming rare human diseases and improving physical and mental well-being.
Acknowledgments
We thank Shoko Taniguchi in Okayama University for guiding us and allowing us to take photos of the medicinal plants in the Medical Botanical Garden, Okayama University (Figure 2). Okayama University Medicinal Botanical Gardens are closed to the public in principle. We thank Yang Yang for introducing us to Shanghai Chenshan Botanical Garden. We thank Qing Zhao and Chi Sun for their great help with this manuscript.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SL: Writing – review & editing, Writing – original draft, Visualization, Resources, Project administration, Investigation, Conceptualization. TM: Writing – review & editing, Project administration, Conceptualization. CM: Writing – review & editing. TA: Writing – review & editing, Visualization, Conceptualization. JC: Writing – review & editing. CS: Writing – review & editing. QZ: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Liu J, Zhao Y, Zhang J, Kong Y, Liu P, Fang Y, et al. Production of species-specific anthocyanins through an inducible system in plant hairy roots. Metab Eng. (2024) 81:182–96. doi: 10.1016/j.ymben.2023.12.005, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Pei T, Yan M, Huang Y, Wei Y, Martin C, Zhao Q. Specific flavonoids and their biosynthetic pathway in Scutellaria baicalensis. Front Plant Sci. (2022) 13. doi: 10.3389/fpls.2022.866282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe T. Synthetic strategies for the construction of C3-N1' bisindoles. Org Biomol Chem. (2024) 22:1756–64. doi: 10.1039/D3OB02089D, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Abe T, Aoyama S, Ohmura M, Taniguchi M, Yamada K. Revisiting Furodiindolines: one-pot synthesis of Furodiindolines using indole 2,3-epoxide surrogates and their synthetic applications. Org Lett. (2019) 21:3367–71. doi: 10.1021/acs.orglett.9b01108, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Poulaki V, Kim SJ, Eldred WD, Kane S, Gingerich M, et al. Implantation and extraction of penetrating electrode arrays in Minipig retinas. Transl Vis Sci Technol. (2020) 9:19. doi: 10.1167/tvst.9.5.19, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphries A, Bowman L, Nguyen T, So J, Duff M, Grover S, et al. Quality of life analysis in patients with retinitis Pigmentosa. Ophthalmic Res. (2024) 67:348–57. doi: 10.1159/000539116, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Matsuo T, Abe T. Revisiting cryptocyanine dye, Nk-4, as an old and new drug: review and future perspectives. Int J Mol Sci. (2023) 24:4411. doi: 10.3390/ijms24054411, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Miyaji M, Hosoya O, Matsuo T. Effect of Nk-5962 on gene expression profiling of retina in a rat model of retinitis Pigmentosa. Int J Mol Sci. (2021) 22:2. doi: 10.3390/ijms222413276, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuo T, Uchida T. Photoelectric dye-based retinal prosthesis (OureP) as a novel type of artificial retina. Internal Med Rev. (2021) 7:2. doi: 10.18103/imr.v7i1.916 [DOI] [Google Scholar]
- 10.Sun C, Chen S. Gene augmentation for autosomal dominant Crx-associated retinopathies. Adv Exp Med Biol. (2023) 1415:135–41. doi: 10.1007/978-3-031-27681-1_21, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan L, Cookson MR, Petrucelli L, La Spada AR. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci. (2018) 21:1300–9. doi: 10.1038/s41593-018-0237-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech. (2017) 10:499–502. doi: 10.1242/dmm.030205, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. (2010) 14:457–87. doi: 10.1111/j.1582-4934.2010.01010.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wareham LK, Liddelow SA, Temple S, Benowitz LI, Di Polo A, Wellington C, et al. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener. (2022) 17:23. doi: 10.1186/s13024-022-00524-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Matsuo T, Matsuo C, Abe T. Traditional Chinese medicines and prescriptions brought from China to Japan by a monk (Jianzhen, Japanese: Ganjin): a historical review. Compounds. (2022) 2:267–84. doi: 10.3390/compounds2040022 [DOI] [Google Scholar]
- 16.Mohan KV, Mishra A, Muniyasamy A, Sinha P, Sahu P, Kesarwani A, et al. Immunological consequences of compromised ocular immune privilege accelerate retinal degeneration in retinitis pigmentosa. Orphanet J Rare Dis. (2022) 17:378. doi: 10.1186/s13023-022-02528-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami Y, Ishikawa K, Nakao S, Sonoda K-H. Innate immune response in retinal homeostasis and inflammatory disorders. Prog Retin Eye Res. (2020) 74:100778. doi: 10.1016/j.preteyeres.2019.100778, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Molday RS, Moritz OL. Photoreceptors at a glance. J Cell Sci. (2015) 128:4039–45. doi: 10.1242/jcs.175687, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Curr Biol. (2010) 20:R114–24. doi: 10.1016/j.cub.2009.12.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon SG, Lennie P. The machinery of colour vision. Nat Rev Neurosci. (2007) 8:276–86. doi: 10.1038/nrn2094 [DOI] [PubMed] [Google Scholar]
- 21.Hussey KA, Hadyniak SE, Johnston RJ. Patterning and development of photoreceptors in the human retina. Front Cell Develop Biol. (2022) 10:2. doi: 10.3389/fcell.2022.878350, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. (1990) 292:497–523. doi: 10.1002/cne.902920402 [DOI] [PubMed] [Google Scholar]
- 23.Ali MU, Rahman MSU, Cao J, Yuan PX. Genetic characterization and disease mechanism of retinitis pigmentosa; current scenario. 3 Biotech. (2017) 7:251. doi: 10.1007/s13205-017-0878-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbakel SK, Van Huet RAC, Boon CJF, Den Hollander AI, Collin RWJ, Klaver CCW, et al. Non-syndromic retinitis pigmentosa. Prog Retin Eye Res. (2018) 66:157–86. doi: 10.1016/j.preteyeres.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 25.Consugar MB, Navarro-Gomez D, Place EM, Bujakowska KM, Sousa ME, Fonseca-Kelly ZD, et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genet Med. (2015) 17:253–61. doi: 10.1038/gim.2014.172, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang F, Wang H, Tuan HF, Nguyen DH, Sun V, Keser V, et al. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet. (2014) 133:331–45. doi: 10.1007/s00439-013-1381-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daiger SP, Bowne SJ, Sullivan LS. Genes and mutations causing autosomal dominant retinitis Pigmentosa. Cold Spring Harb Perspect Med. (2014) 5:a017129. doi: 10.1101/cshperspect.a017129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. (2011) 12:238–49. doi: 10.2174/138920211795860107, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun C, Chen S. Disease-causing mutations in genes encoding transcription factors critical for photoreceptor development. Front Mol Neurosci. (2023) 16:3. doi: 10.3389/fnmol.2023.1134839, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, et al. Gene therapy for leber congenital amaurosis caused by Rpe65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. (2012) 130:9–24. doi: 10.1001/archophthalmol.2011.298, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulaklak K, Gersbach CA. The once and future gene therapy. Nat Commun. (2020) 11:5820. doi: 10.1038/s41467-020-19505-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen XT, Moekotte L, Plomp AS, Bergen AA, Van Genderen MM, Boon CJF. Retinitis Pigmentosa: current clinical management and emerging therapies. Int J Mol Sci. (2023) 24:3. doi: 10.3390/ijms24087481, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campochiaro PA, Iftikhar M, Hafiz G, Akhlaq A, Tsai G, Wehling D, et al. Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase I trial. J Clin Invest. (2020) 130:1527–41. doi: 10.1172/JCI132990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivares-González L, Salom D, González-García E, Hervás D, Mejía-Chiqui N, Melero M, et al. Nutraret: effect of 2-year nutraceutical supplementation on redox status and visual function of patients with retinitis Pigmentosa: a randomized, double-blind, placebo-controlled trial. Front Nutr. (2022) 9:847910. doi: 10.3389/fnut.2022.847910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Feng K, Liu R, Pan J, Zhang L, Lu X. Vitamins and mineral supplements for retinitis Pigmentosa. J Ophthalmol. (2019) 2019:8524607. doi: 10.1155/2019/8524607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tagaris A, Kollias D, Stafylopatis A, Tagaris G, Kollias S. Machine learning for neurodegenerative disorder diagnosis — survey of practices and launch of benchmark dataset. Int J Artificial Intelligence Tools. (2018) 27:1850011. doi: 10.1142/S0218213018500112 [DOI] [Google Scholar]
- 37.Menghini M, Cehajic-Kapetanovic J, Maclaren RE. Monitoring progression of retinitis pigmentosa: current recommendations and recent advances. Expert Opin Orphan Drugs. (2020) 8:67–78. doi: 10.1080/21678707.2020.1735352, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q. Retinitis Pigmentosa: Progress and perspective. Asia-Pacific J Ophthalmol. (2016) 5:3. doi: 10.1097/APO.0000000000000227 [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Matsuo T, Hosoya O, Uchida T. Photoelectric dye used for Okayama University-type retinal prosthesis reduces the apoptosis of photoreceptor cells. J Ocul Pharmacol Ther. (2017) 33:149–60. doi: 10.1089/jop.2016.0093, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Matsuo T, Miyaji M, Hosoya O. The effect of cyanine dye Nk-4 on photoreceptor degeneration in a rat model of early-stage retinitis Pigmentosa. Pharmaceuticals. (2021) 14:694. doi: 10.3390/ph14070694, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuo T, Liu S, Uchida T, Onoue S, Nakagawa S, Ishii M, et al. Photoelectric dye, Nk-5962, as a potential drug for preventing retinal neurons from apoptosis: pharmacokinetic studies based on review of the evidence. Life (Basel). (2021) 11:3. doi: 10.3390/life11060591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuo T, Uchida T, Nitta M, Yamashita K, Takei S, Ido D, et al. Subretinal implantation of Okayama University-type retinal prosthesis (OureP(tm)) in canine eyes by vitrectomy. J Vet Med Sci. (2017) 79:1939–46. doi: 10.1292/jvms.17-0450, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahraman NS, Oner A. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: a 6-month follow-up results of a phase 3 trial. Int J Ophthalmol. (2020) 13:1423–9. doi: 10.18240/ijo.2020.09.14, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wadley L, Sievers C, Bamford M, Goldberg P, Berna F, Miller C. Middle Stone age bedding construction and settlement patterns at Sibudu, South Africa. Science. (2011) 334:1388–91. doi: 10.1126/science.1213317, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Hardy K. Paleomedicine and the evolutionary context of medicinal plant use. Rev Bras. (2021) 31:1–15. doi: 10.1007/s43450-020-00107-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leping J. The Kuahuqiao site and culture. A Companion to Chinese Archaeology. (2013) 537–54. doi: 10.1002/9781118325698.ch26 [DOI] [Google Scholar]
- 47.Qiu Z, Shang X, Ferguson DK, Jiang H. Archaeobotanical analysis of diverse plant food resources and palaeovegetation at the Zhumucun site, a late Neolithic settlement of the Liangzhu culture in East China. Quat Int. (2016) 426:75–85. doi: 10.1016/j.quaint.2016.01.010 [DOI] [Google Scholar]
- 48.Verpoorte R. Exploration of nature's chemodiversity: the role of secondary metabolites as leads in drug development. Drug Discov Today. (1998) 3:232–8. doi: 10.1016/S1359-6446(97)01167-7 [DOI] [Google Scholar]
- 49.Calixto JB. The role of natural products in modern drug discovery. An Acad Bras Cienc. (2019) 91:e20190105. doi: 10.1590/0001-3765201920190105 [DOI] [PubMed] [Google Scholar]
- 50.Atanasov AG, Zotchev SB, Dirsch VM, Orhan IE, Banach M, Rollinger JM, et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. (2021) 20:200–16. doi: 10.1038/s41573-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huynh TP, Mann SN, Mandal NA. Botanical compounds: effects on major eye diseases. Evid Based Complement Alternat Med. (2013) 2013:549174. doi: 10.1155/2013/549174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han J, Dinculescu A, Dai X, Du W, Smith WC, Pang J. Review: the history and role of naturally occurring mouse models with Pde6b mutations. Mol Vis. (2013) 19:2579–89. PMID: [PMC free article] [PubMed] [Google Scholar]
- 53.Kalloniatis M, Nivison-Smith L, Chua J, Acosta ML, Fletcher EL. Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: a review. Exp Eye Res. (2016) 150:106–21. doi: 10.1016/j.exer.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 54.Carter-Dawson LD, Lavail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. (1978) 17:489–98. PMID: [PubMed] [Google Scholar]
- 55.Chen Y, Dong Y, Yan J, Wang L, Yu S, Jiao K, et al. Single-cell transcriptomic profiling in inherited retinal degeneration reveals distinct metabolic pathways in rod and cone photoreceptors. Int J Mol Sci. (2022) 23:4. doi: 10.3390/ijms232012170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hackam AS, Strom R, Liu D, Qian J, Wang C, Otteson D, et al. Identification of gene expression changes associated with the progression of retinal degeneration in the rd1 mouse. Invest Ophthalmol Vis Sci. (2004) 45:2929–42. doi: 10.1167/iovs.03-1184, PMID: [DOI] [PubMed] [Google Scholar]
- 57.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vis Res. (2002) 42:517–25. doi: 10.1016/S0042-6989(01)00146-8 [DOI] [PubMed] [Google Scholar]
- 58.Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and erg study. J Comp Neurol. (2007) 500:222–38. doi: 10.1002/cne.21144, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dryja TP, Mcgee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. (1990) 343:364–6. doi: 10.1038/343364a0, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Sohocki MM, Daiger SP, Bowne SJ, Rodriquez JA, Northrup H, Heckenlively JR, et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat. (2001) 17:42–51. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian X, Liang T, Liu Y, Ding G, Zhang F, Ma Z. Extraction, structural characterization, and biological functions of Lycium Barbarum polysaccharides: a review. Biomol Ther. (2019) 9:4. doi: 10.3390/biom9090389, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang R-F, Zhao C, Chen X, Chan S-W, Wu J-Y. Chemical properties and bioactivities of goji (Lycium barbarum) polysaccharides extracted by different methods. J Funct Foods. (2015) 17:903–9. doi: 10.1016/j.jff.2015.06.045 [DOI] [Google Scholar]
- 63.Amagase H, Farnsworth NR. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (goji). Food Res Int. (2011) 44:1702–17. doi: 10.1016/j.foodres.2011.03.027 [DOI] [Google Scholar]
- 64.Kwok SS, Bu Y, Lo AC, Chan TC, So KF, Lai JS, et al. A systematic review of potential therapeutic use of Lycium Barbarum polysaccharides in disease. Biomed Res Int. (2019) 2019:4615745. doi: 10.1155/2019/4615745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lakshmanan Y, Wong FSY, So KF, Chan HH. Potential role of Lycium barbarum polysaccharides in glaucoma management: evidence from preclinical in vivo studies. Neural Regen Res. (2023) 18:2623–32. doi: 10.4103/1673-5374.355977, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niu Y, Zhang G, Sun X, He S, Dou G. Distinct role of Lycium barbarum L. polysaccharides in oxidative stress-related ocular diseases. Pharmaceuticals (Basel). (2023) 16:215. doi: 10.3390/ph16020215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang T, Li Y, Wang Y, Zhou R, Ma L, Hao Y, et al. Lycium barbarum polysaccharide prevents focal cerebral ischemic injury by inhibiting neuronal apoptosis in mice. PLoS One. (2014) 9:e90780. doi: 10.1371/journal.pone.0090780, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang D, So KF, Lo AC. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin Experiment Ophthalmol. (2017) 45:717–29. doi: 10.1111/ceo.12950, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Liu L, Sha XY, Wu YN, Chen MT, Zhong JX. Lycium barbarum polysaccharides protects retinal ganglion cells against oxidative stress injury. Neural Regen Res. (2020) 15:1526–31. doi: 10.4103/1673-5374.274349, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu F, Zhang J, Xiang Z, Xu D, So KF, Vardi N, et al. Lycium Barbarum polysaccharides protect retina in rd1 mice during photoreceptor degeneration. Invest Ophthalmol Vis Sci. (2018) 59:597–611. doi: 10.1167/iovs.17-22881, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan HH, Lam HI, Choi KY, Li SZ, Lakshmanan Y, Yu WY, et al. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J Ethnopharmacol. (2019) 236:336–44. doi: 10.1016/j.jep.2019.03.023, PMID: [DOI] [PubMed] [Google Scholar]
- 72.Wang K, Xiao J, Peng B, Xing F, So K-F, Tipoe GL, et al. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci Rep. (2014) 4:7601. doi: 10.1038/srep07601, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao Y, Kou R, Huang X, Wang N, Di D, Wang H, et al. Separation of polysaccharides from Lycium barbarum L. by high-speed countercurrent chromatography with aqueous two-phase system. Int J Biol Macromol. (2024) 256:128282. doi: 10.1016/j.ijbiomac.2023.128282 [DOI] [PubMed] [Google Scholar]
- 74.He G, Chen G, Liu W, Ye D, Liu X, Liang X, et al. Salvianolic acid B: a review of pharmacological effects, safety, combination therapy, new dosage forms, and novel drug delivery routes. Pharmaceutics. (2023) 15:15. doi: 10.3390/pharmaceutics15092235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahalakshmi B, Huang C-Y, Lee S-D, Maurya N, Kiefer R, Bharath Kumar V. Review of Danshen: from its metabolism to possible mechanisms of its biological activities. J Funct Foods. (2021) 85:104613. doi: 10.1016/j.jff.2021.104613 [DOI] [Google Scholar]
- 76.Bi SJ, Dong XY, Wang ZY, Fu SJ, Li CL, Wang ZY, et al. Salvianolic acid B alleviates neurological injury by upregulating stanniocalcin 1 expression. Ann Transl Med. (2022) 10:739. doi: 10.21037/atm-21-4779, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao Z, Liu W, Mu YP, Zhang H, Wang XN, Zhao CQ, et al. Pharmacological effects of Salvianolic acid B against oxidative damage. Front Pharmacol. (2020) 11:572373. doi: 10.3389/fphar.2020.572373, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao D, Chen Y, Guo Y, Wang C, Liu N, Gong Q, et al. Salvianolic acid B improves chronic mild stress-induced depressive behaviors in rats: involvement of Ampk/Sirt1 signaling pathway. J Inflamm Res. (2020) 13:195–206. doi: 10.2147/JIR.S249363, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang H, Pan C-S, Mao X-W, Liu Y-Y, Yan L, Zhou C-M, et al. Role of Nadph oxidase in Total Salvianolic acid injection attenuating ischemia-reperfusion impaired cerebral microcirculation and neurons: implication of Ampk/Akt/Pkc. Microcirculation. (2014) 21:615–27. doi: 10.1111/micc.12140, PMID: [DOI] [PubMed] [Google Scholar]
- 80.Lv H, Wang L, Shen J, Hao S, Ming A, Wang X, et al. Salvianolic acid B attenuates apoptosis and inflammation via Sirt1 activation in experimental stroke rats. Brain Res Bull. (2015) 115:30–6. doi: 10.1016/j.brainresbull.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 81.Liu X, Xavier C, Jann J, Wu H. Salvianolic acid B (Sal B) protects retinal pigment epithelial cells from oxidative stress-induced cell death by activating Glutaredoxin 1 (Grx1). Int J Mol Sci. (2016) 17:6. doi: 10.3390/ijms17111835, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong Y, Zhao J, Zheng X, Xue T, Ma W, Cao P, et al. Exploration of the therapeutic potential of Salvianolic acid B against senile cataracts based on network pharmacology and experimental validation. Nat Prod Commun. (2024) 19:1934578X241272458. doi: 10.1177/1934578X241272458 [DOI] [Google Scholar]
- 83.Zhou Y, Xu W, Liu A, Tao Y, Wang Q, Yang Y, et al. Protective effect of Salvianolic acid a against N-methyl-N-Nitrosourea-induced retinal degeneration. Evid Based Complement Alternat Med. (2022) 2022:1219789. doi: 10.1155/2022/1219789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qu H-B, Zhai X-J, Shao Q, Cheng Y-Y. Simultaneous determination of seven active compounds in Radix Salviae Miltiorrhizae by temperature-controlled ultrasound-assisted extraction and Hplc. Chromatographia. (2007) 66:21–7. doi: 10.1365/s10337-007-0244-4 [DOI] [Google Scholar]
- 85.Zeng X, Deng Y, Yuan M, He Q, Wu Y, Li S. Study on the antioxidant effect of Tanshinone Iia on diabetic retinopathy and its mechanism based on integrated pharmacology. Evid Based Complement Alternat Med. (2022) 2022:9990937. doi: 10.1155/2022/9990937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alzhrani RM, Alhadidi Q, Bachu RD, Shah Z, Dey S, Boddu SH. Tanshinone Iia inhibits Vegf secretion and Hif-1α expression in cultured human retinal pigment epithelial cells under hypoxia. Curr Eye Res. (2017) 42:1667–73. doi: 10.1080/02713683.2017.1355467, PMID: [DOI] [PubMed] [Google Scholar]
- 87.Cai Y, Zhang W, Chen Z, Shi Z, He C, Chen M. Recent insights into the biological activities and drug delivery systems of tanshinones. Int J Nanomedicine. (2016) 11:121–30. doi: 10.2147/IJN.S84035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang H, Song C, Chang J. Synthesis and biological activity study of Tanshinone derivatives: a literature and patent review. Curr Top Med Chem. (2020) 20:2520–34. doi: 10.2174/1568026620666200922115109, PMID: [DOI] [PubMed] [Google Scholar]
- 89.Ou C, Jiang P, Tian Y, Yao Z, Yang Y, Peng J, et al. Fructus Lycii and Salvia miltiorrhiza Bunge extract alleviate retinitis pigmentosa through Nrf2/Ho-1 signaling pathway. J Ethnopharmacol. (2021) 273:113993. doi: 10.1016/j.jep.2021.113993, PMID: [DOI] [PubMed] [Google Scholar]
- 90.Gullón B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G. Rutin: a review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Technol. (2017) 67:220–35. doi: 10.1016/j.tifs.2017.07.008 [DOI] [Google Scholar]
- 91.Isai M, Sakthivel M, Ramesh E, Thomas PA, Geraldine P. Prevention of selenite-induced cataractogenesis by rutin in Wistar rats. Mol Vis. (2009) 15:2570–7. [PMC free article] [PubMed] [Google Scholar]
- 92.Sasikala V, Rooban BN, Sahasranamam V, Abraham A. Rutin ameliorates free radical mediated cataract by enhancing the chaperone activity of α-crystallin. Graefes Arch Clin Exp Ophthalmol. (2013) 251:1747–55. doi: 10.1007/s00417-013-2281-z, PMID: [DOI] [PubMed] [Google Scholar]
- 93.Gupta SK, Sharma HP, Das U, Velpandian T, Saklani R. Effect of rutin on retinal Vegf, Tnf-α, aldose reductase, and total antioxidant capacity in diabetic rats: molecular mechanism and ocular pharmacokinetics. Int Ophthalmol. (2020) 40:159–68. doi: 10.1007/s10792-019-01165-x, PMID: [DOI] [PubMed] [Google Scholar]
- 94.Ola MS, Ahmed MM, Ahmad R, Abuohashish HM, Al-Rejaie SS, Alhomida AS. Neuroprotective effects of Rutin in Streptozotocin-induced diabetic rat retina. J Mol Neurosci. (2015) 56:440–8. doi: 10.1007/s12031-015-0561-2, PMID: [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Cheng Z, Wang K, Zhu X, Ali Y, Shu W, et al. Procyanidin B2 and rutin in Ginkgo biloba extracts protect human retinal pigment epithelial (Rpe) cells from oxidative stress by modulating Nrf2 and Erk1/2 signalling. Exp Eye Res. (2021) 207:108586. doi: 10.1016/j.exer.2021.108586, PMID: [DOI] [PubMed] [Google Scholar]
- 96.Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, et al. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res. (2018) 62:6. doi: 10.1002/mnfr.201700447, PMID: [DOI] [PubMed] [Google Scholar]
- 97.D'andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. (2015) 106:256–71. doi: 10.1016/j.fitote.2015.09.018, PMID: [DOI] [PubMed] [Google Scholar]
- 98.Chen L, Teng H, Xie Z, Cao H, Cheang WS, Skalicka-Woniak K, et al. Modifications of dietary flavonoids towards improved bioactivity: an update on structure–activity relationship. Crit Rev Food Sci Nutr. (2018) 58:513–27. doi: 10.1080/10408398.2016.1196334, PMID: [DOI] [PubMed] [Google Scholar]
- 99.Zhu Y, Yu J, Jiao C, Tong J, Zhang L, Chang Y, et al. Optimization of quercetin extraction method in Dendrobium officinale by response surface methodology. Heliyon. (2019) 5:e02374. doi: 10.1016/j.heliyon.2019.e02374, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyamoto N, Izumi H, Miyamoto R, Kondo H, Tawara A, Sasaguri Y, et al. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/Nrf1 transcription pathway. Invest Ophthalmol Vis Sci. (2011) 52:1055–63. doi: 10.1167/iovs.10-5777 [DOI] [PubMed] [Google Scholar]
- 101.Shao Y, Yu H, Yang Y, Li M, Hang L, Xu X. A solid dispersion of quercetin shows enhanced Nrf2 activation and protective effects against oxidative injury in a mouse model of dry age-related macular degeneration. Oxidative Med Cell Longev. (2019) 2019:1479571. doi: 10.1155/2019/1479571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu X-R, Yu H-T, Yang Y, Hang L, Yang X-W, Ding S-H. Quercetin phospholipid complex significantly protects against oxidative injury in Arpe-19 cells associated with activation of Nrf2 pathway. Eur J Pharmacol. (2016) 770:1–8. doi: 10.1016/j.ejphar.2015.11.050, PMID: [DOI] [PubMed] [Google Scholar]
- 103.Chai GR, Liu S, Yang HW, Chen XL. Quercetin protects against diabetic retinopathy in rats by inducing heme oxygenase-1 expression. Neural Regen Res. (2021) 16:1344–50. doi: 10.4103/1673-5374.301027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R, Jha KA, et al. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res. (2014) 125:193–202. doi: 10.1016/j.exer.2014.06.009, PMID: [DOI] [PubMed] [Google Scholar]
- 105.Chang YY, Lee YJ, Hsu MY, Wang M, Tsou SC, Chen CC, et al. Protective effect of quercetin on sodium iodate-induced retinal apoptosis through the reactive oxygen species-mediated mitochondrion-dependent pathway. Int J Mol Sci. (2021) 22:6. doi: 10.3390/ijms22084056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koyama Y, Kaidzu S, Kim YC, Matsuoka Y, Ishihara T, Ohira A, et al. Suppression of light-induced retinal degeneration by quercetin via the Ap-1 pathway in rats. Antioxidants (Basel). (2019) 8:6. doi: 10.3390/antiox8040079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piano I, D’antongiovanni V, Testai L, Calderone V, Gargini C. A nutraceutical strategy to slowing down the progression of cone death in an animal model of retinitis Pigmentosa. Front Neurosci. (2019) 13:6. doi: 10.3389/fnins.2019.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao FJ, Zhang SH, Xu P, Yang BQ, Zhang R, Cheng Y, et al. Quercetin declines apoptosis, ameliorates mitochondrial function and improves retinal ganglion cell survival and function in in vivo model of Glaucoma in rat and retinal ganglion cell culture in vitro. Front Mol Neurosci. (2017) 10:285. doi: 10.3389/fnmol.2017.00285, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ho TY, Lo HY, Liu IC, Lin KA, Liao YF, Lo YC, et al. The protective effect of quercetin on retinal inflammation in mice: the involvement of tumor necrosis factor/nuclear factor-κB signaling pathways. Food Funct. (2020) 11:8150–60. doi: 10.1039/D0FO01324B, PMID: [DOI] [PubMed] [Google Scholar]
- 110.Zhao L, Wang H, Du X. The therapeutic use of quercetin in ophthalmology: recent applications. Biomed Pharmacother. (2021) 137:111371. doi: 10.1016/j.biopha.2021.111371, PMID: [DOI] [PubMed] [Google Scholar]
- 111.Kumar Kashyap P, Singh S, Kumar Singh M, Gupta A, Tandon S, Shanker K, et al. An efficient process for the extraction of lutein and chemical characterization of other organic volatiles from marigold (Tagetes erecta L.) flower. Food Chem. (2022) 396:133647. doi: 10.1016/j.foodchem.2022.133647, PMID: [DOI] [PubMed] [Google Scholar]
- 112.Manzoor S, Rashid R, Prasad Panda B, Sharma V, Azhar M. Green extraction of lutein from marigold flower petals, process optimization and its potential to improve the oxidative stability of sunflower oil. Ultrason Sonochem. (2022) 85:105994. doi: 10.1016/j.ultsonch.2022.105994, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saini RK, Prasad P, Lokesh V, Shang X, Shin J, Keum Y-S, et al. Carotenoids: dietary sources, extraction, encapsulation, bioavailability, and health benefits—a review of recent advancements. Antioxidants. (2022) 11:795. doi: 10.3390/antiox11040795, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iyer S, Bhat I, Bangera Sheshappa M. Lutein and the underlying neuroprotective promise against neurodegenerative diseases. Mol Nutr Food Res. (2024) 68:e2300409. doi: 10.1002/mnfr.202300409, PMID: [DOI] [PubMed] [Google Scholar]
- 115.Johra FT, Bepari AK, Bristy AT, Reza HM. A mechanistic review of β-carotene, lutein, and Zeaxanthin in eye health and disease. Antioxidants. (2020) 9:1046. doi: 10.3390/antiox9111046, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K. Neuroprotective effects of lutein in the retina. Curr Pharm Des. (2012) 18:51–6. doi: 10.2174/138161212798919101, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, Ni M, Wu R, Yang Z, Zhu X, Chen J. The level and efficacy of lutein in patients with age-related macular degeneration: a comprehensive systematic review and meta-analysis. Ann Transl Med. (2022) 10:299. doi: 10.21037/atm-22-173, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma L, Dou HL, Wu YQ, Huang YM, Huang YB, Xu XR, et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr. (2012) 107:350–9. doi: 10.1017/S0007114511004260, PMID: [DOI] [PubMed] [Google Scholar]
- 119.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, et al. Dietary carotenoids, vitamins a, C, and E, and advanced age-related macular degeneration. JAMA. (1994) 272:1413–20. doi: 10.1001/jama.1994.03520180037032, PMID: [DOI] [PubMed] [Google Scholar]
- 120.Zhang HJ, Liu XB, Chen XM, Kong QH, Liu YS, So KF, et al. Lutein delays photoreceptor degeneration in a mouse model of retinitis pigmentosa. Neural Regen Res. (2022) 17:1596–603. doi: 10.4103/1673-5374.330622, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bahrami H, Melia M, Dagnelie G. Lutein supplementation in retinitis pigmentosa: pc-based vision assessment in a randomized double-masked placebo-controlled clinical trial [Nct00029289]. BMC Ophthalmol. (2006) 6:23. doi: 10.1186/1471-2415-6-23, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adackapara CA, Sunness JS, Dibernardo CW, Melia BM, Dagnelie G. Prevalence of cystoid macular edema and stability in Oct retinal thickness in eyes with retinitis Pigmentosa during a 48-week lutein trial. Retina. (2008) 28:103–10. doi: 10.1097/IAE.0b013e31809862aa [DOI] [PubMed] [Google Scholar]
- 123.Aleman TS, Duncan JL, Bieber ML, De Castro E, Marks DA, Gardner LM, et al. Macular pigment and lutein supplementation in retinitis Pigmentosa and usher syndrome. Invest Ophthalmol Vis Sci. (2001) 42:1873–81. PMID: [PubMed] [Google Scholar]
- 124.Nanda S, Madan K. The role of Safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: a systematic review. Heliyon. (2021) 7:e06117. doi: 10.1016/j.heliyon.2021.e06117, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fernández-Albarral JA, Ramírez AI, De Hoz R, López-Villarín N, Salobrar-García E, López-Cuenca I, et al. Neuroprotective and anti-inflammatory effects of a hydrophilic saffron extract in a model of Glaucoma. Int J Mol Sci. (2019) 20:6. doi: 10.3390/ijms20174110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hecht I, Achiron A, Bartov E, Maharshak I, Mendel L, Pe'er L, et al. Effects of dietary and lifestyle recommendations on patients with glaucoma: a randomized controlled pilot trial. Eur J Integr Med. (2019) 25:60–6. doi: 10.1016/j.eujim.2018.12.002 [DOI] [Google Scholar]
- 127.Jabbarpoor Bonyadi MH, Yazdani S, Saadat S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: a pilot study. BMC Complement Altern Med. (2014) 14:399. doi: 10.1186/1472-6882-14-399, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bisti S, Maccarone R, Falsini B. Saffron and retina: neuroprotection and pharmacokinetics. Vis Neurosci. (2014) 31:355–61. doi: 10.1017/S0952523814000108, PMID: [DOI] [PubMed] [Google Scholar]
- 129.Corso L, Cavallero A, Baroni D, Garbati P, Prestipino G, Bisti S, et al. Saffron reduces Atp-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. (2016) 12:161–74. doi: 10.1007/s11302-015-9490-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Marco S, Carnicelli V, Franceschini N, Di Paolo M, Piccardi M, Bisti S, et al. Saffron: a multitask neuroprotective agent for retinal degenerative diseases. Antioxidants (Basel). (2019) 8:6. doi: 10.3390/antiox8070224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lashay A, Sadough G, Ashrafi E, Lashay M, Movassat M, Akhondzadeh S. Short-term outcomes of saffron supplementation in patients with age-related macular degeneration: a double-blind, placebo-controlled, randomized trial. Med Hypothesis Discov Innov Ophthalmol. (2016) 5:32–8. PMID: [PMC free article] [PubMed] [Google Scholar]
- 132.Marangoni D, Falsini B, Piccardi M, Ambrosio L, Minnella AM, Savastano MC, et al. Functional effect of saffron supplementation and risk genotypes in early age-related macular degeneration: a preliminary report. J Transl Med. (2013) 11:228. doi: 10.1186/1479-5876-11-228, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piccardi M, Marangoni D, Minnella AM, Savastano MC, Valentini P, Ambrosio L, et al. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid Based Complement Alternat Med. (2012) 2012:429124. doi: 10.1155/2012/429124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fernández-Sánchez L, Lax P, Esquiva G, Martín-Nieto J, Pinilla I, Cuenca N. Safranal, a saffron constituent, attenuates retinal degeneration in P23H rats. PLoS One. (2012) 7:e43074. doi: 10.1371/journal.pone.0043074, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maccarone R, Rapino C, Zerti D, Di Tommaso M, Battista N, Di Marco S, et al. Modulation of Type-1 and Type-2 cannabinoid receptors by saffron in a rat model of retinal neurodegeneration. PLoS One. (2016) 11:e0166827. doi: 10.1371/journal.pone.0166827, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marco FD, Romeo S, Nandasena C, Purushothuman S, Adams C, Bisti S, et al. The time course of action of two neuroprotectants, dietary saffron and photobiomodulation, assessed in the rat retina. Am J Neurodegener Dis. (2013) 2:208–20. [PMC free article] [PubMed] [Google Scholar]
- 137.Schmidt M, Betti G, Hensel A. Saffron in phytotherapy: pharmacology and clinical uses. Wien Med Wochenschr. (2007) 157:315–9. doi: 10.1007/s10354-007-0428-4, PMID: [DOI] [PubMed] [Google Scholar]
- 138.Manasa PSL, Kamble AD, Chilakamarthi U. Various extraction techniques of curcumin-a comprehensive review. Acs Omega. (2023) 8:34868–78. doi: 10.1021/acsomega.3c04205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zielińska A, Alves H, Marques V, Durazzo A, Lucarini M, Alves TF, et al. Properties, extraction methods, and delivery Systems for Curcumin as a natural source of beneficial health effects. Medicina. (2020) 56:7. doi: 10.3390/medicina56070336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Radomska-Leśniewska DM, Osiecka-Iwan A, Hyc A, GóŹDŹ A, Dąbrowska AM, Skopiński P. Therapeutic potential of curcumin in eye diseases. Cent Eur J Immunol. (2019) 44:181–9. doi: 10.5114/ceji.2019.87070, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vasireddy V, Chavali VR, Joseph VT, Kadam R, Lin JH, Jamison JA, et al. Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation. PLoS One. (2011) 6:e21193. doi: 10.1371/journal.pone.0021193, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scott PA, Kaplan HJ, Mccall MA. Prenatal exposure to curcumin protects rod photoreceptors in a transgenic Pro23His swine model of retinitis Pigmentosa. Transl Vis Sci Technol. (2015) 4:5. doi: 10.1167/tvst.4.5.5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Emoto Y, Yoshizawa K, Uehara N, Kinoshita Y, Yuri T, Shikata N, et al. Curcumin suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. In Vivo. (2013) 27:583–90. [PubMed] [Google Scholar]
- 144.Xu XY, Meng X, Li S, Gan RY, Li Y, Li HB. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current Progress, challenges, and perspectives. Nutrients. (2018) 10:7. doi: 10.3390/nu10101553, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Campochiaro PA, Strauss RW, Lu L, Hafiz G, Wolfson Y, Shah SM, et al. Is there excess oxidative stress and damage in eyes of patients with retinitis Pigmentosa? Antioxid Redox Signal. (2015) 23:643–8. doi: 10.1089/ars.2015.6327, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Q-L, Chen X-Y, Zhu L, Chen H-B, Ho H-M, Yeung W-P, et al. Review on Saussurea laniceps, a potent medicinal plant known as “snow lotus”: botany, phytochemistry and bioactivities. Phytochem Rev. (2016) 15:537–65. doi: 10.1007/s11101-015-9452-y [DOI] [Google Scholar]
- 147.Chik WI, Zhu L, Fan LL, Yi T, Zhu GY, Gou XJ, et al. Saussurea involucrata: a review of the botany, phytochemistry and ethnopharmacology of a rare traditional herbal medicine. J Ethnopharmacol. (2015) 172:44–60. doi: 10.1016/j.jep.2015.06.033, PMID: [DOI] [PubMed] [Google Scholar]
- 148.Fan J-Y, Chen H-B, Zhu L, Chen H-L, Zhao Z-Z, Yi T. Saussurea medusa, source of the medicinal herb snow lotus: a review of its botany, phytochemistry, pharmacology and toxicology. Phytochem Rev. (2015) 14:353–66. doi: 10.1007/s11101-015-9408-2 [DOI] [Google Scholar]
- 149.Ng K-W, Cao Z-J, Chen H-B, Zhao Z-Z, Zhu L, Yi T. Oolong tea: a critical review of processing methods, chemical composition, health effects, and risk. Crit Rev Food Sci Nutr. (2018) 58:2957–80. doi: 10.1080/10408398.2017.1347556, PMID: [DOI] [PubMed] [Google Scholar]
- 150.Wei H, Liu S, Liao Y, Ma C, Wang D, Tong J, et al. A systematic review of the medicinal potential of mulberry in treating diabetes mellitus. Am J Chin Med. (2018) 46:1743–70. doi: 10.1142/S0192415X1850088X, PMID: [DOI] [PubMed] [Google Scholar]
- 151.Ahn K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Rep. (2017) 50:111–6. doi: 10.5483/BMBRep.2017.50.3.221, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu C, Lee SL, Taylor C, Li J, Chan YM, Agarwal R, et al. Scientific and regulatory approach to botanical drug development: a U.S. Fda perspective. J Nat Prod. (2020) 83:552–62. doi: 10.1021/acs.jnatprod.9b00949 [DOI] [PubMed] [Google Scholar]
- 153.Haron MH, Zhang J, Chittiboyina AG, Khan IA, Pugh ND. Validation of a toll-like receptor (Tlr)2/Tlr1 activation assay for biological standardization of Arthrospira/Limnospira immune-enhancing potency. J Dietary Supplements. (2024) 21:281–93. doi: 10.1080/19390211.2023.2263566, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fu R, Li J, Yu H, Zhang Y, Xu Z, Martin C. The yin and Yang of traditional Chinese and Western medicine. Med Res Rev. (2021) 41:3182–200. doi: 10.1002/med.21793 [DOI] [PubMed] [Google Scholar]
- 155.Liu M, Gao Y, Yuan Y, Yang K, Shi S, Zhang J, et al. Efficacy and safety of integrated traditional Chinese and Western medicine for Corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. Pharmacol Res. (2020) 158:104896. doi: 10.1016/j.phrs.2020.104896, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang WJ, Zhang T. Integration of traditional Chinese medicine and Western medicine in the era of precision medicine. J Integr Med. (2017) 15:1–7. doi: 10.1016/S2095-4964(17)60314-5, PMID: [DOI] [PubMed] [Google Scholar]
- 157.Jin K, Zhang C. Personalized medicine in ophthalmic diseases: challenges and opportunities. J Pers Med. (2023) 13:7. doi: 10.3390/jpm13060893, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zheng A, Li Y, Tsang SH. Personalized therapeutic strategies for patients with retinitis pigmentosa. Expert Opin Biol Ther. (2015) 15:391–402. doi: 10.1517/14712598.2015.1006192, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.