Abstract
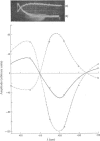
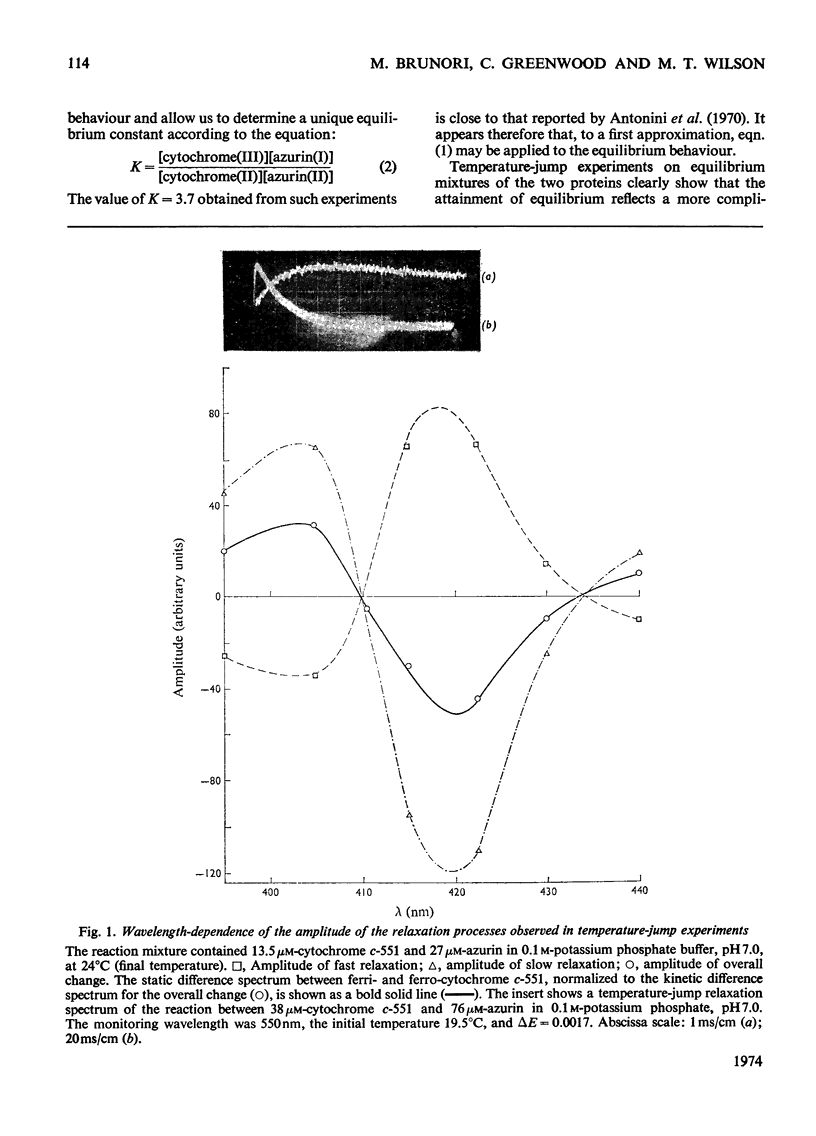
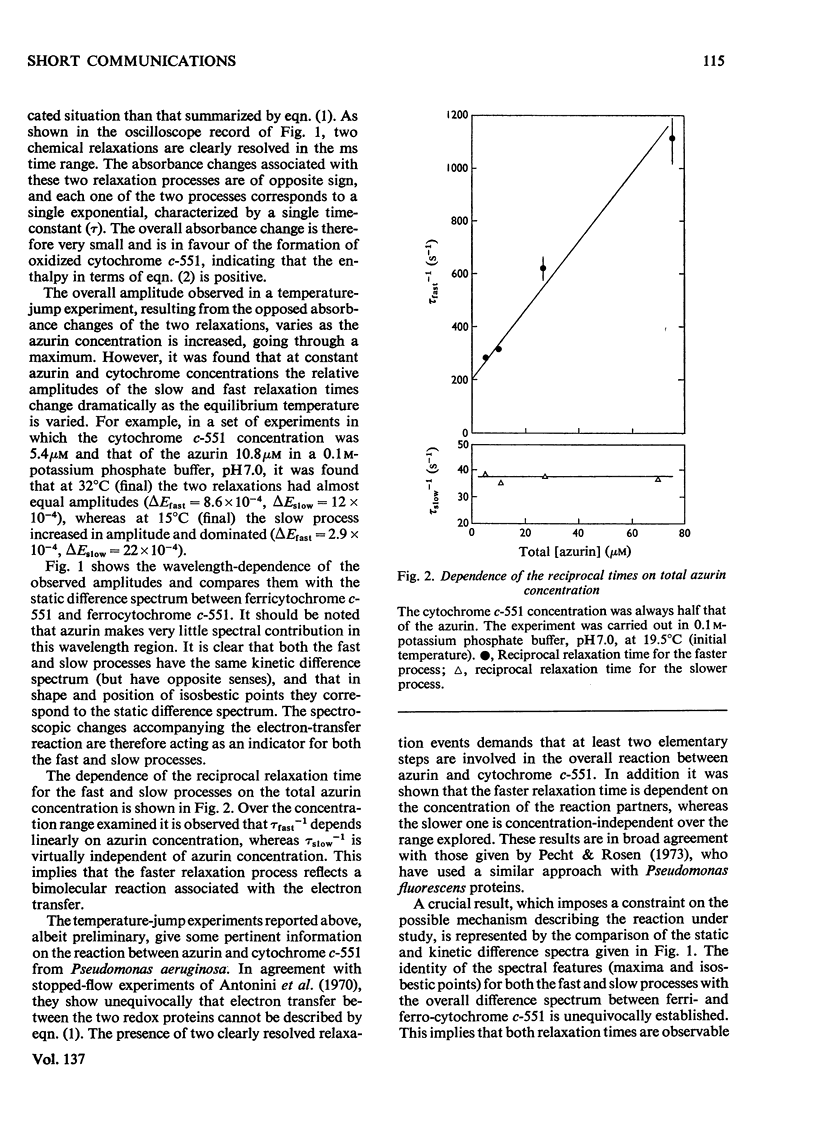
Temperature-jump studies on the electron-transfer reaction between azurin and cytochrome c-551 clearly reveal two chemical relaxations. The amplitudes of these relaxation processes have identical spectral distributions, but the relaxation times show different dependences on the reactant concentrations. These findings are discussed in terms of possible models.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE PURIFICATION AND AMINO ACID COMPOSITION OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:341–349. doi: 10.1042/bj0890341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini E., Finazzi-Agrò A., Avigliano A., Guerrieri P., Rotilio G., Mondovì B. Kinetics of electron transfer between azurin and cytochrome 551 from Pseudomonas. J Biol Chem. 1970 Sep 25;245(18):4847–4849. [PubMed] [Google Scholar]
- Brill A. S., Bryce G. F., Maria H. J. Optical and magnetic properties of Pseudomonas azurins. Biochim Biophys Acta. 1968 Feb 19;154(2):342–351. doi: 10.1016/0005-2795(68)90048-2. [DOI] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., SASAGAWA M., KUSAI K., NAKAI M., OKUNUKI K. Preparation of crystalline Pseudomonas cvtochrome c-551 and its general properties. Biochem J. 1960 Oct;77:194–201. doi: 10.1042/bj0770194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecht I., Rosen P. The kinetics of the cytochrome-c-azurin redox equilibrium. Biochem Biophys Res Commun. 1973 Feb 5;50(3):853–858. doi: 10.1016/0006-291x(73)91323-5. [DOI] [PubMed] [Google Scholar]