Abstract
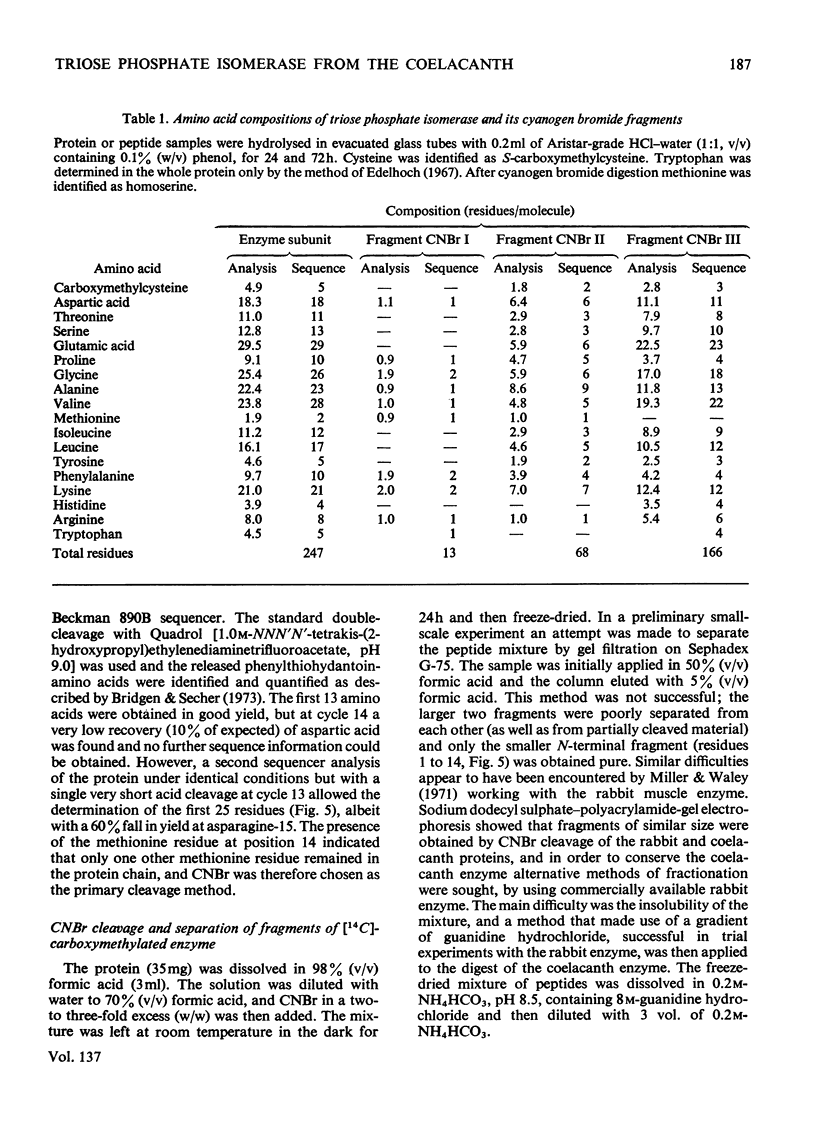
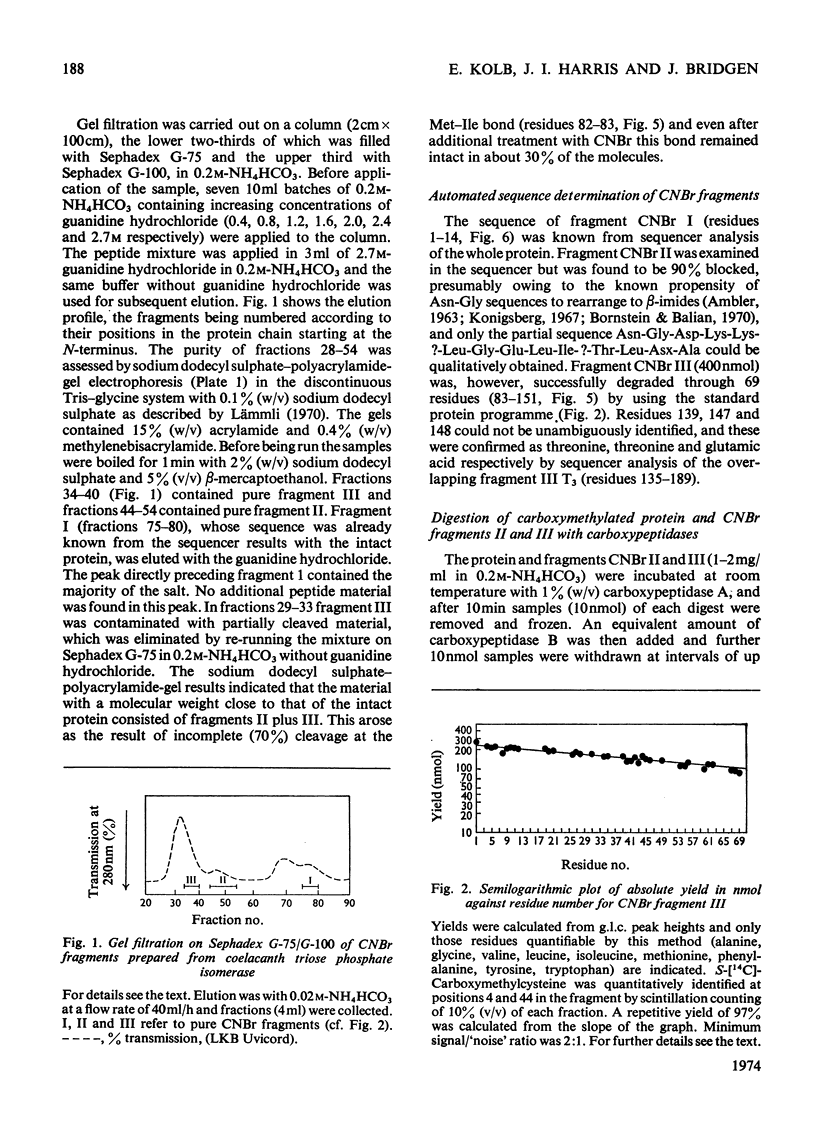
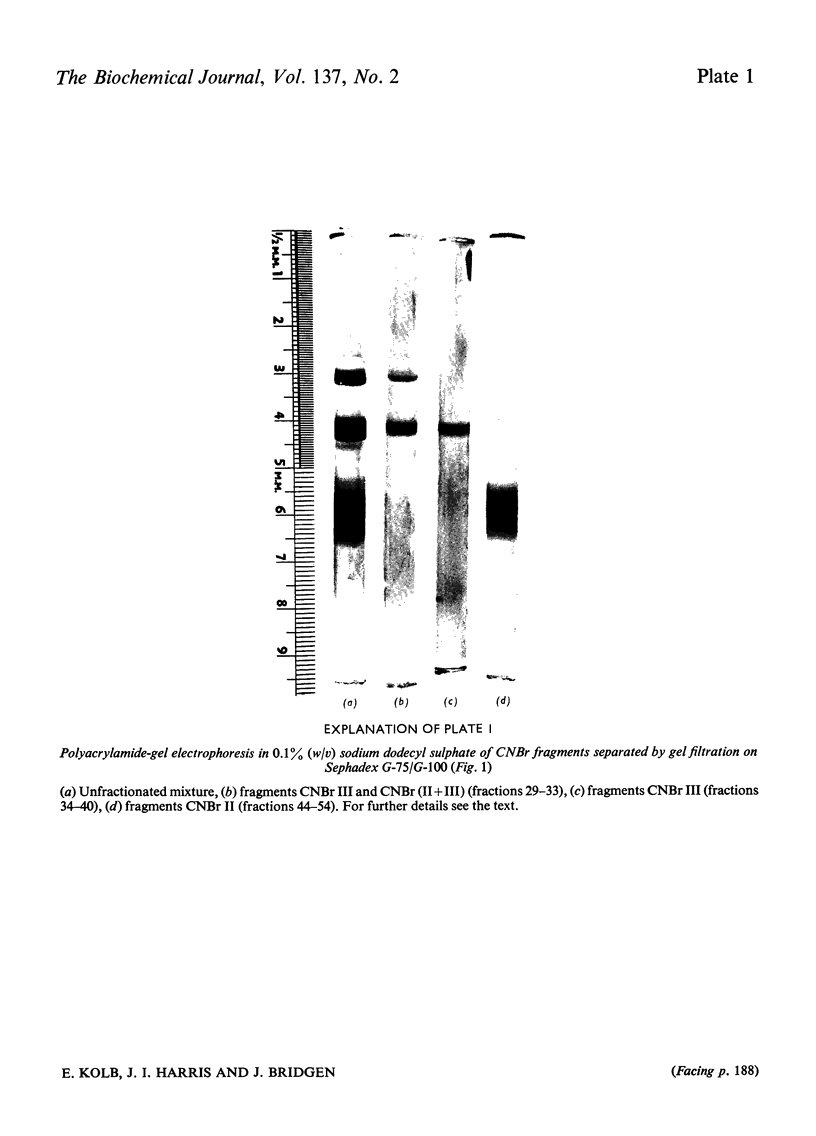
The preparation and purification of cyanogen bromide fragments from [14C]carboxymethylated coelacanth triose phosphate isomerase is presented. The automated sequencing of these fragments, the lysine-blocked tryptic peptides derived from them, and also of the intact protein, is described. Combination with results from manual sequence analysis has given the 247-residue amino acid sequence of coelacanth triose phosphate isomerase in 4 months, by using 100mg of enzyme. (Two small adjacent peptides were placed by homology with the rabbit enzyme.) Comparison of this sequence with that of the rabbit muscle enzyme shows that 207 (84%) of the residues are identical. This slow rate of evolutionary change (corresponding to two amino acid substitutions per 100 residues per 100 million years) is similar to that found for glyceraldehyde 3-phosphate dehydrogenase. The reliability of sequence information obtained by automated methods is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Balian G. The specific nonenzymatic cleavage of bovine ribonuclease with hydroxylamine. J Biol Chem. 1970 Sep 25;245(18):4854–4856. [PubMed] [Google Scholar]
- Braunitzer G., Chen R., Schrank B., Stangl A. Automatische Sequenzanalyse eines Proteins (beta-Lactoglobulin AB. Hoppe Seylers Z Physiol Chem. 1972 May;353(5):832–834. [PubMed] [Google Scholar]
- Braunitzer G., Schrank B., Ruhfus A., Petersen S., Petersen U. Zur vollständigen automatischen Sequenzanalyse von Peptiden mit Quadrol. Hoppe Seylers Z Physiol Chem. 1971 Dec;352(12):1730–1732. [PubMed] [Google Scholar]
- Bridgen J., Kolb E., Harris J. I. Amino acid sequence homology in alcohol dehydrogenase. FEBS Lett. 1973 Jun 15;33(1):1–3. doi: 10.1016/0014-5793(73)80144-9. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Secher D. S. Molecular heterogeneity of alkaline phosphatase. FEBS Lett. 1973 Jan 1;29(1):55–57. doi: 10.1016/0014-5793(73)80014-6. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Lebeman R. The use of maleic anhydride for the reversible blocking of amino groups in polypeptide chains. Biochem J. 1969 May;112(5):679–689. doi: 10.1042/bj1120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The amino acid sequence of rabbit muscle triose phosphate isomerase. FEBS Lett. 1973 Feb 15;30(1):97–99. doi: 10.1016/0014-5793(73)80627-1. [DOI] [PubMed] [Google Scholar]
- Davidson B. E., Sajgò M., Noller H. F., Harris J. I. Amino-acid sequence of glyceraldehyde 3-phosphate dehydrogenase from lobster muscle. Nature. 1967 Dec 23;216(5121):1181–1185. doi: 10.1038/2161181a0. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Harris J. I., Perham R. N. Glyceraldehyde 3-phosphate dehydrogenase from pig muscle. Nature. 1968 Sep 7;219(5158):1025–1028. doi: 10.1038/2191025a0. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Hood L., McKean D., Farnsworth V., Potter M. Mouse immunoglobulin chains. A survey of the amino-terminal sequences of kappa chains. Biochemistry. 1973 Feb;12(4):741–749. doi: 10.1021/bi00728a026. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. M.T., Harris J. I. Glyceraldehyde 3-phosphate dehydrogenase: Amino acid sequence of enzyme from baker's yeast. FEBS Lett. 1972 May 1;22(2):185–189. doi: 10.1016/0014-5793(72)80040-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landon M., Langley T. J., Smith E. L. Sequence of bovine liver glutamate dehydrogenase. VI. Peptides from tryptic and thermolysin hydrolysates of two large cyanogen bromide fragments. J Biol Chem. 1971 Jun 25;246(12):3807–3816. [PubMed] [Google Scholar]
- Laursen R. A., Horn M. J., Bonner A. G. Solid-phase Edman degradation. The use of p-phenyl diisothiocyanate to attach lysine- and arginine-containing peptides to insoluble resins. FEBS Lett. 1972 Mar;21(1):67–70. doi: 10.1016/0014-5793(72)80165-0. [DOI] [PubMed] [Google Scholar]
- Miller J. C., Waley S. G. Amino acid sequences around the cysteine residues of rabbit muscle triose phosphate isomerase. Biochem J. 1971 Apr;122(2):209–218. doi: 10.1042/bj1220209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niall H. D. Automated sequence analysis of proteins and peptides. J Agric Food Chem. 1971 Jul-Aug;19(4):638–644. doi: 10.1021/jf60176a004. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- SMITH I. Colour reactions on paper chromatograms by a dipping technique. Nature. 1953 Jan 3;171(4340):43–44. doi: 10.1038/171043a0. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Evidence for the amino acid sequence of porcine pancreatic elastase. Biochem J. 1973 Apr;131(4):643–675. doi: 10.1042/bj1310643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Evidence for the amino acid sequence of porcine pancreatic elastase. Biochem J. 1973 Apr;131(4):643–675. doi: 10.1042/bj1310643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhorst C., Möller W., Laursen R., Wittmann-Liebold B. The primary structure of an acidic protein from 50-S ribosomes of Escherichia coli which is involved in GTP hydrolysis dependent on elongation factors G and T. Eur J Biochem. 1973 Apr 2;34(1):138–152. doi: 10.1111/j.1432-1033.1973.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Wingard M., Matsueda G., Wolfe R. S. Myxobacter AL-1 protease II: specific peptide bond cleavage on the amino side of lysine. J Bacteriol. 1972 Nov;112(2):940–949. doi: 10.1128/jb.112.2.940-949.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]