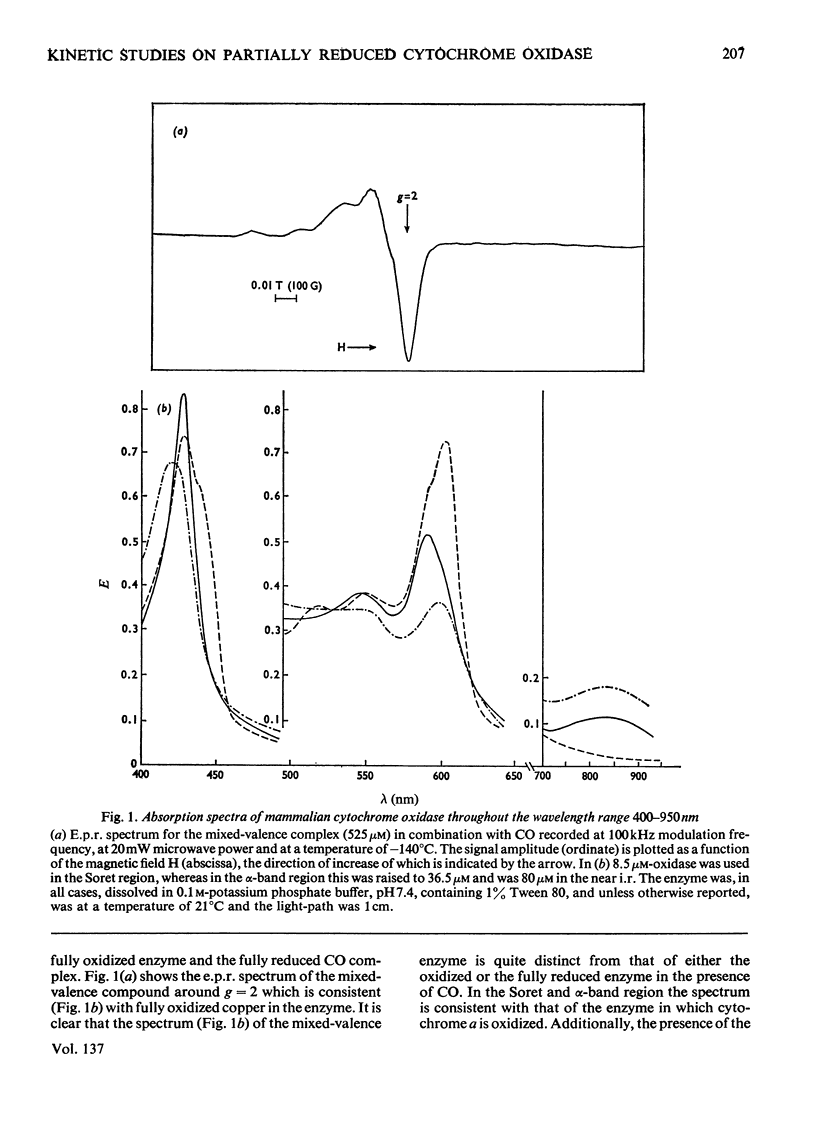
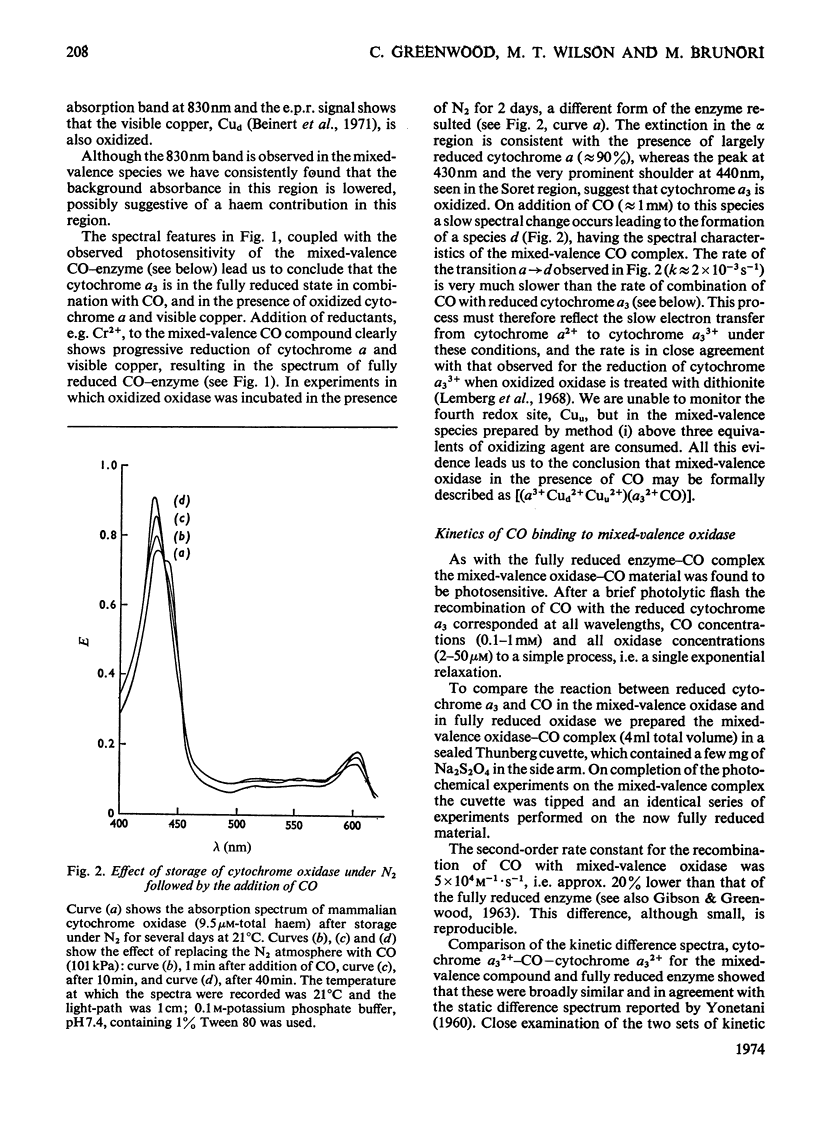
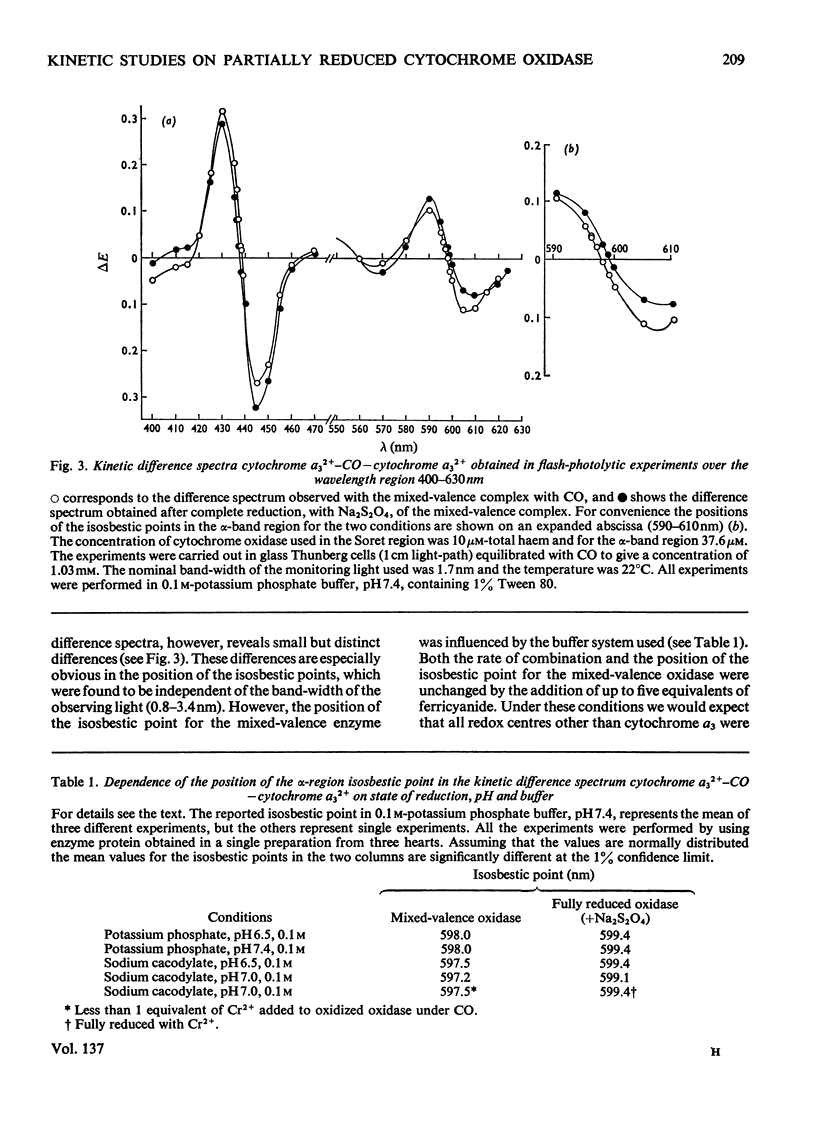
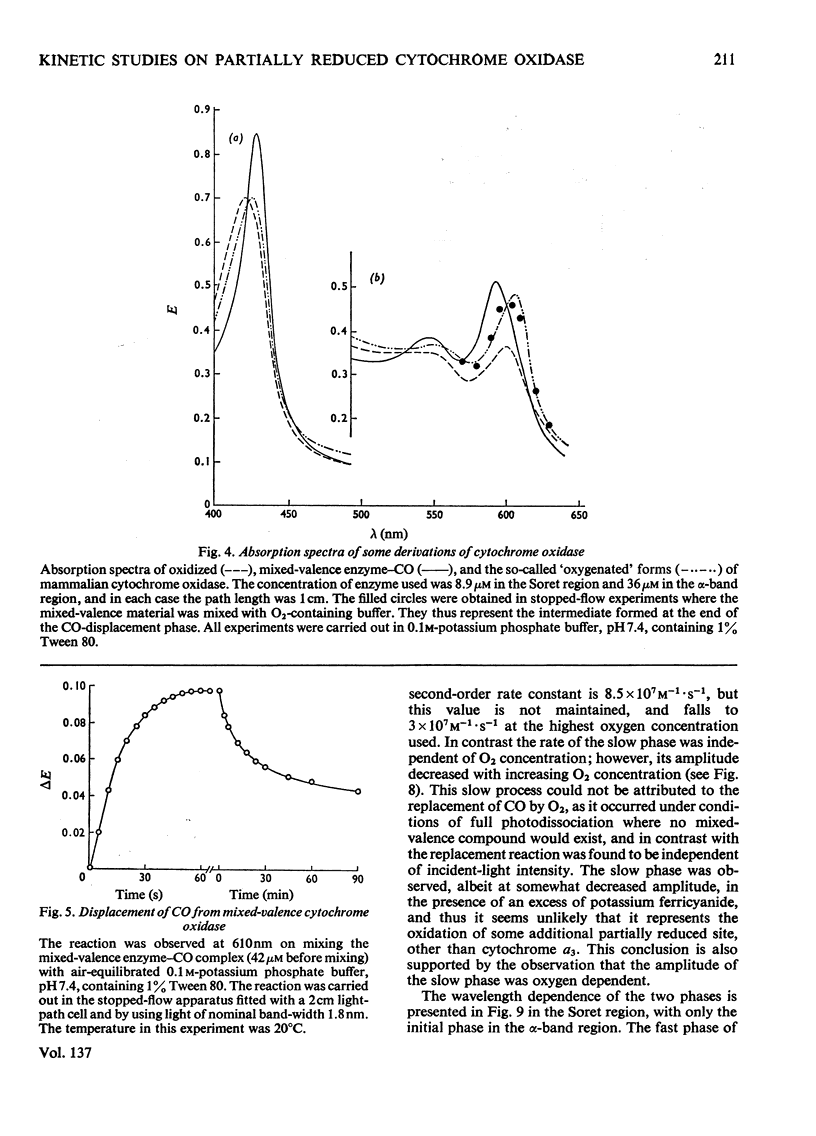
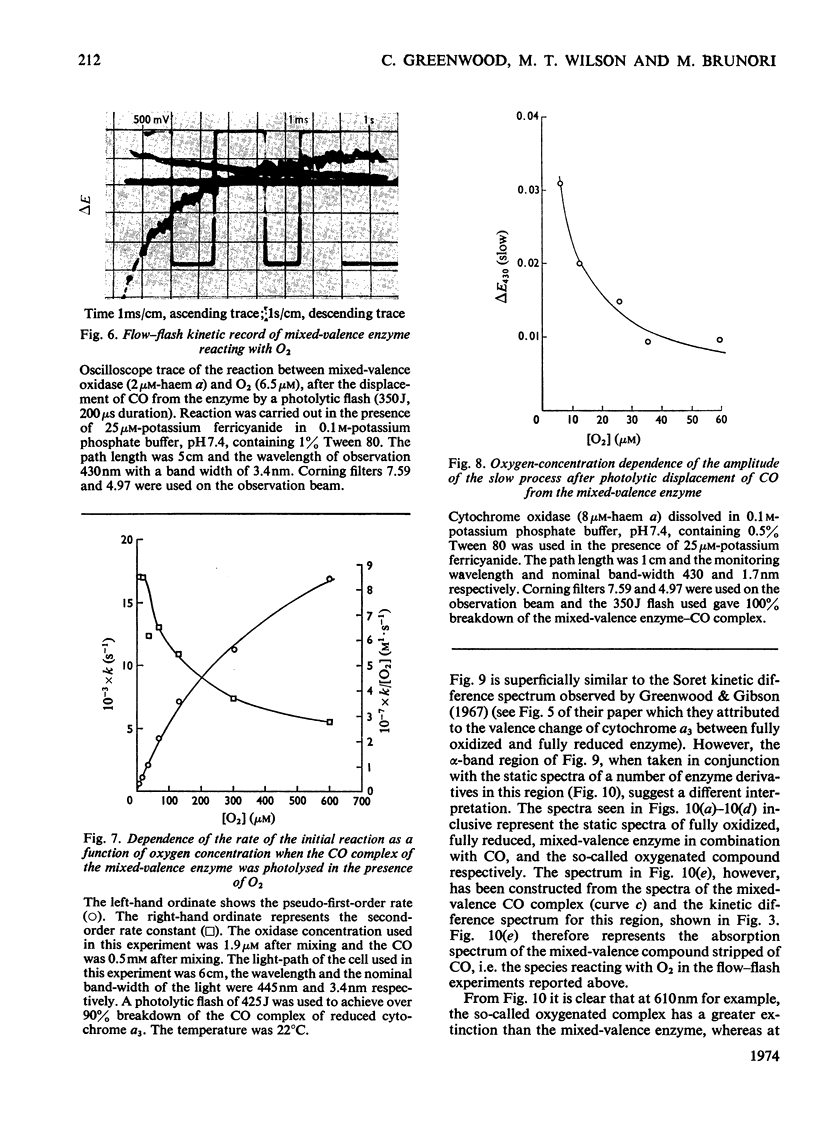
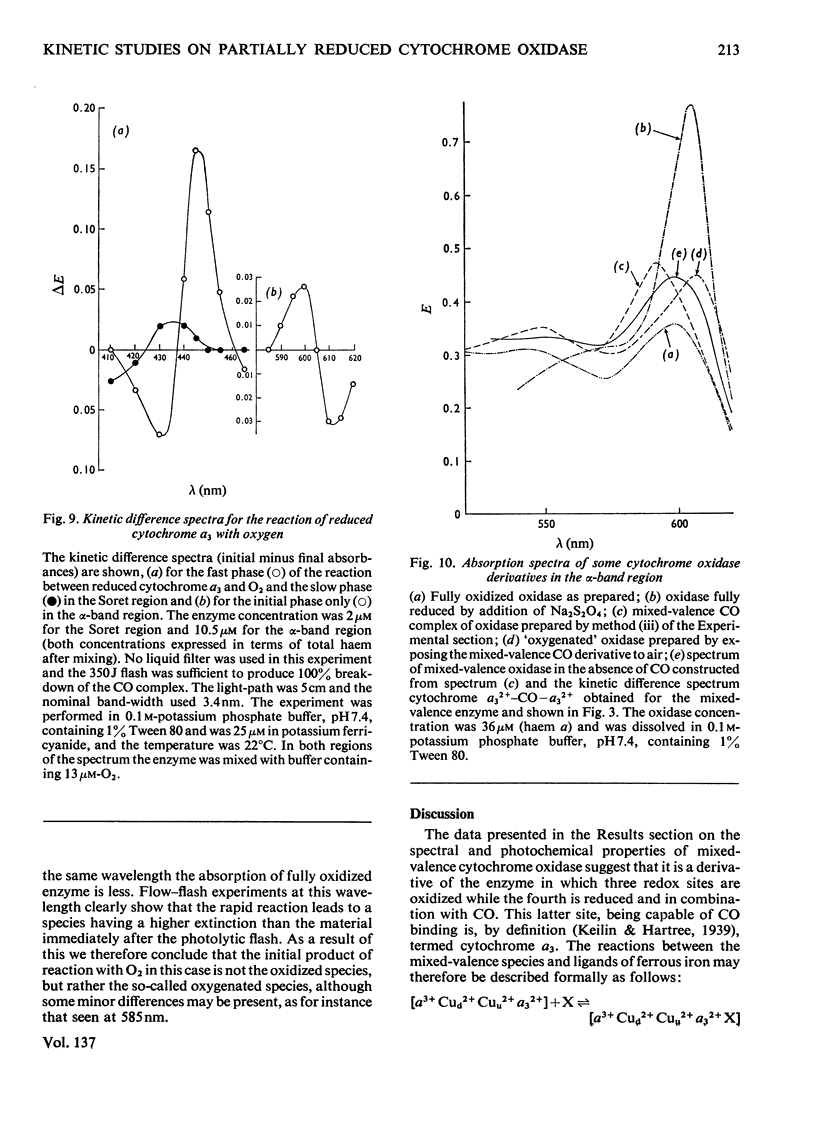
Abstract
A number of methods were used to prepare a species of mammalian cytochrome oxidase (EC 1.9.3.1, ferrocytochrome c–oxygen oxidoreductase) in which only cytochrome a3 is reduced and in combination with CO. The kinetics of CO binding by cytochrome a32+ in this species is significantly different from that exhibited by cytochrome a32+ in the fully reduced enzyme. The second-order rate constant for combination was 5×104m−1·s−1 and the `off' constant was 3×10−2s−1. The kinetic difference spectra cytochrome a32+–cytochrome a32+–CO reveal further differences between the mixed-valence and the fully reduced enzyme. The reaction between cytochrome a32+ and oxygen in the mixed-valence species was followed in flow–flash experiments and reveals a fast, oxygen-dependent (8×107m−1·s−1 at low oxygen) rate followed by a slow process, whose rate is independent of oxygen but whose amplitude is dependent on [O2]. The fast oxygen-dependent reaction yields as the first product the so-called `oxygenated' enzyme. We conclude from these experiments that the ligand-binding behaviour of cytochrome a3 depends on the redox state of its partners, a fact which represents clear evidence for site–site interaction in this enzyme. The fact that oxygen reacts rapidly with this enzyme species in which only one component, namely cytochrome a3, is reduced represents clear and unequivocal evidence that this is indeed the O2-binding site in cytochrome oxidase and may indicate that reduction of oxygen can proceed via single electron steps.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H., GRIFFITHS D. E., WHARTON D. C., SANDS R. H. Properties of the copper associated with cytochrome oxidase as studied by paramagnetic resonance spectroscopy. J Biol Chem. 1962 Jul;237:2337–2346. [PubMed] [Google Scholar]
- Dawson J. W., Gray H. B., Holwerda R. A., Westhead E. W. Kinetics of the reduction of metalloproteins by chromous ion (laccase-cytochrome c-plastocyanins-temperature-rate constants). Proc Natl Acad Sci U S A. 1972 Jan;69(1):30–33. doi: 10.1073/pnas.69.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., AINSWORTH S. Photosensitivity of haem compounds. Nature. 1957 Dec 21;180(4599):1416–1417. doi: 10.1038/1801416b0. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963 Mar;86:541–554. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. THE SPECTRA AND SOME PROPERTIES OF CYTOCHROME OXIDASE COMPONENTS. J Biol Chem. 1964 Feb;239:586–590. [PubMed] [Google Scholar]
- GIBSON Q. H., PALMER G., WHARTON D. C. THE BINDING OF CARBON MONOXIDE BY CYTOCHROME C OXIDASE AND THE RATIO OF THE CYTOCHROMES A AND A3. J Biol Chem. 1965 Feb;240:915–920. [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C., Gibson Q. H. The reaction of reduced cytochrome C oxidase with oxygen. J Biol Chem. 1967 Apr 25;242(8):1782–1787. [PubMed] [Google Scholar]
- Knowles P. F., Gibson J. F., Pick F. M., Bray R. C. Electron-spin-resonance evidence for enzymic reduction of oxygen to a free radical, the superoxide ion. Biochem J. 1969 Jan;111(1):53–58. doi: 10.1042/bj1110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muijsers A. O., Tiesjema R. H., Henderson R. W., Van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VII. The effect of cytochrome c on the oxidation-reduction potential of isolated cytochrome aa 3 . Biochim Biophys Acta. 1972 Apr 20;267(1):216–221. doi: 10.1016/0005-2728(72)90154-5. [DOI] [PubMed] [Google Scholar]
- Myer Y. P. The "oxygenated complex" of cytochrome c oxidase. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1194–1200. doi: 10.1016/0006-291x(72)90595-5. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Tiesjema R. H., Muijsers A. O., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . IV. Some properties of oxygenated cytochrome aa 3 . Biochim Biophys Acta. 1972 Jan 21;256(1):32–42. doi: 10.1016/0005-2728(72)90160-0. [DOI] [PubMed] [Google Scholar]
- Tiesjema R. H., Muijsers A. O., van Gelder B. F. Biochemical and biophysical studies on cytochrome c oxidase. X. Spectral and potentiometric properties of the hemes and coppers. Biochim Biophys Acta. 1973 Apr 27;305(1):19–28. doi: 10.1016/0005-2728(73)90227-2. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Lindsay J. G., Brocklehurst E. S. Heme-heme interaction in cytochrome oxidase. Biochim Biophys Acta. 1972 Feb 28;256(2):277–286. doi: 10.1016/0005-2728(72)90058-8. [DOI] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. III. Improved preparation and some properties. J Biol Chem. 1961 Jun;236:1680–1688. [PubMed] [Google Scholar]
- Yong F. C., King T. E. Studies on cytochrome oxidase. IX. Heme-copper interaction. J Biol Chem. 1972 Oct 25;247(20):6384–6388. [PubMed] [Google Scholar]