Abstract
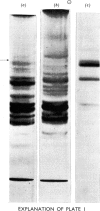
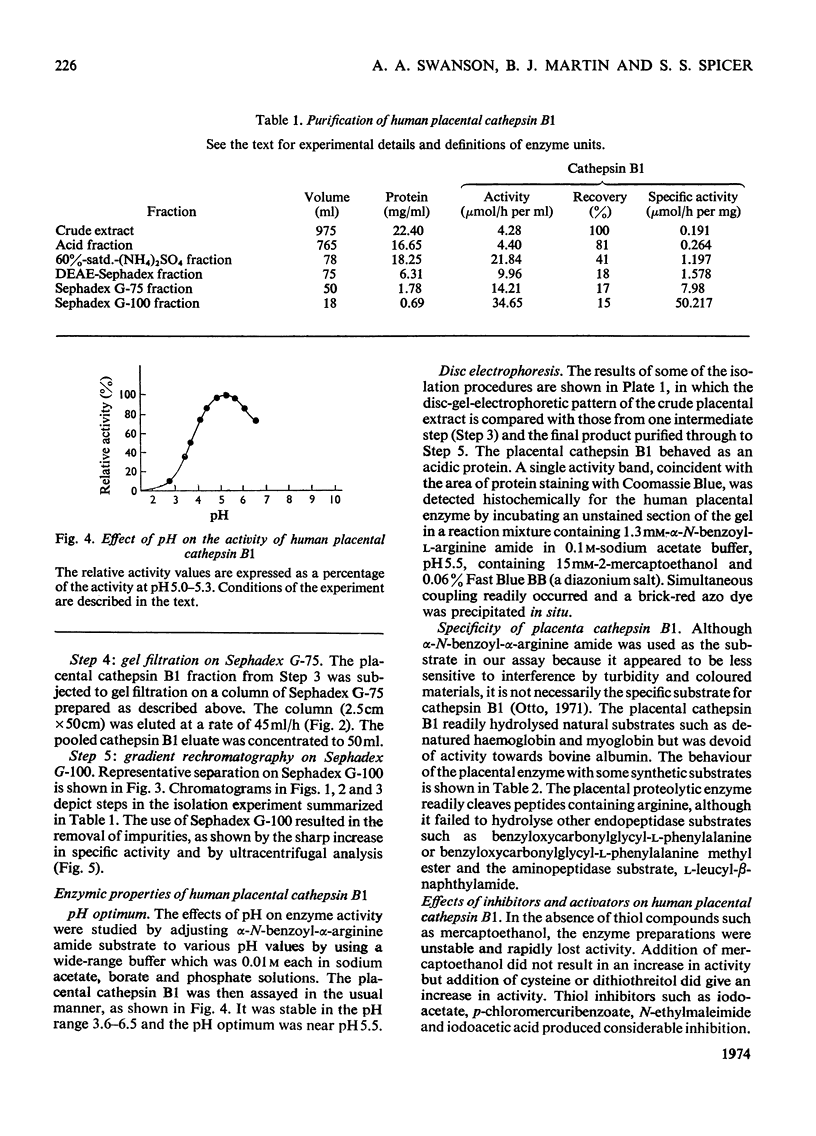
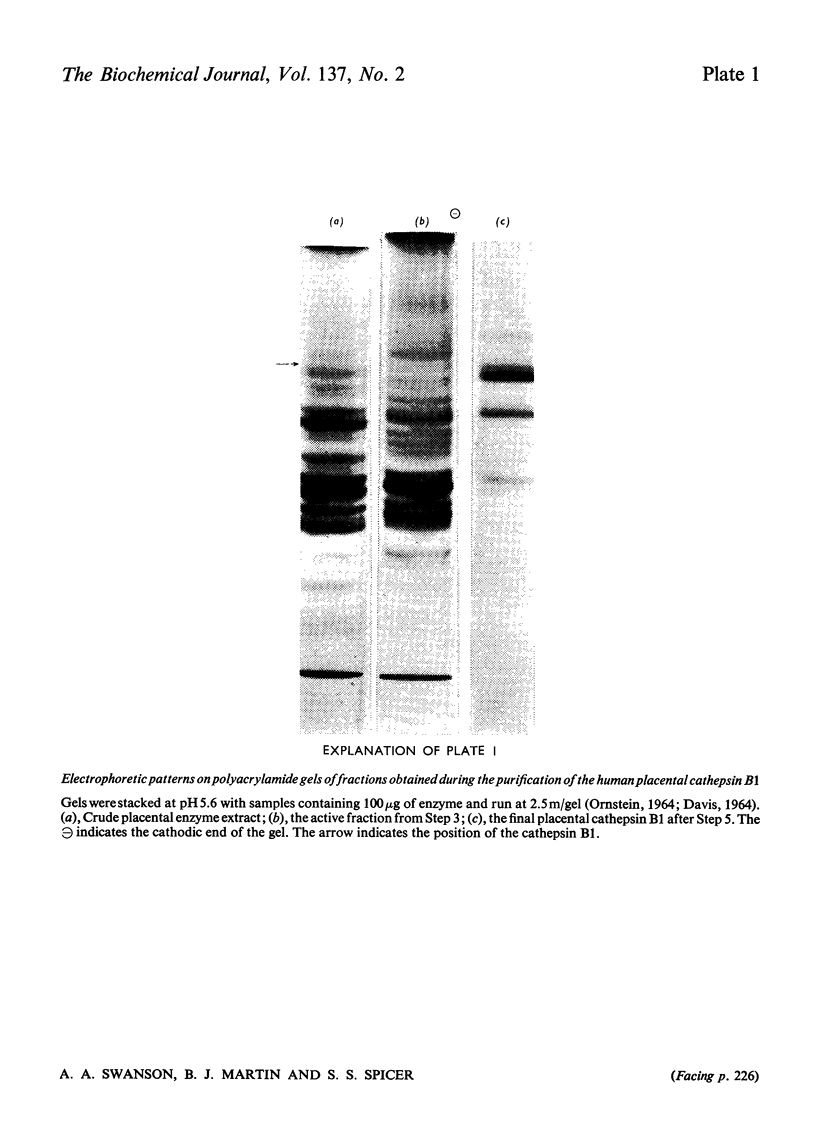
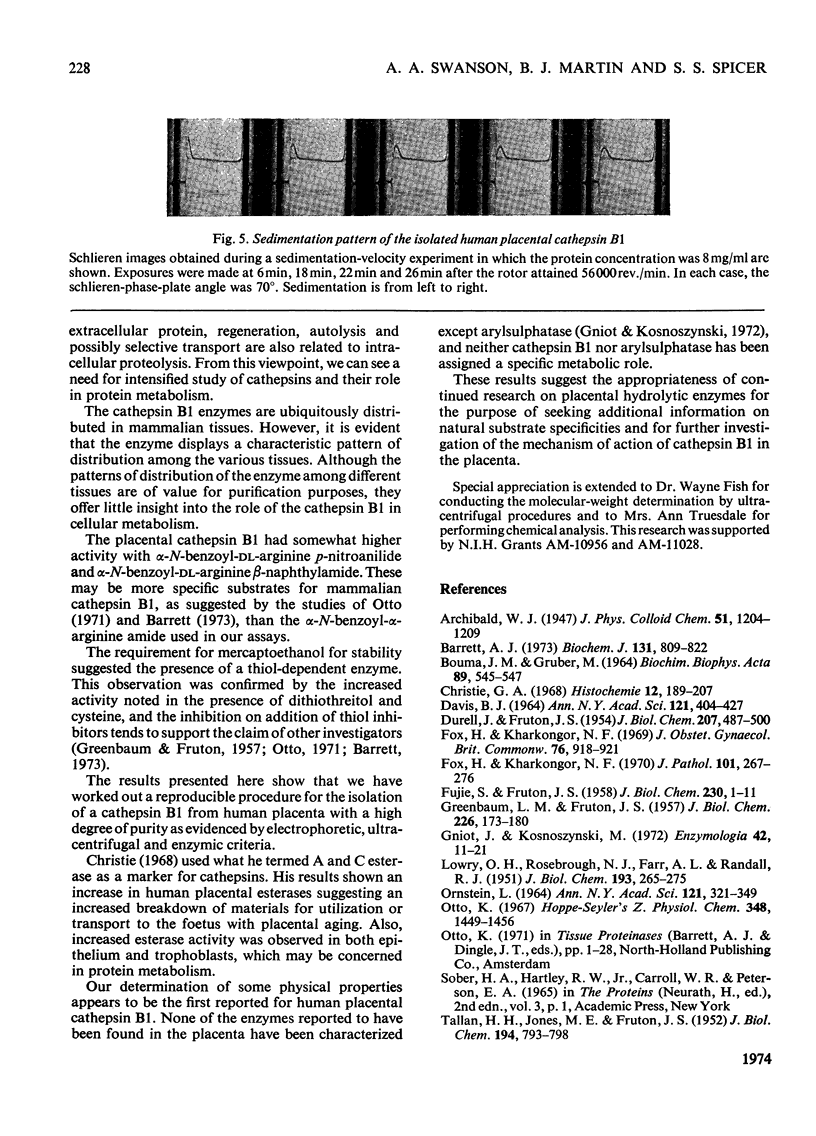
A reproducible procedure for the isolation, from human placenta, of a cathepsin B1 in a homogeneous state, demonstrated by electrophoretic, ultracentrifugal and enzymic criteria, was carried out. The pH optimum was near pH5.5. The placental enzyme catalysed the release of acid-soluble u.v.-dense products from haemoglobin and myoglobin. It was inhibited by heavy metals and several compounds which react with the thiol groups. The optimum temperature was between 37° and 42°C. The molecular weight of the enzyme was calculated to be 24250.
Full text
PDF
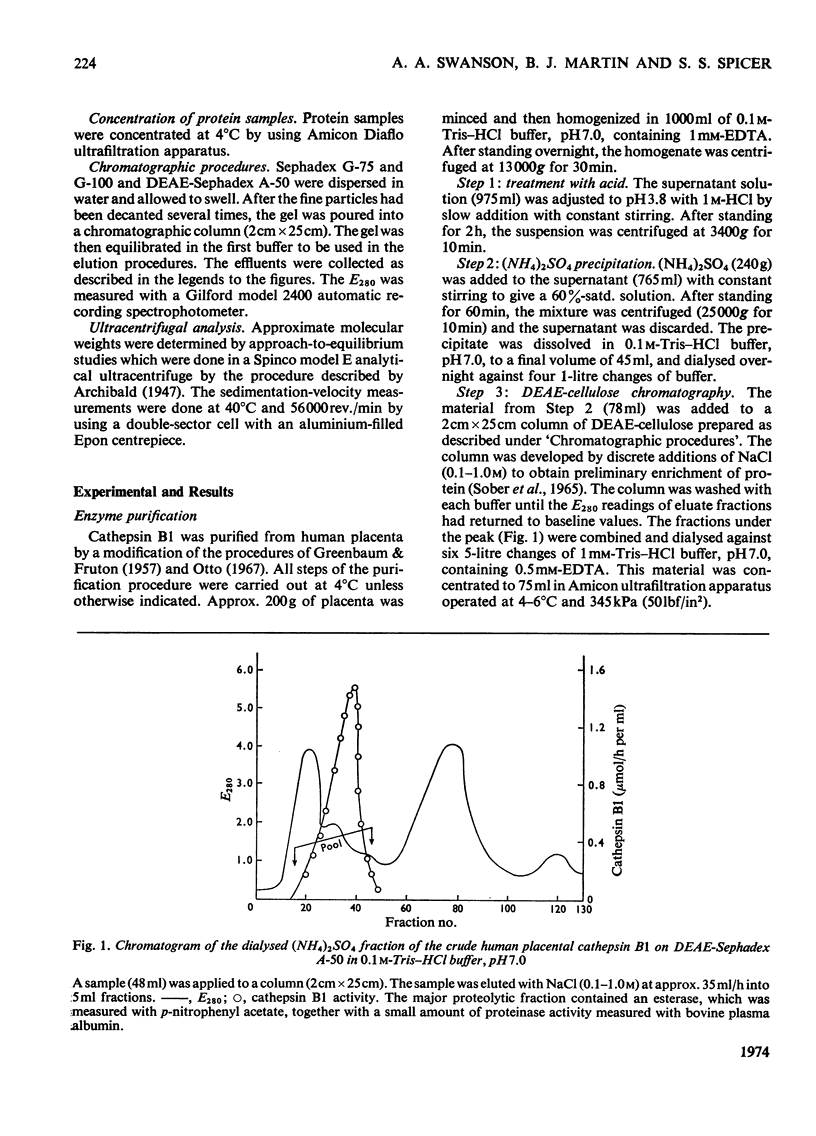
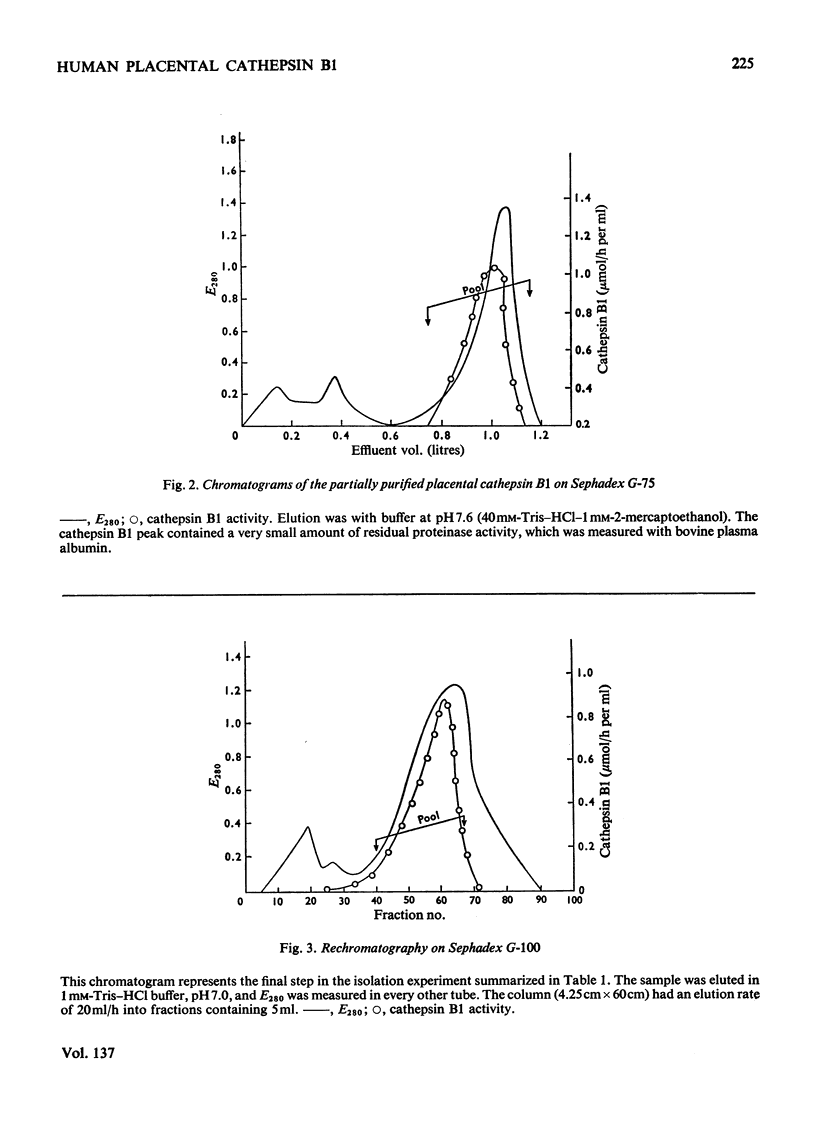

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOUMA J. M., GRUBER M. THE DISTRIBUTION OF CATHEPSINS B AND C IN RAT TISSUES. Biochim Biophys Acta. 1964 Sep 18;89:545–547. doi: 10.1016/0926-6569(64)90082-3. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie G. A. Comparative histochemical distribution of acid phosphatase, non-specific esterase and beta-glucuronidase in the placenta and foetal membranes. Histochemie. 1968;12(3):189–207. doi: 10.1007/BF00305996. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DURELL J., FRUTON J. S. Proteinase-catalyzed transamidation and its efficiency. J Biol Chem. 1954 Apr;207(2):487–500. [PubMed] [Google Scholar]
- FUJII S., FRUTON J. S. Transamidation reactions catalyzed by cathepsins. J Biol Chem. 1958 Jan;230(1):1–11. [PubMed] [Google Scholar]
- Fox H., Kharkongor F. N. Morphology and enzyme histochemistry of cells derived from placental villi in tissue culture. J Pathol. 1970 Jul;101(3):267–276. doi: 10.1002/path.1711010310. [DOI] [PubMed] [Google Scholar]
- Fox H., Kharkongor N. F. Enzyme histochemistry of the Hofbauer cells of the human placenta. J Obstet Gynaecol Br Commonw. 1969 Oct;76(10):918–921. doi: 10.1111/j.1471-0528.1969.tb15730.x. [DOI] [PubMed] [Google Scholar]
- GREENBAUM L. M., FRUTON J. S. Purification and properties of beef spleen cathepsin B. J Biol Chem. 1957 May;226(1):173–180. [PubMed] [Google Scholar]
- Gniot-Szulzycka J., Komoszyński M. Human placenta sulphohydrolase C subfractions. Enzymologia. 1972 Jan 31;42(1):11–21. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Otto K. Uber ein neues Kathepsin. Reinigung aus Rindermilz, Eigenschaften, sowie Vergleich mit Kathepsin B. Hoppe Seylers Z Physiol Chem. 1967 Nov;348(11):1449–1460. [PubMed] [Google Scholar]
- TALLAN H. H., JONES M. E., FRUTON J. S. On the proteolytic enzymes of animal tissues. X. Beef spleen cathepsin C. J Biol Chem. 1952 Feb;194(2):793–805. [PubMed] [Google Scholar]