Abstract
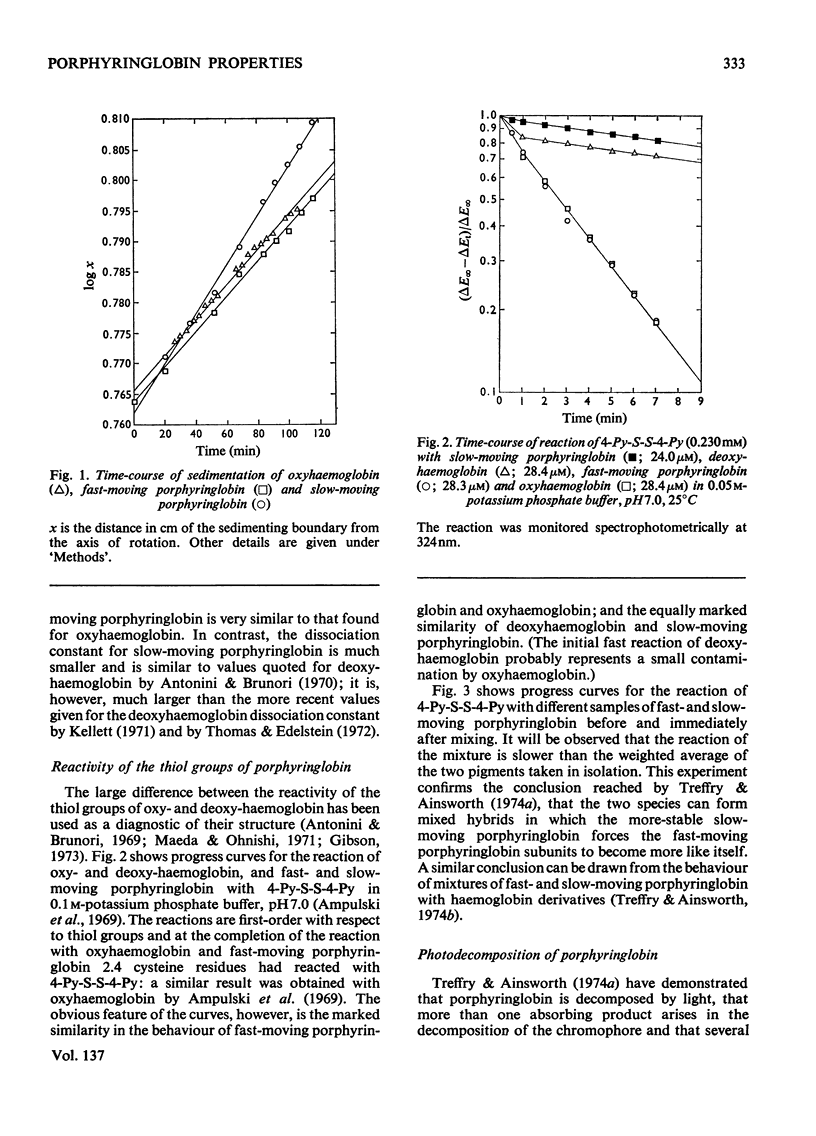
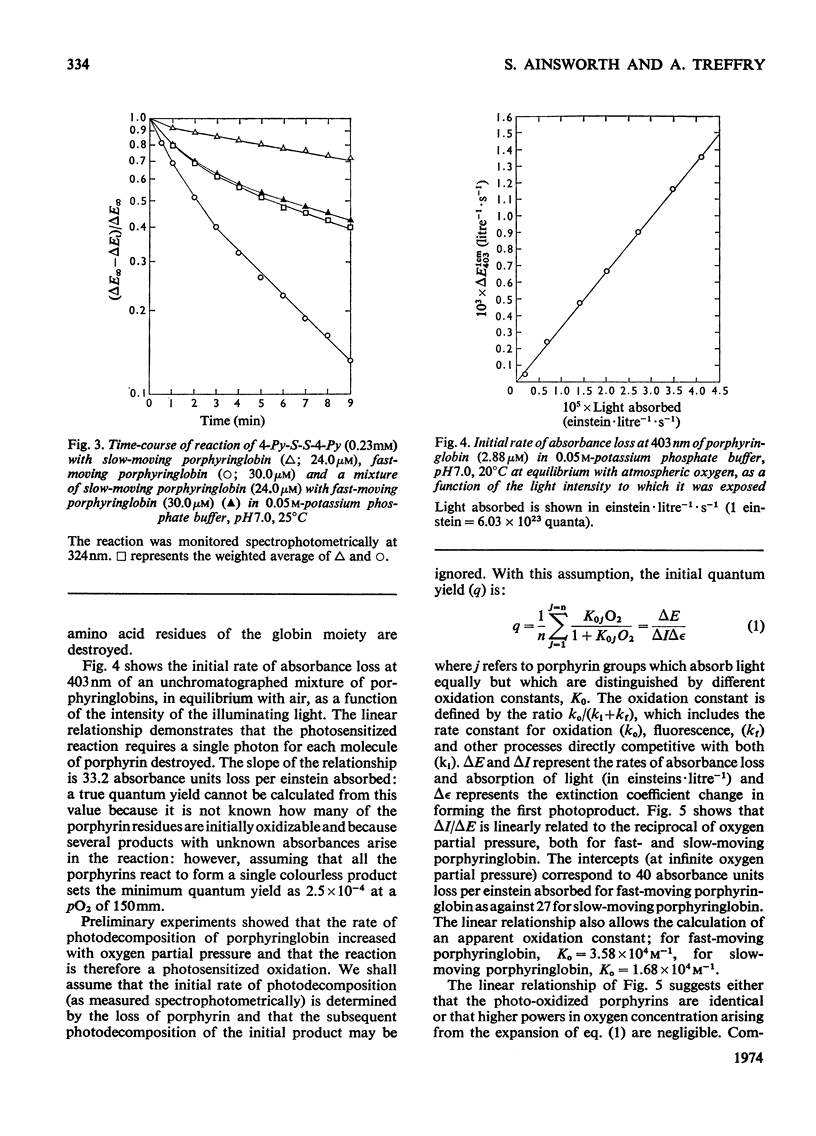
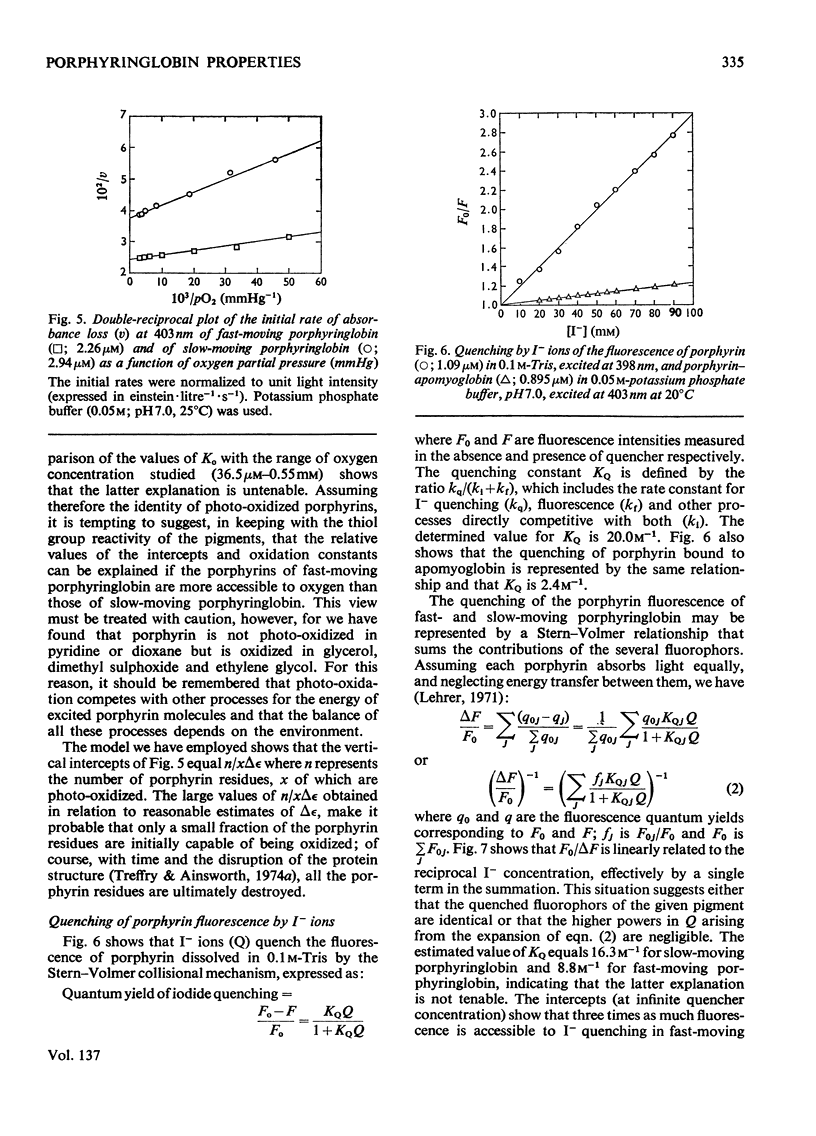
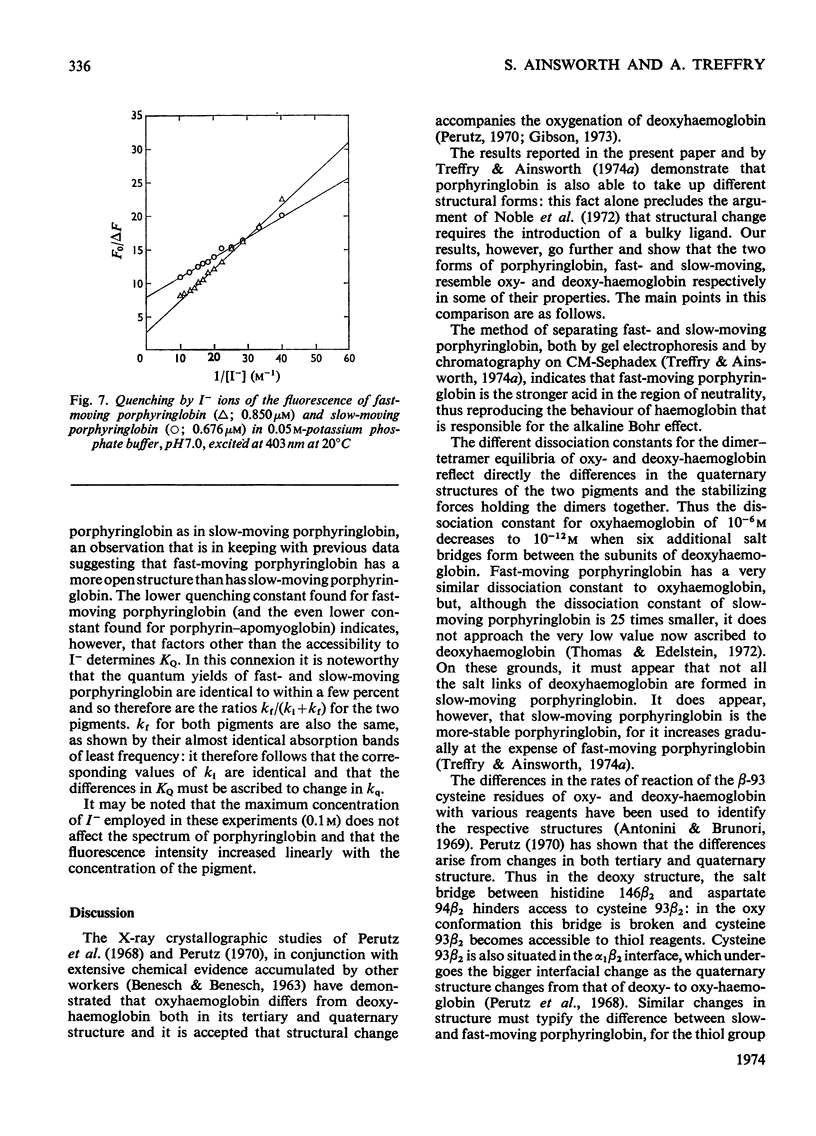
Globin was prepared from the main (A0) component of human haemoglobin and reacted with protoporphyrin IX; the product, when subjected to chromatography on CM-Sephadex, separated into fast- and slow-moving species. These were isolated for examination. The dissociation constant for the tetramer–dimer equilibrium of fast-moving porphyringlobin was determined at 2.8×10−6m; this is to be compared with values of 2.2×10−6m and 8×10−8m determined for oxyhaemoglobin and the slow-moving porphyringlobin respectively. It was also shown that the thiol groups of fast-moving porphyringlobin react with 4,4′dithiodipyridine at an identical rate with those of oxyhaemoglobin; in comparison, the rates of reaction of deoxyhaemoglobin and porphyringlobin are much slower but are again identical with one another. The quenching of porphyringlobin fluorescence by I− ions was also studied. The quenching could not be represented by a simple Stern–Volmer relationship (whereas that of porphyrin–apomyoglobin is), but was represented by a model in which the fluorescence of fast-moving porphyringlobin was more accessible to the quencher than that of the slow-moving component. Similarly, fast-moving porphyringlobin was photodecomposed more rapidly by oxygen than the slow-moving species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampulski R. S., Ayers V. E., Morell S. A. Determination of the reactive sulfhydryl groups in heme proteins with 4,4'-dipyridinedisulfide. Anal Biochem. 1969 Oct 15;32(1):163–169. doi: 10.1016/0003-2697(69)90118-3. [DOI] [PubMed] [Google Scholar]
- Antonini E., Brunori M. Hemoglobin. Annu Rev Biochem. 1970;39:977–1042. doi: 10.1146/annurev.bi.39.070170.004553. [DOI] [PubMed] [Google Scholar]
- Antonini E., Brunori M. On the rate of a conformation change associated with ligand binding in hemoglobin. J Biol Chem. 1969 Jul 25;244(14):3909–3912. [PubMed] [Google Scholar]
- Chiancone E. Dissociation of hemoglobin into subunits. II. Human oxyhemoglobin: gel filtration studies. J Biol Chem. 1968 Mar 25;243(6):1212–1219. [PubMed] [Google Scholar]
- Gibson Q. H. p-Mercuribenzoate as an indicator of conformation change in hemoglobin. J Biol Chem. 1973 Feb 25;248(4):1281–1284. [PubMed] [Google Scholar]
- Kellett G. L. Dissociation of hemoglobin into subunits. Ligand-linked dissociation at neutral pH. J Mol Biol. 1971 Aug 14;59(3):401–424. doi: 10.1016/0022-2836(71)90307-x. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Maeda T., Onishi S. I. Kinetic evidence for propagation of conformational changes in the alpha subunit to the beta subunit of hemoglobin. Biochemistry. 1971 Mar 30;10(7):1177–1180. doi: 10.1021/bi00783a013. [DOI] [PubMed] [Google Scholar]
- Noble R. W., Rossi G. L., Berni R. A strctural comparison of ligand-saturated hemoglobin with protoporphyrin globin. I. Reaction with bromothymol blue and sulfhydryl reagents. J Mol Biol. 1972 Oct 14;70(3):689–696. doi: 10.1016/0022-2836(72)90567-0. [DOI] [PubMed] [Google Scholar]
- Parkhurst L. J., Geraci G., Gibson Q. H. Kinetics of the reaction of hybrid-heme hemoglobins with carbon monoxide. J Biol Chem. 1970 Aug 25;245(16):4131–4135. [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Steinhardt J. Stabilization of horse globin by protoporphyrin IX and hemin. J Biol Chem. 1970 Oct 25;245(20):5395–5403. [PubMed] [Google Scholar]
- Thomas J. O., Edelstein S. J. Observation of the dissociation of unliganded hemoglobin. J Biol Chem. 1972 Dec 25;247(24):7870–7874. [PubMed] [Google Scholar]
- Treffry A., Ainsworth S. A study of the properties of hybrids of oxyhaemoglobin and deoxyhaemoglobin with two porphyringlobin species. Biochem J. 1974 Feb;137(2):339–348. doi: 10.1042/bj1370339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffry A., Ainsworth S. A study of the reaction of protoporphyrin IX with human globin. Biochem J. 1974 Feb;137(2):319–329. doi: 10.1042/bj1370319. [DOI] [PMC free article] [PubMed] [Google Scholar]


