Abstract
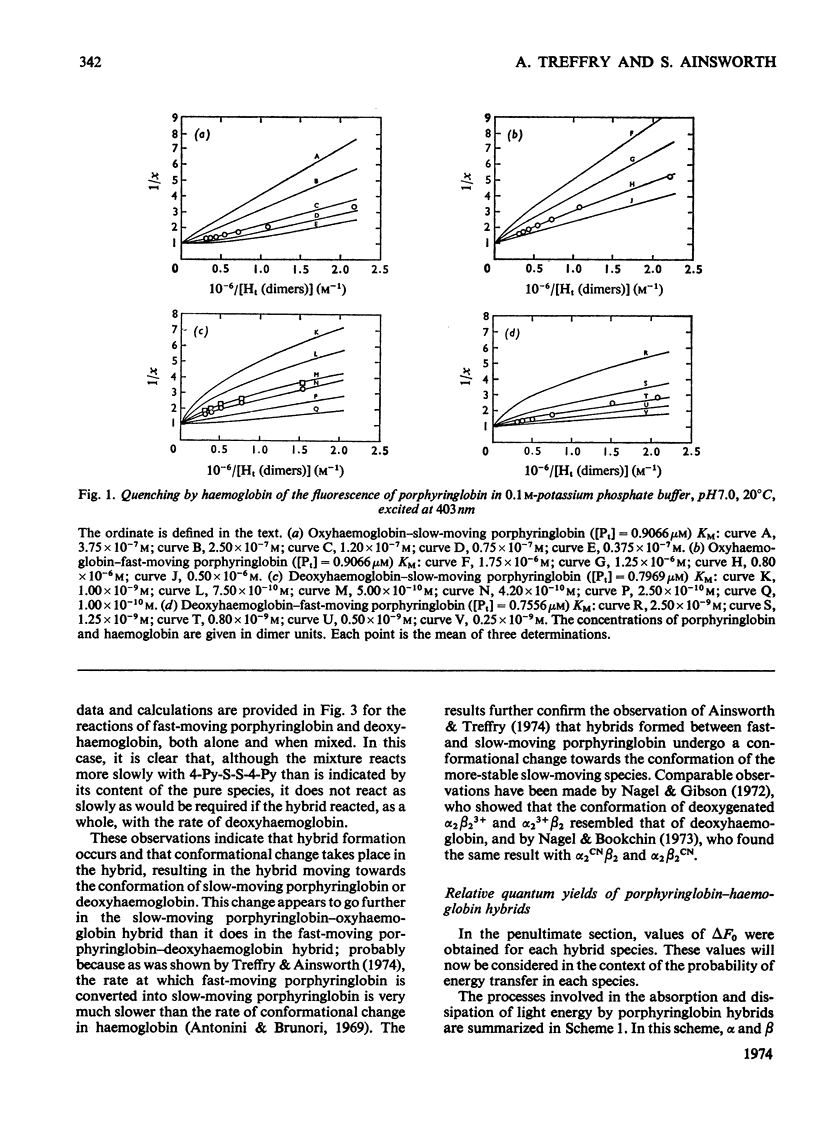
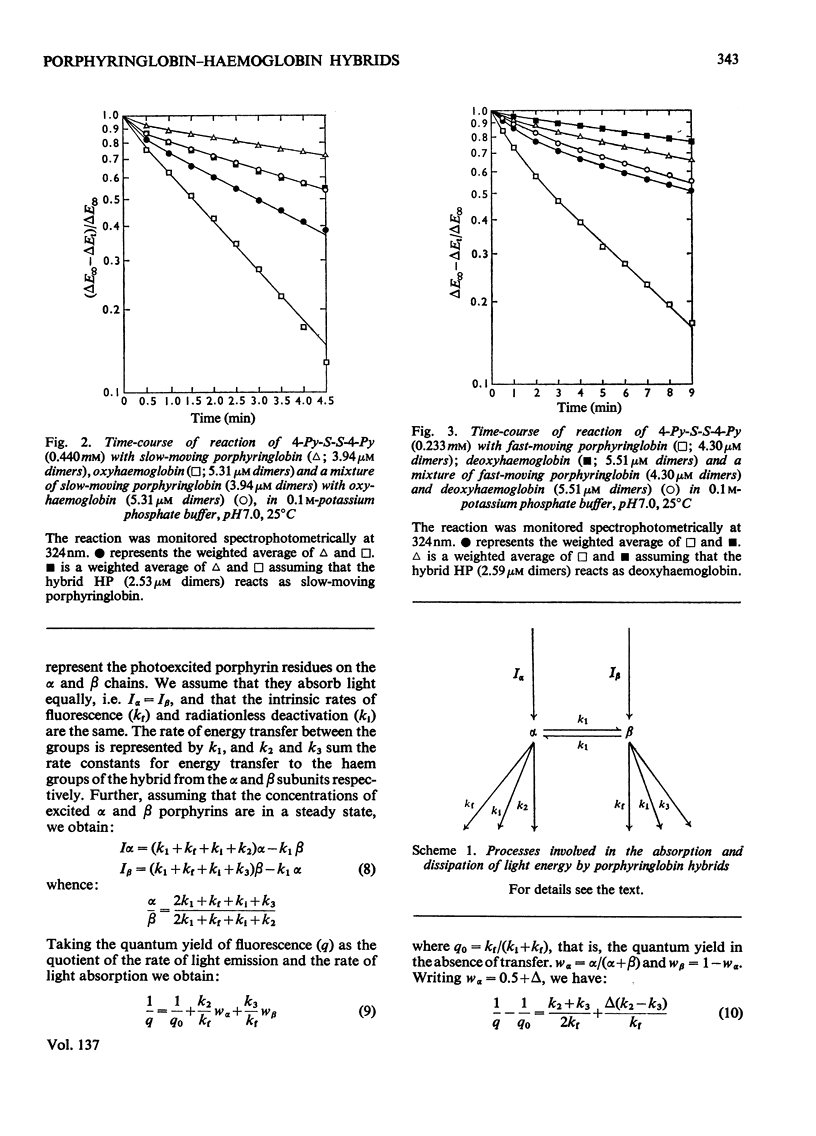
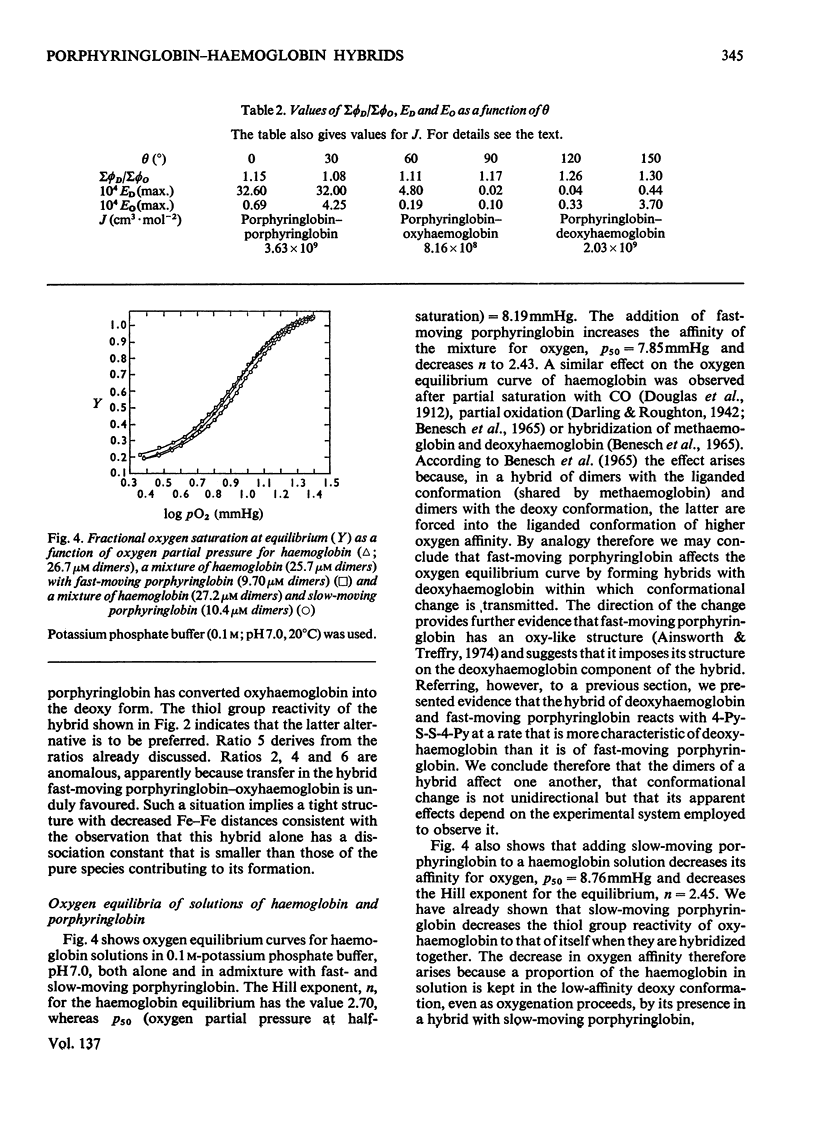
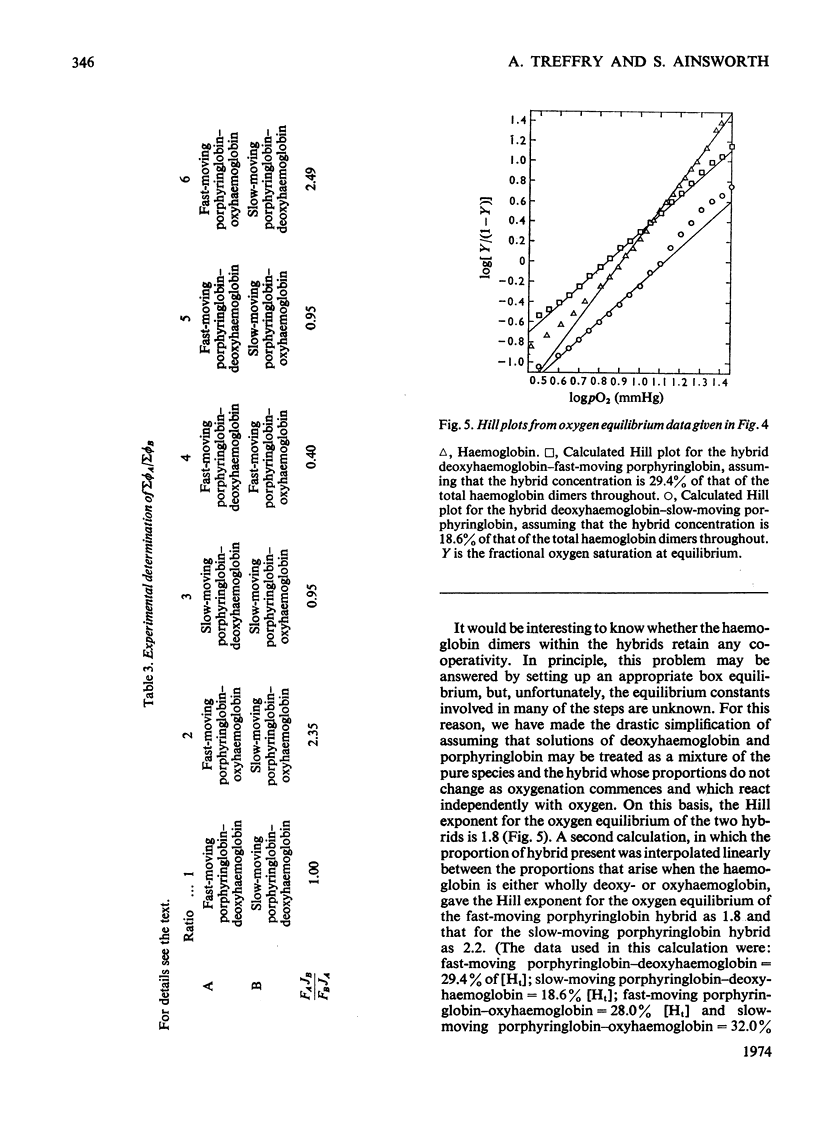
The fluorescence of porphyringlobin is quenched on adding haemoglobin to its solutions. It is suggested that this result indicates the formation of hybrids (comprising a dimer of porphyringlobin and a dimer of haemoglobin) in which quenching occurs by energy transfer from the porphyrin to the haem groups of the protein. From an analysis of fluorescence quenching, dissociation constants were calculated for the hybrids of oxy- and deoxyhaemoglobin with the fast- and slow-moving porphyringlobin species isolated by chromatography on CM-Sephadex (Treffry & Ainsworth, 1974). The values obtained are: deoxyhaemoglobin–fast-moving porphyringlobin, 0.8×10−9m; deoxyhaemoglobin–slow-moving porphyringlobin, 5×10−10m; oxyhaemoglobin–fast-moving porphyringlobin, 0.8×10−6m; oxyhaemoglobin–slow-moving porphyringlobin, 1.2×10−7m. The rates of reactions of solutions of haemoglobin and porphyringlobin, containing hybrids, with the thiol reagent 4,4′-dithiodipyridine showed that the thiol groups of the hybrids deoxyhaemoglobin–fast-moving porphyringlobin and oxyhaemoglobin–slow-moving porphyringlobin react more slowly than expected on the basis of composition alone: this result indicates that the deoxy and slow-moving conformations are the more stable, imposing themselves partially on to the fast-moving or oxy dimer of the hybrid. Also the rate of the reaction of CO with deoxyhaemoglobin is decreased when slow-moving porphyringlobin is added to its solutions: this is reflected in a movement of the oxygen equilibrium curve of such a mixture to higher oxygen partial pressures. Similar experiments with deoxyhaemoglobin solutions containing fast-moving porphyringlobin, showed an initial increase in the rate of CO uptake. Correspondingly, the oxygen equilibrium curve of the mixture showed an increased affinity for oxygen. Approximate calculations to determine the oxygen equilibria of the hybrids indicate that a functional dimer retains co-operative characteristics even when the dimer accompanying it within the tetramer has the reacted conformation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainsworth S., Treffry A. A study of the properties of two porphyringlobin species formed in the reaction of protoporphyrin IX with human globin. Biochem J. 1974 Feb;137(2):331–337. doi: 10.1042/bj1370331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen M. E., Moffat J. K., Gibson Q. H. The kinetics of ligand binding and of the association-dissociation reactions of human hemoglobin. Properties of deoxyhemoglobin dimers. J Biol Chem. 1971 May 10;246(9):2796–2807. [PubMed] [Google Scholar]
- Antonini E., Brunori M. On the rate of a conformation change associated with ligand binding in hemoglobin. J Biol Chem. 1969 Jul 25;244(14):3909–3912. [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Macduff G. Subunit exchange and ligand binding: a new hypothesis for the mechanism of oxygenation of hemoglobin. Proc Natl Acad Sci U S A. 1965 Aug;54(2):535–542. doi: 10.1073/pnas.54.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Tyuma I. Subunit exchange and ligand binding. II. The mechanism of the allosteric effect in hemoglobin. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1268–1274. doi: 10.1073/pnas.56.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- Douglas C. G., Haldane J. S., Haldane J. B. The laws of combination of haemoglobin with carbon monoxide and oxygen. J Physiol. 1912 Jun 12;44(4):275–304. doi: 10.1113/jphysiol.1912.sp001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Morimoto H., Kotani M., Watari H., Hirata W. Studies on the function of abnormal hemoglobins. I. An improved method for automatic measurement of the oxygen equilibrium curve of hemoglobin. Biochim Biophys Acta. 1970 Feb 17;200(2):189–196. doi: 10.1016/0005-2795(70)90163-7. [DOI] [PubMed] [Google Scholar]
- Kellett G. L. Dissociation of hemoglobin into subunits. Ligand-linked dissociation at neutral pH. J Mol Biol. 1971 Aug 14;59(3):401–424. doi: 10.1016/0022-2836(71)90307-x. [DOI] [PubMed] [Google Scholar]
- Kellett G. L. Ligand-free haemoglobin dimers. Nat New Biol. 1971 Dec 8;234(49):189–191. doi: 10.1038/newbio234189a0. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Bookchin R. M. Conformation of mixed liganded hybrids as detected by their interaction with Hb C Harlem . Nat New Biol. 1973 Jan 31;241(109):143–144. doi: 10.1038/newbio241143a0. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Gibson Q. H. The hemoglobin-haptoglobin reaction as a probe of hemoglobin conformation. Biochem Biophys Res Commun. 1972 Aug 21;48(4):959–966. doi: 10.1016/0006-291x(72)90702-4. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Edelstein S. J. Observation of the dissociation of unliganded hemoglobin. J Biol Chem. 1972 Dec 25;247(24):7870–7874. [PubMed] [Google Scholar]
- Treffry A., Ainsworth S. A study of the reaction of protoporphyrin IX with human globin. Biochem J. 1974 Feb;137(2):319–329. doi: 10.1042/bj1370319. [DOI] [PMC free article] [PubMed] [Google Scholar]


