Abstract
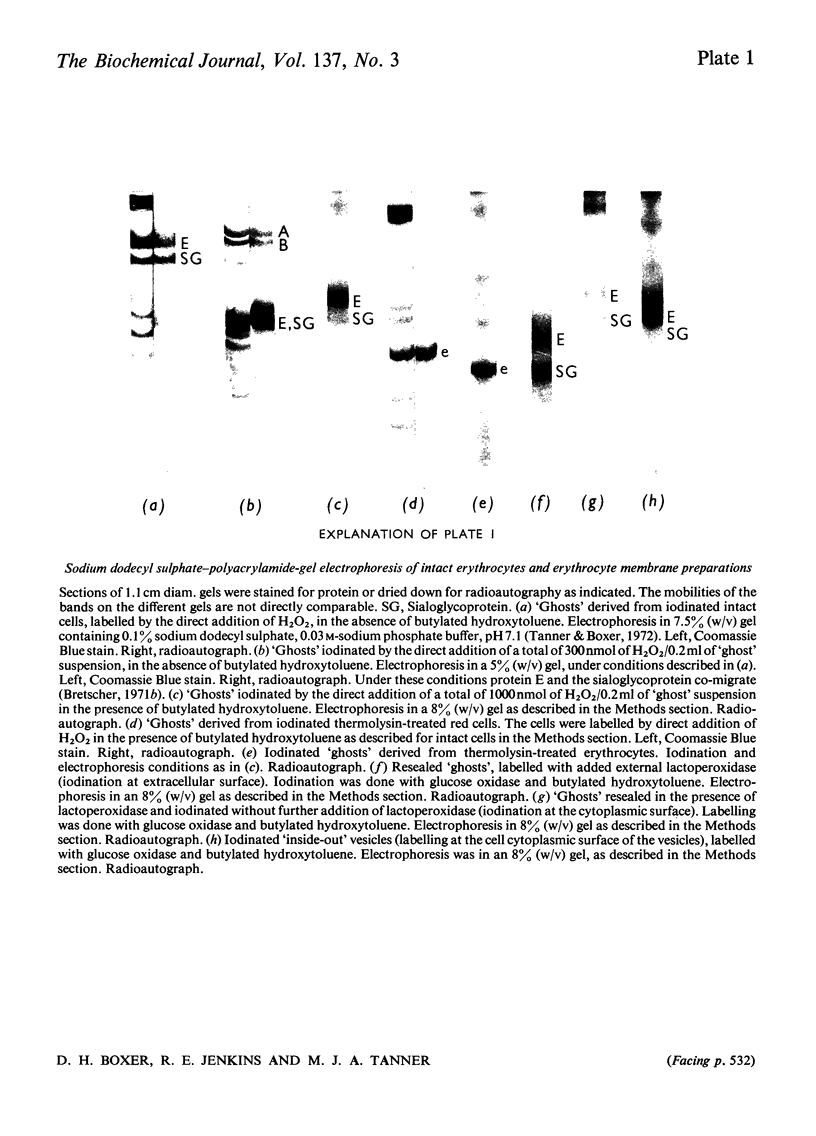
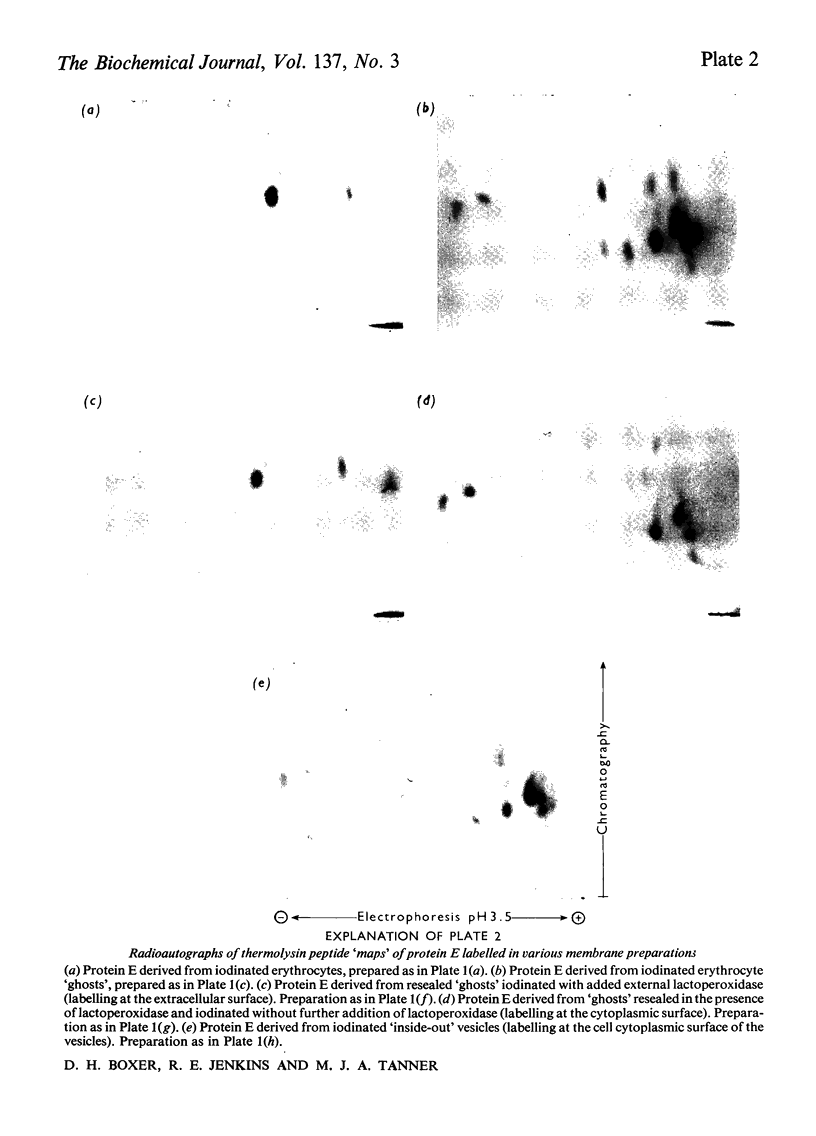
The enzyme lactoperoxidase was used to catalyse the radioiodination of membrane proteins in intact human erythrocytes and in erythrocyte `ghosts'. Two major proteins of the erythrocyte membrane were isolated after iodination of these two preparations, and the peptide `maps' of each protein so labelled were compared. Peptides from both proteins are labelled in the intact cell. In addition, further mobile peptides derived from one of the proteins are labelled only in the `ghost' preparation. Various sealed `ghost' preparations were also iodinated, lactoperoxidase being present only at either the cytoplasmic or extra-cellular surface of the membrane. The peptide `maps' of protein E (the major membrane protein) labelled in each case were compared. Two discrete sets of labelled peptides were consistently found. One group is obtained when lactoperoxidase is present at the extra-cellular surface and the other group is found when the enzyme is accessible only to the cytoplasmic surface of the membrane. The results support the assumption that the organization of protein E in the membrane of the intact erythrocyte is unaltered on making erythrocyte `ghosts'. They also confirm previous suggestions that both the sialoglycoprotein and protein E extend through the human erythrocyte membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W. W., Garan H., Berg H. C. Proteins of the human erythrocyte membrane as modified by pronase. J Mol Biol. 1971 Jun 28;58(3):783–797. doi: 10.1016/0022-2836(71)90040-4. [DOI] [PubMed] [Google Scholar]
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Human erythrocyte membranes: specific labelling of surface proteins. J Mol Biol. 1971 Jun 28;58(3):775–781. doi: 10.1016/0022-2836(71)90039-8. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant J. A., Steck T. L. Cation-impermeable inside-out and right-side-out vesicles from human erythrocyte membranes. Nat New Biol. 1972 Nov 1;240(96):26–28. doi: 10.1038/newbio240026a0. [DOI] [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exterior proteins on the human erythrocyte membrane. Biochem Biophys Res Commun. 1971 Nov;45(4):1103–1108. doi: 10.1016/0006-291x(71)90451-7. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Kahane I., Jackson R. L., Marchesi V. T. Major glycoprotein of the human erythrocyte membrane: evidence for an amphipathic molecular structure. Arch Biochem Biophys. 1973 Mar;155(1):167–183. doi: 10.1016/s0003-9861(73)80019-0. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H. Separation and some properties of the major proteins of the human erythrocyte membrane. Biochem J. 1972 Sep;129(2):333–347. doi: 10.1042/bj1290333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Gray W. R. The isolation and functional identification of a protein from the human erythrocyte 'ghost'. Biochem J. 1971 Dec;125(4):1109–1117. doi: 10.1042/bj1251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett R. B., Carraway K. L. Proteolytic digestion of erythrocytes, resealed ghosts, and isolated membranes. Biochemistry. 1972 Jul 18;11(15):2897–2903. doi: 10.1021/bi00765a024. [DOI] [PubMed] [Google Scholar]
- Welton A. F., Aust S. D. Lipid peroxidation during enzymatic iodination of rat liver endoplasmic reticulum. Biochem Biophys Res Commun. 1972 Nov 1;49(3):661–666. doi: 10.1016/0006-291x(72)90462-7. [DOI] [PubMed] [Google Scholar]